Abstract
Neuroinflammatory conditions such as traumatic brain injury, aging, Alzheimer’s disease, and Down syndrome are often associated with cognitive dysfunction. Much research has targeted inflammation as a causative mediator of these deficits, although the diverse cellular and molecular changes that accompany these disorders obscure the link between inflammation and impaired memory. Therefore, we used a transgenic mouse model with a dormant human IL-1β excisional activation transgene to direct overexpression of IL-1β with temporal and regional control. Two weeks of hippocampal IL-1β overexpression impaired long-term contextual and spatial memory in both male and female mice, while hippocampal-independent and short-term memory remained intact. Human IL-1β overexpression activated glia, elevated murine IL-1β protein and PGE2 levels, and increased proinflammatory cytokine and chemokine mRNAs specifically within the hippocampus, while having no detectable effect on inflammatory mRNAs in the liver. Sustained neuroinflammation also reduced basal and conditioning-induced levels of the plasticity-related gene Arc.
Keywords: interleukin-1, inflammation, learning, memory, hippocampus, gender, activity regulated cytoskeletal protein
Introduction
Neuroinflammatory conditions such as traumatic brain injury, aging, Alzheimer’s disease, and Down syndrome are often associated with cognitive dysfunction, including memory deficits (Casadesus et al., 2007; Griffin et al., 1989; Montine et al., 1999). Much research has focused on anti-inflammatory treatments to try and prevent these memory deficits with mixed results (ADAPT Research Group et al., 2007; Hurley et al., 2002; Rogers et al., 1993). It is clear that acute inflammation, caused by injection of lipopolysaccharide or interleukin-1β (IL-1β) itself, impairs memory (Gibertini et al., 1995; Hein et al., 2007; Oitzl et al., 1993; Pugh et al., 1998; Pugh et al., 2001; Thomson and Sutherland, 2005). However, a lack of experimental models has made the study of the effects of chronic neuroinflammation on memory difficult, especially when trying to parse the potential influences of resulting sickness or neurodegeneration on memory. Therefore, we utilized a recently developed mouse model, which can maintain sustained IL-1β overexpression with temporal and regional control, to study the effects of prolonged IL-1β overexpression and ensuing neuroinflammation on learning and memory processes.
This mouse model retains a dormant human IL-1β excisional activation transgene (IL-1βXAT). When activated by microinjection of a virus expressing Cre, astrocytes local to the injection site express human IL-1β (Shaftel et al., 2007b). Human IL-1β binds to the murine IL-1β receptor and signals downstream pathways to induce inflammation. Research in this model has shown that a single microinjection of virus expressing Cre into the hippocampus causes sustained IL-1β overexpression for up to nearly a year after injection (Shaftel et al., 2007b). Within 2 weeks of transgene activation, IL-1β overexpression within the hippocampus leads to glial activation, elevated cytokines, elevated chemokines, leukocyte infiltration, and increased vascular permeability (Moore et al., 2009; Shaftel et al., 2007a; Shaftel et al., 2007b). However, after 2 weeks or 2 months of IL-1β overexpression, no overt loss of neurons or neuronal integrity is apparent within the hippocampus as measured by apoptotic stains and neuronal, synaptophysin, and acetylcholinesterase labeling (Moore et al., 2009; Shaftel et al., 2007a). Therefore, this model allows us to study the role of chronic IL-1β driven neuroinflammation on learning and memory processes.
Initial behavioral studies in this mouse model revealed spatial learning deficits in the Morris water maze 2 weeks following transgene activation (Moore et al., 2009). Here we further characterize these deficits by testing hippocampal-dependent and -independent, as well as short- and long-term, fear memory and potential gender differences in fear conditioning and the Morris water maze. We also further characterize the central and peripheral inflammatory responses to sustained hippocampal IL-1β overexpression and find changes in one plasticity-related gene, activity-regulated cytoskeleton-associated protein (Arc; also Arg 3.1).
Materials
hIL-1βXAT construct
Creation and genotyping of IL-1βXAT mice on a C57BL/6 background has been described previously (Shaftel et al., 2007b). Briefly, a construct with a murine GFAP promoter, loxP flanked transcriptional stop, and downstream signal sequence for the human IL-1RA was fused to the cDNA of human mature IL-1β to allow extracellular release of IL-1β.
Feline immunodeficiency virus (FIV)
The construction and packaging of FIV-Cre has been described previously (Lai et al., 2006). Briefly, the FIV-Cre virus encodes a modified Cre recombinase protein with a nuclear localization sequence, and V5 epitope tag under the control of a cytomegalovirus promoter. FIV-Cre mediated excision of the transcriptional stop activates the IL-1βXAT transgene. FIV-Cre and FIV-green fluorescent protein (GFP) (System Biosciences, Mountain View, CA) used in these studies had final titers of ~1×107 infectious viral particles per milliliter.
Animals
Experiment 1
Male IL-1βXAT B/b transgenic and wild-type (WT) littermate mice were used for experiment 1. Mice were housed in temperature (23 ± 3 °C) and light (12:12 light:dark) controlled rooms with free access to chow and water. All animal procedures were reviewed and approved by the University Committee on Animal Resources of the University of Rochester Medical Center for compliance with federal regulations prior to the initiation of the study. FIV-Cre injected WT and FIV-GFP injected IL-1βXAT mice served as controls for both genotype and viral injection. Since no differences were found between these groups only FIV-Cre injected WT mice were used as controls in experiments 2 and 3.
Experiment 2
Male and female IL-1βXAT B/b transgenic and WT littermate mice were used for experiment 2. Animal colonies were maintained as described above. After viral microinjections, mice were shipped to Denver, CO and allowed to recover for 7-10 days before behavioral experiments began. In Denver, animals were housed at the Center for Laboratory Animal Care at the University of Colorado Denver on a 12:12 h light/dark cycle with ad libitum access to food and water. Experiments were under the approval of the University of Colorado’s Animal Care and Use Committee.
Experiment 3
Male and female IL-1βXAT B/b transgenic mice were used for experiment 3 and maintained as in experiment 1.
Microinjections
Intrahippocampal microinjections were described previously (Shaftel et al., 2007b). At 8-16 weeks of age, mice received bilateral, hippocampal FIV-Cre or FIV-GFP injections in experiments 1 and 2. In experiment 3, mice were injected with FIV-Cre in one side of the hippocampus and FIV-GFP in the other to create a within subjects design. Under 1.75% isoflurane, in 30% oxygen and 70% nitrogen gas, mice were placed into a Kopf stereotaxic apparatus in a biosafety level 2 approved facility. A 0.5 mm burr hole was drilled at AP: -1.8 mm and ML: ±1.8 mm relative to bregma and a 33 gauge needle attached to a 10 μl syringe was lowered 1.8 mm over 2 min. A Micro-1 microsyringe pump controller (World Precision Instruments) injected 1.5 μl of virus at a constant rate over 10 min and 5 min was allowed for diffusion, resulting in delivery of approximately 1.5 × 104 infectious viral particles to the mouse hippocampus. The needle was raised over 2 min and burr hole sealed with bone wax. The procedure was then repeated on the opposite side to deliver virus bilaterally. Following both injections, the scalp incision was closed with tissue adhesive (Vetbond). Behavioral experiments began 2 weeks after FIV microinjections.
Behavioral Procedures
Experiment 1
Mice underwent contextual and auditory fear conditioning to assess hippocampal-dependent and -independent memory processes (see figure 1 A for timeline). For 3 days before fear conditioning, mice were transported from the colony room to the testing room, handled for 2 min each, and returned to the colony room to acclimate them to experimenter manipulation. On conditioning day, mice were allowed to explore the conditioning context, which consisted of a Plexiglas chamber and metal floor grid (model H10-11M; Coulbourn Instruments, Whitehall, PA, USA). After 3 min, 15 s of white noise (80 dB) was presented co-terminating with a 2 s, 0.75 mA foot shock. This noise-shock pairing was repeated twice for a total of 3 shocks with an interval of 30 s between shocks. One or 24 hours later to test short-term or long-term memory respectively, mice were re-exposed to the conditioning chamber for 6 min each. Mice that had been tested for long-term contextual fear memory were also tested for freezing to a novel context and the auditory stimulus 4 hours after initial testing. Mice were placed in a novel context consisting of a 15 cm open-topped plastic cylinder with bedding on the floor for 3 min followed by re-exposure to the white noise for 3 min. All data were video recorded using FreezeFrame Video-Based Conditioned Fear System and analyzed by Actimetrics Software (Coulbourn Instruments). In a pilot study, automated scoring in this program showed greater than 95% concordance with human scoring.
Figure 1.
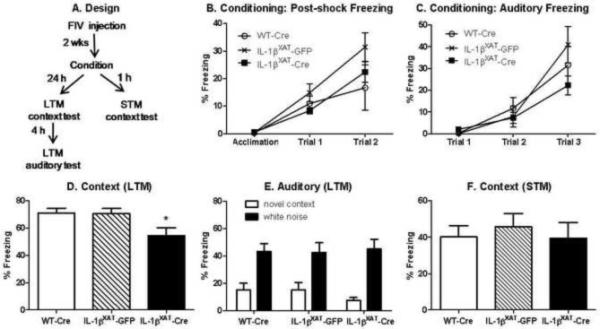
Design for behavioral studies in experiment 1 (A). Mean percent freezing (±SEM) during the acquisition, post-shock intervals, and auditory stimuli during conditioning increased with repeated pairing but did not differ amongst groups (B, C). In the long-term memory (LTM) tests, IL-1βXAT-Cre injected mice show reduced freezing to the conditioned context compared to both WT-Cre and IL-1βXAT-GFP injected mice (D, * p < 0.03). All mice showed low freezing to a novel context and strong freezing to a conditioned white noise stimulus (E). No differences in freezing to the conditioned context were found 1 h after conditioning, indicating intact short-term memory (STM).
Experiment 2
Fear Conditioning
The protocol for these experiments was based on previous work with the Ts65Dn mouse model for Down syndrome (Costa et al., 2008). Mice were allowed to explore a novel conditioning chamber (Med Associates, St. Albans, VT, Modular Mouse Test Chamber) for 3 min before the onset of 15 s of white noise (80 dB) followed by a 2 s, 0.7 mA foot shock. Twenty-four hours later, mice were re-exposed to the conditioning chamber and freezing measured every 9 s for 3 min by two observers blind to mouse genotype. Four hours later, mice were placed in a novel context for 3 min followed by re-exposure to the white noise for 3 min and freezing was assessed. All experiments were videotaped for archival purposes.
Morris water maze
The protocol for these experiments was also based on previous work with the Ts65Dn mouse model for Down syndrome (Stasko and Costa, 2004). After contextual and auditory fear testing, animals were allowed to rest for 2 days before water maze training began. A circular pool (95 × 17 cm) was filled with opaque water (24 ± 5 °C) and a platform (6.5 cm diameter circle) placed 1 cm below the surface of the water in the center of 1 of the 4 quadrants. For each training trial, a mouse was placed randomly in 1 of 4 assigned quadrants and allowed to swim freely. Once the platform was located, the mouse was allowed to remain for 10 s. If the platform was not located after 60 s, the mouse was gently guided to the platform and allowed to remain for 10 s. Trials were separated by ~20 min and mice dried between trials to prevent hypothermia. Potential differences in swim speed and motivation were first assessed during visible training, where the platform’s location was made conspicuous by the presence of a flag. Mice received 3 blocks of 4 training trials for the visible platform. Two hours after the last visible training trial, hidden platform training began. Each animal received 9 blocks of 4 training trials for the hidden platform (2 blocks/day). At the end of this training, a probe test was completed where the platform was removed from the pool and each mouse was allowed to search freely for 60 s. A video-tracking system was used to monitor and quantify performance (Videomex-V and Water Maze Program version 5, Columbus Instruments, Columbus, OH, acquisition rate = 30 frames/s).
Experiment 3
Mice were handled for 3 days as described in experiment 1. Following handling, half of the mice underwent contextual fear conditioning and half remained in their home cage as unconditioned controls. Mice were sacrificed 15 minutes after conditioning by cervical dislocation, brains removed, and hippocampi rapidly dissected and frozen in isopentane for RNA analysis.
Immunohistochemistry
Mice were anesthetized with ketamine and xylazine (i.p., 60-90 and 4-8 mg/kg, respectively) and perfused with 0.15 M phosphate buffer (PB) containing 2 IU/ml heparin and 0.5% w/v sodium nitrite followed by 4% paraformaldehyde in 0.15 M PB (pH 7.2). Brains were removed, postfixed for 2 h, sunk in 30% sucrose overnight, frozen in cold isopentane, and stored at -80 °C. Brains were sectioned at 30 μm on a sliding microtome and free-floating sections stored in cryoprotectant until assayed. Sections were washed in 0.15 M PB, reacted in 3% H2O2, blocked with 10% normal serum (Vector), and incubated in rat anti-mouse MHCII (Cd74; BD Pharmingen, clone M5/114.17.2, 1:6000) or rabbit anti-bovine GFAP (Dako 1:6000) for 48 h. A biotinylated rabbit anti-rat mouse absorbed or goat anti-rabbit IgG (Vector, 1:2000) secondary antibody was used followed by incubation in ABC Elite (Vector) and Ni-DAB reaction. Sections were mounted, cleared, and coverslipped. Light microscopic images were acquired on an Axioplan IIi (Zeiss) microscope equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments).
IL-1β and PGE2 Immunoassays
Mice were anesthetized with ketamine and xylazine (i.p., 60-90 and 4-8 mg/kg, respectively) and perfused with 0.15 M PB containing 2 IU/ml heparin, 0.5% w/v sodium nitrite, and indomethacin (1:10,000). Brains were removed and hippocampi dissected, rapidly frozen in cold isopentane, and stored at -80 °C. Hippocampi were weighed and homogenized in Tissue Protein Extraction Reagent (T-PER; Pierce, Rockford, IL, USA) with protease (EMD Biosciences, San Diego, CA, USA) and phosphatase (Sigma) inhibitors. T-PER was added at a volume of 1 ml per 50 mg of frozen tissue. Homogenate was briefly sonicated and vortexed. Murine IL-1β protein was measured using the Quantikine Immunoassay (R&D Systems, Minneapolis, MN, USA) according to manufacturer’s directions. Total protein concentrations were determined using a bicinchoninic acid assay (Pierce).
PGE2 was extracted from the tissue homogenate with 70% cold methanol, briefly vortexed, and then centrifuged for 20 min at 15,000 g. The supernatant was evaporated to dryness and the pellet was resuspended in assay buffer. PGE2 concentration was determined by enzyme immunoassay according to the manufacturer’s protocol (Cayman Chemical, Ann Arbor, MI, USA).
Real-time RT-PCR
RNA extraction and cDNA synthesis
Animals were treated and hippocampi were removed as described above, except that a piece of liver was removed before perfusion, frozen in cold isopentane, and stored at -80 °C in experiment 1 to assess the peripheral inflammatory state in these transgenic mice. Total RNA was extracted using the TRIzol (Invitrogen, Carlsbad, CA, USA) method. Hippocampi or liver were homogenized in 1 ml TRIzol using an Omni 2000 tissue homogenizer. After incubation at room temperature for 5 min, 200 μl chloroform was added, tubes were vortexed, and samples were incubated for 3 min. Samples were then centrifuged (11,900 x g) for 15 min at 4 °C to achieve phase separation of nucleic acid. Isopropyl alcohol (500 μl) was added to the aqueous phase to precipitate nucleic acid. Samples were vortexed briefly and incubated for 10 min followed by centrifugation (11,900 x g) for 15 min at 4 °C. Nucleic acid precipitate was washed in 75% ethanol (1 ml) and centrifuged (7500 x g) for 5 min at 4 °C. Ethanol was decanted and samples air dried. Nucleic acid pellet was resuspended in 20 μl RNase-free water. UV spectrophotometric analysis of nucleic acid was performed at 260 nm to determine concentration. Samples were DNase-treated (DNA-free kit, Ambion) to remove contaminating DNA. cDNA synthesis was completed using the SuperScript III First Strand Synthesis System (Invitrogen).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed using custom-designed primers (Invitrogen, see table 1) and FAM 490 probes (Biosearch Technologies) or proprietary predesigned primer/probe sets (Applied Biosystems) and the iCycler (Bio-Rad). For custom-designed sequences, optimal concentrations were determined empirically. Standard curves were generated from serial dilutions of expected products over 5 orders of magnitude. PCR reactions were carried out in a final volume of 20 μl using iQ Supermix (Bio-Rad) and 5 nM FITC dye. PCR reaction conditions were generally as follows: initial denaturation at 95 °C for 3 minutes, followed by 50 cycles of amplification by denaturing at 95 °C for 30 s and annealing/extension at 60 °C for 30 s (55 °C for TNFα). Reaction efficiency (E) was determined from standard curves. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was assessed as a house-keeping gene and used to normalize data. Threshold cycle (Ct) values were transformed using (1 + E)Ct to determine the relative differences in mRNA expression.
Table 1.
RT-PCR primer and probe sequences used for amplification of cDNA. Chemokine (C-X-C motif) ligand (CXCL), chemokine (C-C motif) ligand 2 (CCL2), glial fibrillary acidic protein (GFAP), major histocompatibility complex II (MHCII), tumor necrosis factor α (TNFα)
| Gene | Primer Sequence Upper 5′ → 3′ | Primer Sequence Lower 5′ → 3′ | Probe Sequence 5′ → 3′ |
|---|---|---|---|
| Arc | Applied Biosystems | cctggcccccagcagtgattcatac | |
| CCL2 | ggctcagccagatgcagttaa | cctactcattgggatcatcttgct | ccccactcacctgctgctactcattca |
| CXCL1 | gctaaaaggtgtccccaagtaa | taggaccctcaaaagaaattgta | ctgctctgatggcaccgtctggt |
| CXCL2 | caagaacatccagagcttgagtgt | ttttgaccgcccttgagagt | cccactgcgcccagacagaagtcat |
| COX-1 | gtgccagaaccagggtgtct | gtagcccgtgcgagtacaatc | cgctttggcctcgacactaccagtg |
| COX-2 | tgacccccaaggctcaaata | cccaggtcctcgcttatgatc | ctttgcccgcacttcacccatcagtt |
| GAPDH | cccaatgtgtccgtcgtg | cctgcttcaccaccttcttg | tgtcatcatacttggcaggtttctccagg |
| GFAP | ctggaggtggagagggacaa | ggttggtttcatcttggagctt | tttgcacaggacctcggcaccc |
| mIL-1α | aaggagagccgggtgacagt | gaaactcagccgtctcttcttca | cagcaacgtcaagcaacgggaagattc |
| mIL-1β | tcgctcagggtcacaagaaa | atcagaggcaaggaggaaacac | catggcacattctgttcaaagagagcctg |
| IL-6 | Applied Biosystems | aatgagaaaagagttgtgcaatggc | |
| MHCII | agtcagtcgcagacggtgttt | gataagacagcttgtggaaggaatagt | tgagaccagcttcttcgtcaaccgtg |
| TNFα | gacaaggctgccccgacta | tttctcctggtatgagatagcaaatc | ctcctcacccacaccgtcagcc |
Statistical analyses
Data was analyzed in Prism (GraphPad Software, San Diego, CA, USA) using 1-way, 2-way, or 2-way repeated measures analysis of variance (ANOVA). Analyses were considered significant when p < 0.05. Bonferroni post-hoc tests were used to further analyze significant ANOVAs.
Results
Experiment 1: Sustained and localized hippocampal IL-1β expression leads to neuroinflammation and memory impairments
In the first experiment, WT-Cre (n = 10) and IL-1βXAT-GFP injected mice (n = 9) served as controls for the IL-1βXAT-Cre injected experimental mice (n = 13). These 3 groups of mice underwent contextual and auditory fear conditioning 2 weeks after viral microinjection (figure 1 A). No significant differences in freezing behavior between groups were found during the 3 min acclimation period prior to shock presentation (figure 1 B). Freezing during the intervals following shock presentation increased across trials (F (2,66) = 32.95, p < 0.0001), but did not vary by genotype or viral injection, suggesting that all animals processed the shock similarly. Likewise, freezing to the auditory stimulus increased across trials (F (2,64) = 37.33, p < 0.0001), but did not vary by genotype or viral injection, suggesting that the white noise stimulus was processed and the noise-shock pairing was formed by all groups (figure 1 C).
When tested the following day for long-term contextual fear memory, IL-1βXAT-Cre injected mice were significantly impaired as shown by reduced levels of freezing to the conditioned context (figure 1 D). A one-way ANOVA revealed a significant main effect of treatment group (F (2,29) = 4.09, p < 0.03) and post-hoc analyses showed significantly reduced freezing in IL-1βXAT-Cre injected mice compared to both WT-Cre and IL-1βXAT-GFP injected mice (p < 0.05). All treatment groups showed equally elevated freezing behavior to the white noise stimulus compared to a novel context (F (1,60) = 46.5, p < 0.0001), with no differences between any groups found in either measure (figure 1 E). Therefore, 2 weeks of hippocampal IL-1β overexpression in IL-1βXAT-Cre injected mice impairs hippocampal-dependent but not - independent long-term memory processes.
In a separate set of animals, short-term context memory was assessed 1 h after contextual fear conditioning (n = 6 for all groups). No significant differences between any groups were found in short-term memory (figure 1 F). All groups froze equally well to the context 1 h after conditioning, indicating that IL-1β overexpression does not impair acquisition of the context itself. Rather, some aspect of sustained hippocampal inflammation specifically impairs long-term memory processes.
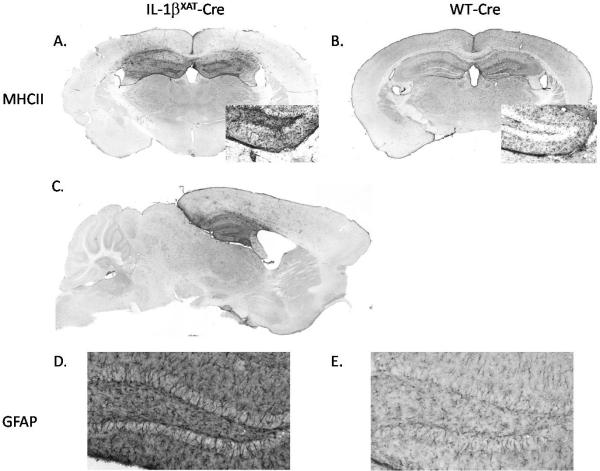
We examined MHCII and GFAP immunoreactivity (IR) within the hippocampi of WT-Cre and IL-1βXAT-Cre injected mice after behavioral experimentation. MHCII-IR, which labels activated microglia/macrophages, was highly elevated in IL-1βXAT-Cre compared to WT-Cre injected mice (figure 2 A and B). MHCII staining in sagittal sections showed that the inflammation extends throughout the hippocampus in the rostral/caudal axis and, importantly, this increased activation of microglia/macrophages was primarily localized to the hippocampus with only sparse staining in surrounding cortex (figure 2 A, C). GFAP-IR was also increased in IL-1βXAT-Cre compared to WT-Cre injected mice, indicating increased astrocytic activation with IL-1β overexpression (figure 2 D and E).
Figure 2.
MHCII- and GFAP-IR following sustained IL-1β overexpression. Activation of microglia/macrophages and astrocytes, as measured by MHCII- and GFAP-IR, is increased within the hippocampi of IL-1βXAT-Cre compared to WT-Cre injected mice. MHCII-IR is increased in IL-1βXAT-Cre (A, C) compared to WT-Cre injected mice (B) and this increased IR is restricted to the hippocampus, indicating regional specificity of inflammation. MHCII inset shows 20x magnification of the dentate gyrus. GFAP-IR is also increased in the dentate gyrus of IL-1βXAT-Cre (D) compared to WT-Cre (E) injected mice (10x magnification).
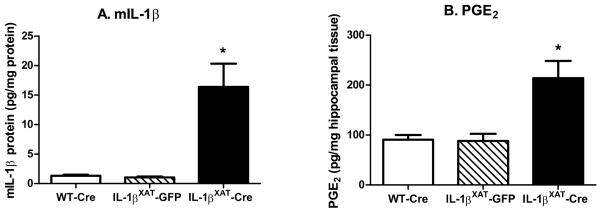
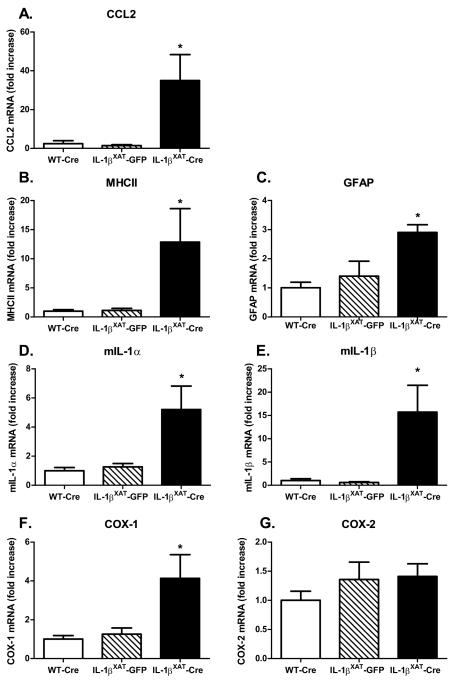
In order to quantify the increased inflammation in IL-1βXAT-Cre injected mice, we examined hippocampal IL-1β protein, PGE2, and mRNA levels of multiple indicators of inflammation after fear conditioning (n = 8, 10, and 7 for WT-Cre, IL-1βXAT-GFP, and IL-1βXAT-Cre injected mice respectively). Murine IL-1β (mIL-1β) and PGE2 levels were significantly elevated after prolonged IL-1β overexpression in IL-1βXAT-Cre injected mice (figure 3 A & B; F (2,19) = 6.92, p < 0.006, F (2,20) = 6.36, p < 0.008). IL-1βXAT-Cre injected mice also had significantly increased mRNA levels of CCL2 (F (2,20) = 7.9, p < 0.003), MHCII (F (2,22) = 5.5, p < 0.012), GFAP (F (2,18) = 6.813, p < 0.008), IL-1α (F (2,20) = 8.7, p < 0.002), IL-1β (F (2,22) = 8.9, p < 0.002), and COX-1 (F (2,22) = 6.8, p = 0.005) compared to both WT-Cre and IL-1βXAT-GFP injected mice (figure 4 A-F;). COX-2 was the only gene examined for which increased mRNA was not found following IL-1β overexpression (figure 4 G), suggesting that COX-1 may have a greater contribution to the elevation in hippocampal PGE2. No differences were found between WT-Cre and IL-1βXAT-GFP injected mice for IL-1β protein, PGE2, or any gene examined.
Figure 3.
Hippocampal murine IL-1β protein and PGE2 levels following sustained IL-1β overexpression. IL-1βXAT-Cre injected mice have elevated levels of hippocampal murine IL-1β protein (A) and PGE2 (B) compared to both WT-Cre and IL-1βXAT-GFP injected mice (* p < 0.008). Error bars show SEM.
Figure 4.
Hippocampal pro-inflammatory mRNAs following sustained IL-1β overexpression (A-G). Messenger RNA levels are increased for all inflammatory genes measured except for COX-2. Error bars show SEM. * Significantly increased from levels in WT-Cre and IL-1βXAT-GFP injected mice.
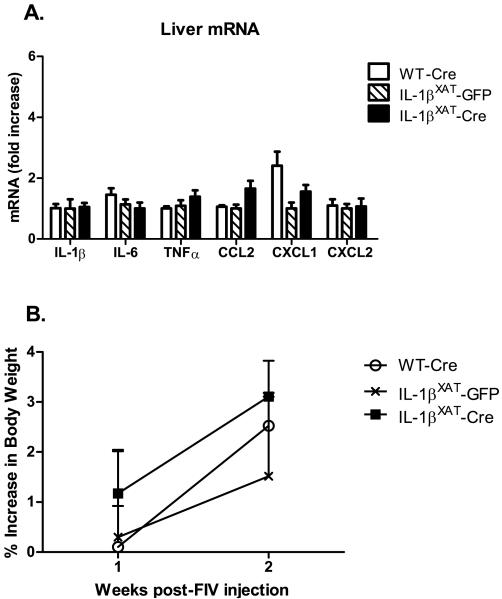
We examined the liver for changes in mRNA levels of the cytokines IL-1β, IL-6, and TNF and the chemokines CCL2, CXCL2, and CXCL1 to investigate whether a peripheral state of inflammation had been induced by sustained hippocampal IL-1β overexpression. Messenger RNA levels in the livers of IL-1βXAT-Cre injected mice did not differ significantly from either control group for any gene examined (figure 5 A). We also measured body weights before and after surgery to see if hippocampal IL-1β overexpression would cause a sickness-induced weight loss. A repeated measures 2-way ANOVA found a main effect of time (F (1,17) = 25.45, p < 0.0001), but no differences between any treatment group after viral microinjections and no interaction (figure 5 B). Therefore, prolonged hippocampal IL-1β overexpression did not appear to induce peripheral inflammation as measured by cytokine and chemokine mRNA levels and body weight. WT-Cre and IL-1βXAT-GFP injected mice did not differ in any behavioral or molecular measure examined in experiment 1. Therefore, only WT-Cre mice were used as controls in experiment 2.
Figure 5.
Liver mRNA levels and body weight measures following two weeks of hippocampal IL-1β overexpression. (A) Cytokine and chemokine mRNA levels and (B) body weight were unchanged by hippocampal IL-1β overexpression. Error bars show SEM.
Experiment 2: Memory impairment and neuroinflammation is not dependent on gender
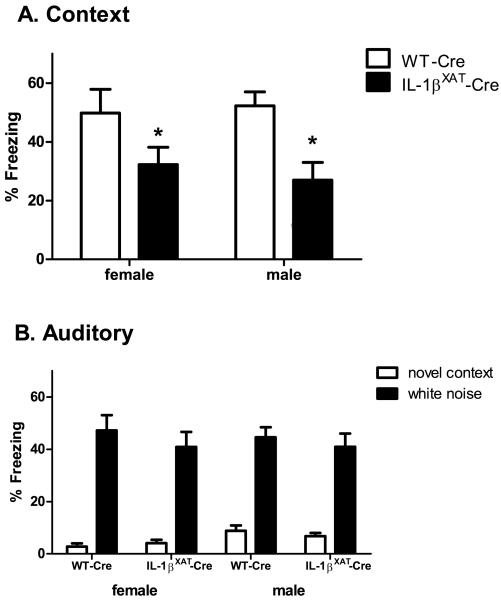
In the second experiment, we examined potential gender differences in behavioral and molecular measures following sustained hippocampal IL-1β overexpression. Two weeks after FIV-Cre microinjection, WT (n females = 9, n males = 13) and IL-1βXAT mice (n females = 11, n males = 11) underwent contextual and auditory fear conditioning. When re-exposed to the conditioned context the following day, IL-1βXAT mice froze significantly less than WT controls (figure 6 A). A 2-way ANOVA revealed a main effect of genotype (F (1,39) = 12.3, p < 0.002), but the effect of gender and the interaction were not significant. Therefore, prolonged hippocampal IL-1β overexpression impairs contextual fear memory equally in male and female mice. Both WT-Cre and IL-1βXAT-Cre mice showed low freezing to a novel context and increased freezing to the conditioned white noise stimulus (figure 6 B; F (1,78) = 207, p < 0.0001). No differences between genotypes or gender were found in this hippocampal-independent task.
Figure 6.
Contextual and auditory fear memory in male and female IL-1βXAT-Cre injected mice. (A) Contextual fear memory is impaired equally in male and female IL-1βXAT-Cre mice (* p < 0.002) and (B) no differences between any groups were found in freezing to a novel context or the white noise stimulus. Error bars show SEM.
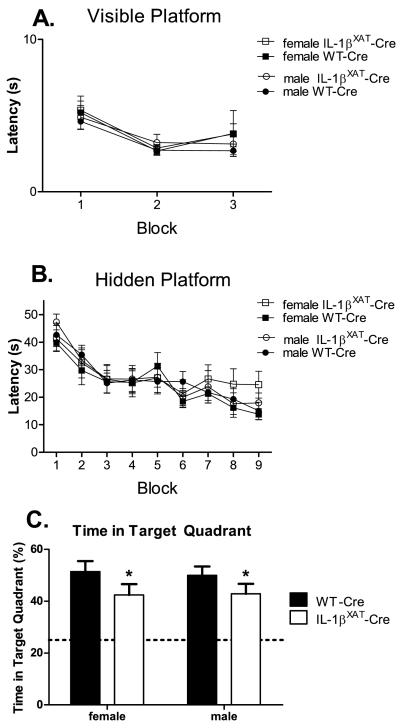
After fear conditioning, WT-Cre and IL-1βXAT-Cre mice were trained in the Morris water maze. WT-Cre (n females = 9, n males = 13) and IL-1βXAT-Cre mice (n females = 11, n males = 10) did not differ in their latencies to find a visible platform over 3 training blocks, suggesting similar visual acuity and motivation between genotypes in this task (figure 7 A). During hidden platform training, all groups of mice showed reduced latencies to find the platform over training blocks (F (8,304) = 21.46, p < 0.0001). Although female IL-1βXAT-Cre mice had slightly longer latencies in the last blocks of hidden training, neither gender nor genotype had a statistically significant effect on these latencies (figure 7 B). However, both male and female IL-1βXAT-Cre mice spent less time searching the target quadrant during the probe trial compared to WT-Cre mice (figure 7 C; F (1,38) = 4.3, p < 0.05). Therefore, male and female IL-1βXAT-Cre mice show equal impairments in spatial memory.
Figure 7.
Effects of hippocampal IL-1β overexpression on spatial memory in the Morris water maze in male and female mice. No differences were found between genotypes or genders during training for the visible platform (A) or the hidden platform (B). However, both male and female IL-1βXAT-Cre mice spent significantly less time in the target quadrant during the probe trial (C). * p < 0.05 compared to WT-Cre. Error bars show SEM.
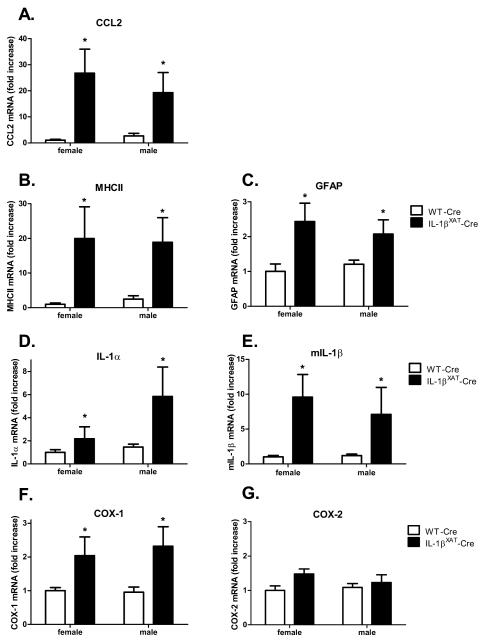
Changes in pro-inflammatory mRNA levels were examined in WT-Cre (n females = 6, n males = 9) and IL-1βXAT-Cre mice (n females = 7, n males = 5) after behavioral experimentation. Similar to experiment 1, we found a significant main effect of genotype with IL-1βXAT-Cre mice having significantly elevated levels of CCL2 (F (1,23) =13.8, p < 0.002), MHCII (F (1,23) =10, p < 0.005), GFAP (F (1,23) =11.26, p < 0.003), IL-1α (F (1,23) =6.2, p < 0.02), IL-1β (F (1,23) =10.5, p < 0.004), and COX-1 (F (1,23) =9.9, p < 0.005) (figure 8 A-F). Again, COX-2 was the only gene examined that did not increase with chronic IL-1β overexpression (figure 8 G). Additionally, no differences between males and females were found for any gene.
Figure 8.
Gender effects on hippocampal pro-inflammatory mRNAs observed following sustained IL-1β overexpression. Increases were found in CCL2, MHCII, GFAP, IL-1α, IL-1β, and COX-1 mRNA in both male and female IL-1βXAT-Cre injected mice. * Significantly increased compared to WT-Cre levels. COX-2 mRNA was not affected by IL-1β overexpression. Error bars show SEM.
Experiment 3: Neuroinflammation is associated with a reduction in behaviorally-induced Arc
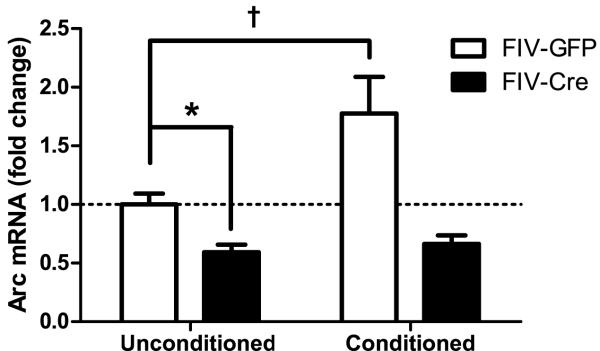
In the third experiment, we measured levels of the immediate early gene Arc in unconditioned control (n = 8) and conditioned IL-1βXAT mice (n = 8) 2 weeks after microinjection with FIV-Cre into one hippocampus and FIV-GFP into the other hippocampus (figure 9). This design has been used previously by our lab to induce unilateral inflammation and we confirmed increased mIL-1β levels in FIV-Cre versus FIV-GFP injected hippocampi (data not shown) (Shaftel et al., 2007a; Shaftel et al., 2007b). A 2-way ANOVA of the Arc mRNA data revealed a main effect of viral injection (F (1,28) = 19.5, p < 0.001) and of conditioning (F (1,28) = 6.11, p < 0.02). The interaction was also significant (F (1,28) = 4.25, p < 0.05), indicating that the effect of conditioning depended on viral injection. In unconditioned mice, IL-1β overexpression significantly reduced basal Arc mRNA levels (p < 0.04). Conditioning caused an increase in Arc mRNA in the GFP-injected hippocampi that did not reach significance with a Bonferroni correction (p < 0.09), but no such trend was seen in the Cre-injected hippocampi. Therefore, prolonged neuroinflammation caused by IL-1β overexpression prevented the conditioning-induced increase in Arc mRNA.
Figure 9.
Fold change (±SEM) in Arc mRNA in unconditioned and conditioned IL-1βXAT mice microinjected with FIV-GFP in one hippocampus and FIV-Cre in the other. IL-1β overexpression reduces basal Arc mRNA (* p < 0.04). In the GFP-injected hemisphere, conditioning increases Arc mRNA († p < 0.09) and IL-1β overexpression in the Cre-injected hemisphere prevents this increase.
Discussion
The present experiments extend and confirm previous work in our lab by showing that sustained hippocampal IL-1β overexpression in a transgenic mouse model impairs contextual and spatial long-term memory, but not hippocampal-independent and short-term memory (Moore et al., 2009). We also found increases in inflammation specifically within the hippocampus, but no measurable changes in inflammatory mRNAs in the liver. Interestingly, the observed behavioral and molecular effects of sustained neuroinflammation did not depend on gender. Finally, we report that sustained hippocampal neuroinflammation reduced basal and conditioning induced levels of the plasticity-related gene Arc.
In experiment 1, we show that 2 weeks of IL-1β overexpression within the hippocampus of male IL-1βXAT-Cre mice impairs long-term hippocampal-dependent contextual fear memory. Overexpression of human IL-1β, which can signal through the murine IL-1 receptor, activated glia, elevated mIL-1β protein and PGE2 levels, and increased pro-inflammatory cytokine and chemokine mRNAs specifically within the injected hippocampi. Importantly, these effects depended on transgene activation as FIV-Cre injected WT and FIV-GFP injected IL-1βXAT mice did not display memory deficits or hippocampal inflammation.
Because we are studying the effects of prolonged inflammation, our animals were inflamed during exposure to the context and shock, the learning event. This necessity raises the potential that hippocampal inflammation may interfere with the initial learning or acquisition of the context and not the long-term storage of the contextual memory. In order to distinguish between these two possibilities, we measured freezing levels during the conditioning session and tested short-term memory in a separate set of IL-1βXAT-Cre mice. We found that sustained IL-1β overexpression did not alter post-shock or auditory freezing during the conditioning session and did not impair short-term contextual fear memory, indicating that mice with hippocampal inflammation do indeed explore and form an initial representation of the context. The intact memory for the auditory stimulus in the IL-1βXAT-Cre mice also supports the conclusion that mice with hippocampal inflammation do not display generally inferior memory or a decreased freezing ability and are capable of forming new memories that do not depend on the hippocampus. Therefore, prolonged hippocampal inflammation appears to interfere specifically with processes necessary for the long-term storage of hippocampal-dependent memories.
It is important to note that the majority of the IL-1β-induced inflammation was restricted to the hippocampus and only sparse MHCII labeling was detected in surrounding cortical structures. Therefore, non-specific effects of inflammation on other brain regions are unlikely to explain the memory deficits we observed. Moreover, we did not detect any signs of peripheral inflammation or general sickness in the IL-1βXAT-Cre mice. Cytokine and chemokine mRNA levels were not elevated in the livers of IL-1βXAT-Cre mice and no weight differences were observed following transgene activation and induction of inflammation. These findings are in contrast with data from Campbell et al. (2007; 2008) who used a model of chronic neuroinflammation induced by intracerebroventricular (ICV) or intra-striatal administration of an adenovirus expressing rat IL-1β. Two hours and eight days after intra-striatal or ICV administration of the adenovirus, they reported marked reductions in body weight and increased hepatic IL-1β and chemokine mRNA levels (Campbell et al., 2007; Campbell et al., 2008). It is not surprising that ICV administration of IL-1β, which circulates throughout the entire brain including the hypothalamus, would reduce body weight since feeding behavior and some aspects of energy metabolism are regulated by the hypothalamus (Guijarro, 2006; Kent et al., 1996; Kent et al., 1994). Inflammation in our model is largely restricted to the hippocampus, therefore, one would expect and, indeed, we find no change in body weight. How ICV or intra-striatal IL-1β overexpression signals to induce chemokines in the liver is unknown, but this signaling pathway does not appear to be activated by the level of IL-1β overexpression induced within the hippocampus of our IL-1βXAT-Cre mice. The amount of IL-1β/inflammation, brain regions affected, duration of inflammation, type of virus injected, and potentially other factors (e.g. breed, age, etc.) may determine whether or not a peripheral immune response is incited.
In experiment 2, we extend the findings from experiment 1 by demonstrating memory deficits in a second hippocampal-dependent task, the Morris water maze. This spatial memory impairment confirms previous work by Moore et al. (2009), showing similar spatial deficits in this IL-1βXAT mouse model. Admittedly, we observed relatively small but significant deficits during the probe trial in this task. Compensatory, anti-inflammatory processes may help explain the relatively modest behavioral effects despite remarkable hippocampal inflammation. Previous studies in these transgenic mice have shown peak elevations in IL-1 receptor antagonist (IL-1ra) mRNA at two weeks following transgene activation (Shaftel et al., 2007b). Elevated IL-1ra may diminish IL-1β signaling and lessen the impact of the inflammatory signaling on memory processes.
We also examined potential gender differences with regards to the effect of inflammation on memory. Here, we report that male and female IL-1βXAT-Cre mice have equally impaired memory and inflamed hippocampi. Although sex hormones have been shown to alter and gender can influence immune cells and the peripheral immune response, few studies have examined the effect of gender on central immune responses (Bird et al., 2008; Bouman et al., 2005; Nalbandian and Kovats, 2005). The data presented here generally agrees with the few studies that have examined the same measures as in our study. Following intranasal LPS, male and female rats show equal increases in IL-1β mRNA within the hippocampus (Tonelli et al., 2008). Similarly, equal levels of microglial activation and CCL2 mRNA were found in male and female mice following traumatic brain injury (Bruce-Keller et al., 2007). We did not examine the stage of estrus of our female mice, an admittedly important factor in gender studies. However, we did not observe increased variability in the behavioral or molecular data from female compared to male mice, suggesting that multiple populations of female mice do not exist within this data.
Finally, we examined levels of the plasticity-related gene Arc following fear conditioning in animals with unilateral hippocampal inflammation. Arc is an immediate early gene whose expression is highly regulated by neuronal activity and localized to activated dendrites (Moga et al., 2004). Arc mRNA and protein have been shown to increase within the hippocampus following fear conditioning and Morris water maze training (Guzowski et al., 2001; Montag-Sallaz and Montag, 2003). Studies inhibiting Arc activity or using Arc knockout mice prevented the maintenance phase, but not induction of long-term potentiation and impaired long-term, but not short-term memory (Guzowski et al., 2000; Plath et al., 2006; Ploski et al., 2008). Interestingly, neuroinflammation has also been shown to regulate Arc expression. Cranial irradiation, which causes local inflammation, prevented the induction in Arc mRNA and protein with spatial exploration (Rosi et al., 2008). Also, chronic neuroinflammation resulting from 28 d of ventricular lipopolysaccharide infusion altered normal Arc induction (Rosi et al., 2005). Given this relationship with neuroinflammation and the observations presented here of impairments in long-term, but not short-term memory in IL-1βXAT-Cre mice, we examined Arc mRNA levels following fear conditioning in our model of neuroinflammation.
We found that sustained hippocampal IL-1β overexpression reduced basal levels of Arc expression and blunted the conditioning induced increase in Arc mRNA. We did not explore potential mechanisms for how prolonged neuroinflammation might have reduced Arc expression and, therefore, have no data to support such mechanisms. However chronic neuroinflammation has been shown to reduce [3H]MK-801 binding sites in the hippocampus and decreased immunolabelling of NMDAR1 within the pyramidal layer of the CA3 and hilar regions of the dentate gyrus and to a somewhat lesser degree within the entorhinal cortex (Rosi et al., 2004). Therefore, one speculation could be that reduced calcium signaling through these channels led to decreased Arc mRNA levels. Regardless of specifically how neuroinflammation affects Arc levels, reduced Arc signaling following IL-1β overexpression provides one potential molecular mechanism for the impaired long-term, but not short-term memory observed in our transgenic mice. The data reported here show an interesting association between reduced Arc induction with neuroinflammation and memory deficits in IL-1βXAT transgenic mice. Further studies are needed to establish a causative role of Arc in the memory deficits in this model.
Together, the results presented here indicate that neuroinflammation resulting from sustained IL-1β overexpression is sufficient to impair long-term contextual and spatial memory formation without the presence of other confounding variables such as infection, brain injury, amyloid beta plaques, or overt neurodegeneration (Moore et al., 2009; Shaftel et al., 2007a). Using this transgenic model, we will now be able to investigate the role of truly chronic neuroinflammation, up to 1 year of sustained IL-1β expression, on learning and memory processes in future studies.
Acknowledgements
We would like to thank S. Kyrkanides and J. Miller for FIV packaging, M. Olschowka and J. Walter for animal colony management, L. Trojancyzk for help with tissue processing, and R. Johnson for assistance with ELISA. The present work was supported by NIH RO1 AG030149, HD056235, and AG028271, the Coleman Institute, and the Anna & John J. Sie Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADAPT Research Group. Lyketsos C, Breitner J, Green R, Martin B, Meinert C, Piantadosi S, Sabbagh M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell. Immunol. 2008;252:57–67. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Dimayuga FO, Reed JL, Wang C, Angers R, Wilson ME, Dimayuga VM, Scheff SW. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma. 2007;24:203–215. doi: 10.1089/neu.2006.0163. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Deacon RM, Jiang Y, Ferrari C, Pitossi FJ, Anthony DC. Overexpression of IL-1beta by adenoviral-mediated gene transfer in the rat brain causes a prolonged hepatic chemokine response, axonal injury and the suppression of spontaneous behaviour. Neurobiol. Dis. 2007;27:151–163. doi: 10.1016/j.nbd.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Zahid I, Losey P, Law S, Jiang Y, Bilgen M, van Rooijen N, Morsali D, Davis AE, Anthony DC. Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology. 2008;55:780–787. doi: 10.1016/j.neuropharm.2008.06.074. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Smith MA, Basu S, Hua J, Capobianco DE, Siedlak SL, Zhu X, Perry G. Increased isoprostane and prostaglandin are prominent in neurons in Alzheimer disease. Mol. Neurodegener. 2007;2:2. doi: 10.1186/1750-1326-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–1632. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein T. Spatial Learning Impairment in Mice Infected with Legionella pneumophila or Administered Exogenous Interleukin-1-ß. Brain. Behav. Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro A, Laviano A, Meguid MM. Chapter 22: hypthalamic integration of immune function and metabolism. Prog. Brain Res. 2006;153:367–405. doi: 10.1016/S0079-6123(06)53022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, Maier SF. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–763. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SD, Olschowka JA, O’Banion MK. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J. Neurotrauma. 2002;19:1–15. doi: 10.1089/089771502753460196. [DOI] [PubMed] [Google Scholar]
- Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci. Biobehav. Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- Kent S, Rodriguez F, Kelley KW, Dantzer R. Reduction in food and water intake induced by microinjection of interleukin-1 beta in the ventromedial hypothalamus of the rat. Physiol. Behav. 1994;56:1031–1036. doi: 10.1016/0031-9384(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Lai YC, Shaftel SS, Miller JN, Tallents RH, Chang Y, Pinkert CA, Olschowka JA, Dickerson IM, Puzas JE, O’Banion MK, Kyrkanides S. Intraarticular induction of interleukin-1beta expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–1197. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learn. Mem. 2003;10:99–107. doi: 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, 2nd, Morrow JD. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–1498. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.08.073. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol. Res. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- Oitzl M, Van Oers H, Schobitz B, Ron de Kloet E. Interleukin-1 ß, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J. Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain. Behav. Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pugh RC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci. Biobehav. Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, Zalinski J, Cofield M, Mansukhani L, Willson P. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–9770. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J Neuroinflammation. 2004;1:12. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J. Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 2007a;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007b;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasko M, Costa A. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav. Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res. Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]