Abstract
The zebrafish has become a useful model organism for research on development and diseases. However, there has been no zebrafish model system for studying oral carcinogenesis. In the present study, we first characterized the histology of the upper gastrointestinal tract of zebrafish. We found that zebrafish tongue was covered by a non-keratinized stratified squamous epithelium, which was similar to the oro-esophageal epithelium in humans. In situ hybridization showed that keratin 5, a marker of the basal cell layer of mammalian oral epithelium, was expressed in the squamous epithelium of zebrafish tongue. A highly conserved promoter of zebrafish keratin 5 was cloned to drive transgenic expression of GFP. GFP was found to be expressed in the periderm of embryos. In adult fish, GFP was also abundantly expressed in the tongue and fin. GFP expression in transgenic fish recapitulated endogenous zebrafish keratin 5 gene expression as shown by in situ hybridization. This study indicated a high fidelity of GFP reporter gene expression in the tongue under the control of zebrafish keratin 5 promoter. This zebrafish transgenic model system may be used for future studies on oral development and cancer.
Keywords: GFP, Keratin 5, Tongue, Zebrafish, Oral cancer
Introduction
Cancers arising in the upper gastrointestinal tract are common worldwide. The prognosis for oral cancer remains dismal, as about 50% of patients succumb to the disease and its complications within five years of diagnosis. 1 Hence it is important to understand the pathogenesis of oral cancer in order to design new and effective strategies for prevention and treatment.
Rodents, especially rats and mice, have been commonly used to model oro-esophageal squamous cell carcinoma with carcinogen treatment or genetic modifications. 2, 3 However, rodent oro-esophagus is dissimilar to human oro-esophagus in many aspects. There is marked keratinization in the squamous epithelium and no submucosal glands in rodent esophagus. Rodent esophagus often shows endophytic epithelial ingrowths that invade the lamina propria of the mucosa but never extend through it, and should not be regarded as precancerous lesions. Oro-esophageal squamous papilloma is frequently seen in rodents, but rarely in humans. Rodents may die from papilloma before squamous cell carcinoma is fully developed. Oroesophageal cancer in rodents almost never metastasize. 4, 5 Therefore, novel model systems are needed for studying mechanism, prevention and therapy of oro-esophageal carcinogenesis.
The zebrafish (Danio rerio) has emerged as a new model organism for studying vertebrate developmental biology. 6–8 Due to its many favorable characteristics, including prolific reproduction, easy availability of eggs and embryos, external development, short generation time, optical transparency of embryos, and easy maintenance of both the adult and the young, it has attracted much attention in recent years as a model system for human diseases. The zebrafish has been found to develop almost any tumor type known for humans with similar morphology and comparable mechanisms. 9 Cancer features such as genomic instability, invasiveness, transplantability, and the existence of cancer stem cells apply to zebrafish tumors as well. Many tumor suppressor genes and oncogenes are highly conserved between zebrafish and veterbrates. 10 These models have been especially useful in the studies of tumor progression in living fish by fluorescence, treatment with chemical compounds, and screening for genetic modifiers. 9 Nevertheless, there has been no zebrafish model system for studying oral carcinogenesis.
It is known that the upper gastrointestinal tract of the zebrafish comprises the oral cavity, the pharynx and the esophagus. The zebrafish is stomachless and the intestine is continuous with the pharynx through a short esophagus. 11 In order to develop a system to study oral cancer, we first characterized the histology of the upper gastrointestinal tract of the zebrafish, and identified the tongue which is covered by a non-keratinized stratified squamous epithelium. A zebrafish keratin 5 (zK5) promoter was cloned to regulate tissue-specific transgenic expression of green fluorescent protein (GFP) in zebrafish tongue. Our data indicated a high fidelity of GFP reporter gene expression in the tongue under the zK5 promoter. Our study will provide a transgenic zebrafish system for future studies on oral carcinogenesis.
Materials and Methods
Zebrafish and histology
Zebrafish were obtained from the Zebrafish International Resource Center (Eugene, OR). To generate pminiTol2-zK5p-EGFP transgenic fish, AB* line was used. Fish were housed in an automatic fish housing system (Aquaneering, San Diego, CA) at 28.5°C and maintained according to the established procedures. 12 Stages of embryos were indicated as dpf (day post-fertilization) at 28.5°C. 13
In order to examine the histology of the upper gastrointestinal tract of zebrafish, we did serial sectioning of a few fish heads. We also dissected out the whole gastrointestinal tract of adult fish using a stereomicroscope. Starting from the oral cavity, periderm and gills were carefully removed with a pair of fine scissors. The gastrointestinal tract could be visualized by the presence of fish food inside. This procedure was used for fish at or more than 2 months old.
The fish head and gastrointestinal tract were fixed with 10% PBS-buffered formalin, processed, and embedded in paraffin. Five-micron sections were stained with H&E and examined using an Olympus BX51 microscope (Center Valley, PA).
In situ hybridization of tissue sections
In situ hybridization using digoxigenin-labeled riboprobes was carried out as previously described. 14 The plasmid DNA, pExpress-1-krt5, pME18S-FL3-krt15 (ATCC, Manassas, VA) and pCMV-Script-EGFP, were linearized with proper restriction enzymes for in vitro transcription with T7 RNA polymerase (pExpress-1-krt5 and pCMV-Script-EGFP), or T3 RNA polymerase (pME18S-FL3-krt15). Sense probes were also transcribed for control experiments. The procedure of in situ hybridization on paraffin sections was based on the protocol of a commercial kit (IsHyb in situ Hybridization kit; Biochain Institute, Hayward, CA). The sections were washed to remove excess stain and covered with cover slips for observation using an Olympus BX51 microscope (Center Valley, PA).
Isolation of zK5 gene promoter and construction of the pminiTol2-zK5p-EGFP plasmid
Based upon the interspecies conservation plot generated by Genome Browser (http://genome.ucsc.edu/cgi-bin/hgGateway, March 2006 assembly), a highly conserved region was identified in the promoter region of the zK5 gene. BAC clones containing the zK5 promoter were used as PCR templates, CH73-305G13, CH73-111M24 and CH73-70M8 (BACPAC Resources, Oakland, CA). The reporter gene vector used in the present study, pminiTol2-EGFP, was a homemade vector. The zK5 promoter PCR product was gel purified and cloned into pminiTol2-EGFP vector in front of the EGFP coding region at the EcoRI and BamHI sites, in order to create the pminiTol2-zK5p-EGFP plasmid. The pT3TS-Tol2 15 vector was cleaved with XbaI and transcribed with T3 RNA polymerase using the Ambion mMessage Machine kit (Austin, TX), to produce capped Tol2 transposase RNA.
Microinjection and detection of GFP expression
Transgenic zebrafish were generated by microinjection of one-cell stage AB* zebrafish embryos with a mixture of pminiTol2-zK5p-EGFP (80 μg/ml) and Tol2 transposase RNA (4 μg/ml) 15. The injected embryos were reared in autoclaved Holtfreter's solution (0.35% NaCl, 0.01% KCl, and 0.01% CaCl2) supplemented with 1μg/ml of methylene blue. Dead and grossly abnormal embryos were removed. Bright-field and fluorescent images were captured using a Nikon SMZ-1500 stereomicroscope (Melville, NY) and an Olympus MVX10 macroview microscope (Center Valley, PA). Stable germ-line transgenic lines were bred to homozygosity in F2 generation.
Whole-mount in situ hybridization
The embryos were fixed with 4% paraformaldehyde, hybridized with a digoxigenin-labeled RNA probe in a hybridization buffer (50% formamide, 5x SSC, 50mg/ml tRNA, and 0.1% Tween 20) at 70°C, followed by sequential incubation with anti-digoxigenin antibody conjugated with alkaline phosphate, and the substrates.
Immunohistochemical staining
Expression of GFP protein was examined with immunohistochemical staining. In brief, paraffin-embedded tissue sections were deparaffinized, rehydrated, and pretreated. Staining was performed with the ABC kit (Vector Labs, Capenteria, CA) according to the manufacturer's instructions with a mouse monoclonal antibody (Clone 3E6; Invitrogen Molecular Probes, Eugene, OR). Normal serum or phosphate buffered saline were used as negative controls, instead of the primary antibodies. Both positive and negative control slides were processed in parallel.
Results
Histology of the upper gastrointestinal tract of zebrafish
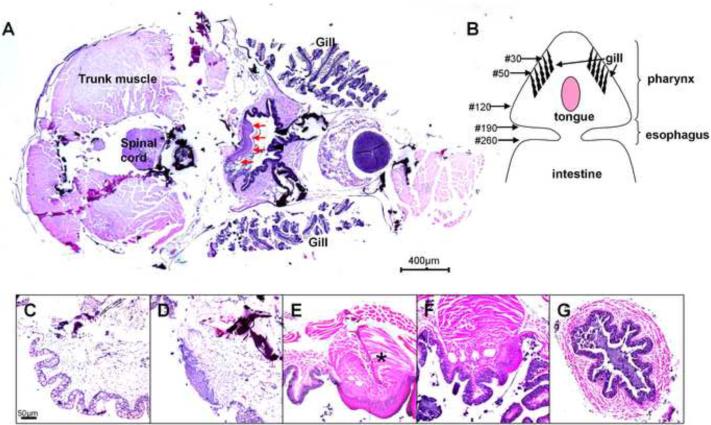
On serial horizontal sections of fish heads, a short segment of stratified squamous epithelium was found on the dorsal side of the pharynx (Figure 1A). To better characterize this area, the whole gastrointestinal tract was dissected out from adult fish. The pharynx was funnel-shaped in connection with a narrow tube of esophagus. In one typical case, serial horizontal sectioning of formalin-fixed paraffin-embedded tissue generated ~260 sections for the upper gastrointestinal tract (Figure 1B). The pharynx was covered by simple columnar epithelium containing many mucous cells (Figure 1C). Section 50 (Figure 1D) to Section 120 (Figure 1F) contained a segment of non-keratinized stratified squamous epithelium. Between these two sections, for example Section 90 (Figure 1E), showed the presence of cylinder-shaped tongue covered by such an epithelium with bulky muscle in the middle. The esophagus was covered by a simple columnar epithelium containing numerous mucous cells (Figure 1G).
Figure 1.
Histology of the tongue of adult zebrafish (H&E staining). Horizontal sectioning of head of an adult fish showed the tongue as indicated by red arrows (A). Sections were numbered on a schematic drawing of serial sectioning of the upper gastrointestinal tract, which was dissected from an adult zebrafish (B). Oral cavity was found to be covered by simple columnar epithelium (C, Section No. 30). The tongue appeared between Section No. 50 (D) and Section No. 120 (F). The squamous epithelium and muscles underneath (asterisk) indicated the presence of a tongue (E, Section No. 90). Esophagus appeared as a tubular structure between Section No. 190 and Section No. 260. It was covered by a simple columnar epithelium (section No. 220; G).
Expression of K5 and K15 in zebrafish tongue
Since keratin 5 and 15 were known to be expressed in the squamous epithelial cells of the upper gastrointestinal tract in mammals, 16 their expression patterns in zebrafish upper gastrointestinal tract were first examined. As shown by in situ hybridization analysis, K5 mRNA was expressed strongly in the squamous epithelium of the tongue, especially in the basal cell layer (Figure 2A). K15 mRNA was only slightly expressed with relatively stronger expression in the parabasal layer (Figure 2B). Little or no staining was found with a sense probe. Similar expression patterns were also observed in the periderm (data not shown).
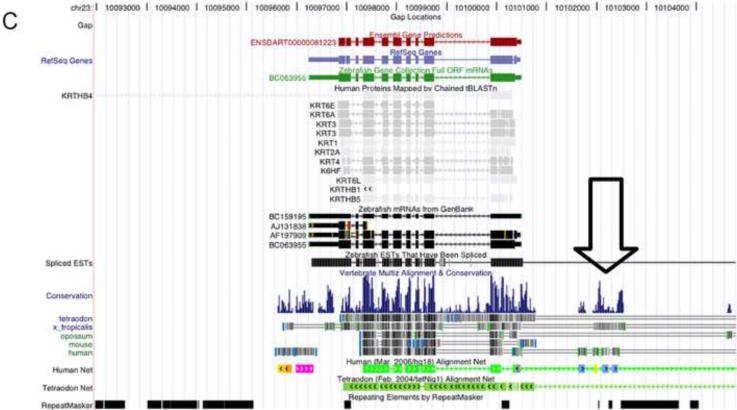
Figure 2.
Tissue-specific expression of endogenous keratins (K5 and K15) and cloning of the promoter of the zebrafish K5 gene. In the adult zebrafish tongue, K5 mRNA was found to be highly expressed (A), while K15 expressed at a low level (B), as detected by in situ hybridization. A highly conserved region in the promoter area of K5 gene was identified by comparing the sequences of K5 among five species (C). This 2,358bp segment was cloned and sequenced. The sequence highlighted by red is the highly conserved DNA sequence in the promoter region. Two regions correspond to PCR primers are underlined (D). pminiTol2-zK5p-EGFP plasmid was constructed (E).
Expression of endogenous K5 and GFP driven by the zK5 promoter
Conservation analysis of zebrafish K5 gene demonstrated the presence of a highly conserved DNA sequence in the promoter region across multiple species (Figure 2C). A 2,358-bp sequence 5' upstream of the K5 translational initiation site was amplified and cloned (Figure 2D). A 7.9-kb transgenic construct, pminiTol2-zK5p-EGFP, was made by cloning zK5 promoter into a pminiTol2-EGFP vector (Figure 2E).
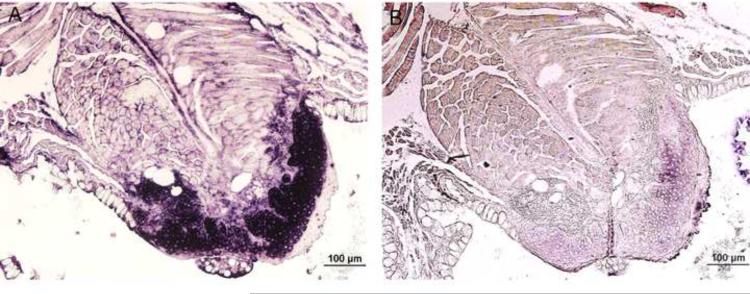
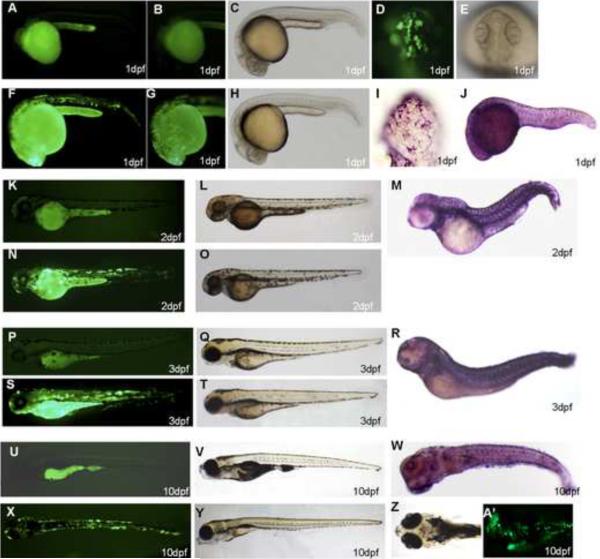
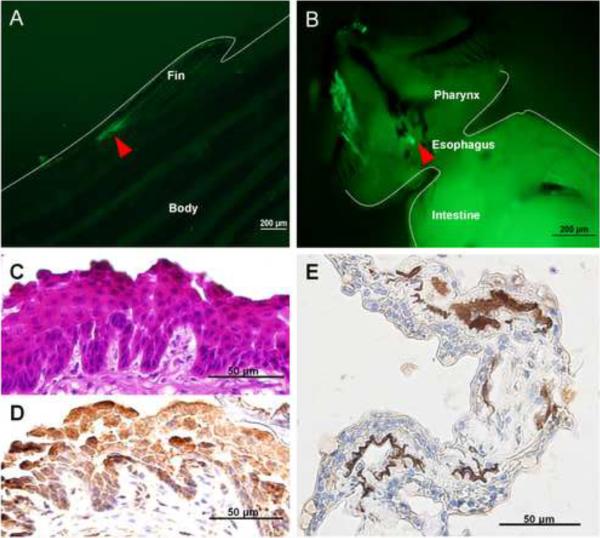
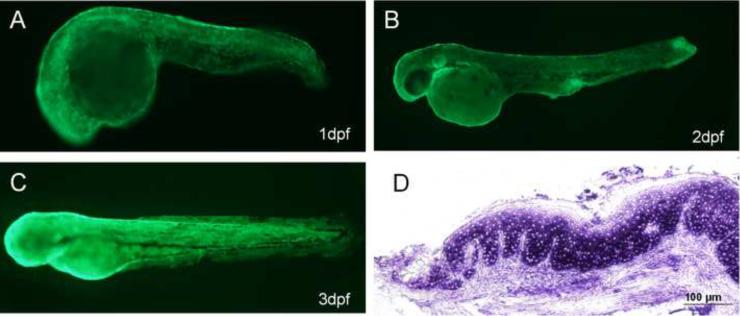
In F0 generation, at 1dpf, the strongest GFP expression was usually found in the yolk sac, the periderm and the cornea (Figure 3D, F, G). The transient transgenic expression was mosaic as it was observed only in some, but not all, peridermal cells. By 2dpf and 3dpf, about 90% of surviving embryos expressed GFP and 100% of GFP-expressing embryos showed expression in the periderm and the cornea (Figure 3N, S). GFP expression was still visible when the embryos were 10dpf (Figure 3X, A'). About 80% of the fish initially expressing GFP remained GFP-positive at this stage without obvious decrease of the expression level. We did not observe any GFP expression in wild-type zebrafish controls at 1dpf (Figure 3A, B), 2dpf (Figure 3K), 3dpf (Figure 3N) and 10dpf (Figure 3U). Bright field image of wild-type zebrafish (Figure 3C, L, Q, V) and transgenic zebrafish (Figure 3H, O, T, Y), showed that transgenic zebrafish developed normally. Wild-type zebrafish embryos were hybridized with K5 antisense riboprobe at 1dpf (Figure 3I, J), 2dpf (Figure 3M), 3dpf (Figure 3R) and 10dpf (Figure 3W). The expression pattern of endogenous K5 mRNA in the periderm was consistent with peridermal GFP expression in transgenic founder embryos. In adult fish, GFP was found to be expressed in the fin (Figure 4A) and the pharynx (Figure 4B). Immunohistochemical staining confirmed GFP expression in both the tongue (Figure 4C, D) and the fin (Figure 4E) of the same fish.
Figure 3.
Expression of GFP in founder zebrafish. GFP expression was observed in F0 founder embryos at 1dpf (D, F, G), 2dpf (N), 3dpf (S), and 10dpf (X, A'). GFP was expressed in yolk sac, the cornea, and the periderm. Wild-type zebrafish controls: 1dpf (A, B), 2dpf (K), 3dpf (N), 10dpf (U). Comparison of the bright field image of wild-type zebrafish (C, L, Q, V) with transgenic zebrafish (H, O, T, Y), showed normal development of transgenic zebrafish. Wild-type zebrafish embryos were hybridized with zK5 antisense riboprobe at 1dpf (I, J), 2dpf (M), 3dpf (R), and 10dpf (W). High-resolution images showed GFP expression and zK5 mRNA in periderm (D and I, respectively). Stages of embryos are indicated in each panel.
Figure 4.
Expression of GFP in the tongue and fin of founder zebrafish. Expression of GFP in the fin (A) and the tongue (B) of founder zebrafish are indicated in the area outlined by dashed lines and arrowhead. H&E staining of the tongue of a zebrafish (2-month-old, F0 generation) showed non-keratinized stratified squamous epithelium (C). GFP protein was expressed in the squamous epithelial cells of zebrafish tongue (D) and fin (E) as detected by immunohistochemical staining.
Generation of zK5p-EGFP stable transgenic zebrafish
To generate germ-line transmitted transgenic zebrafish, pminiTol2-zK5p-EGFP was injected into zebrafish eggs at 1–2 cell stage. Approximately 90% of injected embryos expressed GFP and were raised to the adult stage. Of 18 founder fish screened, three produced GFP-expressing offspring. These F1 generation embryos expressed GFP in an identical pattern to founder embryos, indicating germ-line transmission of the transgene. Meanwhile, F1 generation fish did not exhibit mosaic expression of GFP, and had an expression pattern of GFP mimicking that of the endogenous K5 gene at 1dpf (Figure 5A), 2dpf (Figure 5B) and 3dpf (Figure 5C).
Figure 5.
GFP expression in the periderm and tongue of F1 generation of pminitol-zK5p-EGFP transgenic zebrafish. GFP expression patterns in transgenic zebrafish were observed by fluorescence microscopy at 1dpf (A), 2dpf (B), and 3dpf (C). Expression of GFP mRNA in the squamous epithelial cells of zebrafish tongue was detected by GFP in situ hybridization (2-month-old, F1 generation) (D).
In situ hybridization showed that GFP mRNA was expressed in the squamous epithelial cells in the tongue of adult fish, especially in the basal cell layer (Figure 5D). The expression pattern was the same as that of the endogenous K5 gene (Figure 2A). In conclusion, these data confirmed that the zK5 promoter directed GFP expression to cell types that normally express K5 in zebrafish tongue.
Discussion
In this study, we characterized the histology of the upper gastrointestinal tract of zebrafish, and generated transgenic zebrafish expressing GFP under the control of the zK5 gene promoter. Stable lines of transgenic zebrafish were generated to express GFP in the epithelial cells of the tongue, with an expression pattern consistent with that of the endogenous K5 gene. To our knowledge, this study is the first of its kind in developing a zebrafish model system which can be used for future studies on oral carcinogenesis.
The zebrafish oral cavity can be divided into three zones based on the well-differentiated anatomical relieves as well as the aspect and structure of their epithelial covering. The outer zone contains the lips, the intermediate zone corresponding to the internal valves, and the internal zone corresponding to the tongue. 11 Very few studies in the literature have described zebrafish tongue. It was described as a mediosagittal cylinder-shaped structure by scanning electron microscopy, which was clearly isolated from the jaw and adhered to the lateral walls by differentiated plicae. 11 In the present study, we describe that zebrafish tongue is covered by a non-keratinized stratified squamous epithelium, which is similar to the oro-esophageal epithelium in humans.
Transgenic expression of GFP reporter in germ-line transmitted zebrafish has proven to be a powerful tool to recapitulate gene expression programs, because GFP expression can be detected in live fish due to their external embryonic development and transparency. 17–19 Our current zK5p-EGFP transgenic line is a new addition to the GFP transgenic zebrafish collection. Because transgenic GFP expression was observed in most of the regions normally expressing endogenous zebrafish K5 mRNA, the 2.4-kb 5'-flanking region of the K5 gene cloned in the present study should contain most of the cis-elements required for the temporal and spatial expression of K5 during development and in adulthood. No ectopic expression of transgenic GFP was noted in the present study, suggesting that the promoter was highly tissue-specific and functional in the transgenic zebrafish.
In the cancer research area, zebrafish has been used to study chemical and xenograft carcinogenesis of the gastrointestinal tract. 9, 20, 21 However, there are no reports of zebrafish being used to study oral carcinogenesis. In this study, we developed zK5p-EGFP transgenic zebrafish, which express GFP in the epithelial cells of the tongue, with an expression pattern consistent with that of endogenous K5 gene expression. Faithful expression of GFP under control of the zK5 promoter suggests that this transgenic system can be used for future studies on oral development and diseases. In addition, fish maintenance is more economical than rodents. Chemical agents may be administered to fish through water or food. Nevertheless, the zK5 promoter has potential limitations. Strong peridermal expression of genes driven by zK5 promoter may pose a potential problem in generating an oral cancer model. We are currently testing two separate approaches to address this issue. First, periderm-specific promoters are being analyzed to identify a promoter that drives gene expression only in the periderm, but not the tongue. Such promoters may be used to express small hairpin RNA that can specifically inhibit the expression of the exogenous gene in the periderm. One potential candidate promoter is zebrafish keratin 8 promoter, which is active in the periderm and gastrointestinal system, 17, 22 but not in the tongue (data not shown). Second, we are currently analyzing promoter regions of other genes expressed in zebrafish tongue.
In conclusion, we describe here the generation of transgenic zebrafish expressing GFP under control of the zK5 gene promoter. Such a transgenic system is likely to be useful in future studies on development and diseases of the oral system.
Acknowledgement
The authors thank Dr. Stephen C. Ekker for the generous gift of pminiTol2 and pT3TS-Tol2 vectors, and Ms. Shanta Mackinnon for maintaining zebrafish. This study was supported by NIH grants U56 CA092077 and NS33981.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel V, Leethanakul C, Gutkind JS. New approaches to the understanding of the molecular basis of oral cancer. Crit Rev Oral Biol Med. 2001;12(1):55–63. doi: 10.1177/10454411010120010401. [DOI] [PubMed] [Google Scholar]
- 2.Opitz OG, Harada H, Suliman Y, Rhoades B, Sharpless NE, Kent R, et al. A mouse model of human oral-esophageal cancer. J Clin Invest. 2002;110(6):761–9. doi: 10.1172/JCI15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoner GD, Wang LS, Chen T. Chemoprevention of esophageal squamous cell carcinoma. Toxicol Appl Pharmacol. 2007;224(3):337–49. doi: 10.1016/j.taap.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Yang CS. Barrett's esophagus - preclinical models for investigation. In: Jobe BA, Thomas CR, Hunter JG, Tran N, editors. Esophageal Tumors: Principles and Practice. Demos Medical Publishing; New York: 2009. pp. 61–7. [Google Scholar]
- 5.Pozharisski KM. Tumors of the esophagus. In: Turusov VS, Mohr U, editors. Pathology of Tumors in Laboratory Animals. International Agency for Research on Cancer; Lyon: 1990. pp. 109–29. [Google Scholar]
- 6.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- 7.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125(23):4655–67. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 8.Pack M, Solnica-Krezel L, Malicki J, Neuhauss SC, Schier AF, Stemple DL, et al. Mutations affecting development of zebrafish digestive organs. Development. 1996;123:321–8. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- 9.Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res. 2008;6(5):685–94. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- 10.Langenau DM, Keefe MD, Storer NY, Guyon JR, Kutok JL, Le X, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21(11):1382–95. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbate F, Germana GP, De Carlos F, Montalbano G, Laura R, Levanti MB, et al. The oral cavity of the adult zebrafish (Danio rerio) Anat Histol Embryol. 2006;35(5):299–304. doi: 10.1111/j.1439-0264.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon; Eugene, Oregon: 1995. The Zebrafish Book. [Google Scholar]
- 13.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 14.Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev Dyn. 1998;213(1):92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2(11):e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 17.Gong Z, Ju B, Wang X, He J, Wan H, Sudha PM, et al. Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8. Dev Dyn. 2002;223(2):204–15. doi: 10.1002/dvdy.10051. [DOI] [PubMed] [Google Scholar]
- 18.Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228(1):30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- 19.Lee JA, Cole GJ. Generation of transgenic zebrafish expressing green fluorescent protein under control of zebrafish amyloid precursor protein gene regulatory elements. Zebrafish. 2007;4(4):277–86. doi: 10.1089/zeb.2007.0516. [DOI] [PubMed] [Google Scholar]
- 20.Mizgireuv IV, Majorova IG, Gorodinskaya VM, Khudoley VV, Revskoy SY. Carcinogenic effect of N-nitrosodimethylamine on diploid and triploid zebrafish (Danio rerio) Toxicol Pathol. 2004;32(5):514–8. doi: 10.1080/01926230490496311. [DOI] [PubMed] [Google Scholar]
- 21.Mizgireuv IV, Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66(6):3120–5. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- 22.Imboden M, Goblet C, Korn H, Vriz S. Cytokeratin 8 is a suitable epidermal marker during zebrafish development. C R Acad Sci III. 1997;320(9):689–700. doi: 10.1016/s0764-4469(97)84816-0. [DOI] [PubMed] [Google Scholar]