Abstract
The potent oxidants hypochlorous acid (HOCl) and hypobromous acid (HOBr) are produced extracellularly by myeloperoxidase, following release of this enzyme from activated leukocytes. The subendothelial extracellular matrix is a key site for deposition of myeloperoxidase and damage by myeloperoxidase-derived oxidants, with this damage implicated in the impairment of vascular cell function during acute inflammatory responses and chronic inflammatory diseases such as atherosclerosis. The heparan sulfate proteoglycan perlecan, a key component of the subendothelial extracellular matrix, regulates important cellular processes and is a potential target for HOCl and HOBr. It is shown here that perlecan binds myeloperoxidase via its heparan sulfate side chains and that this enhances oxidative damage by myeloperoxidase-derived HOCl and HOBr. This damage involved selective degradation of the perlecan protein core without detectable alteration of its heparan sulfate side chains, despite the presence of reactive GlcNH2 resides within this glycosaminoglycan. Modification of the protein core by HOCl and HOBr (measured by loss of immunological recognition of native protein epitopes and the appearance of oxidatively-modified protein epitopes) was associated with an impairment of its ability to support endothelial cell adhesion, with this observed at a pathologically-achievable oxidant dose of 425 nmol oxidant/mg protein. In contrast, the heparan sulfate chains of HOCl/HOBr-modified perlecan retained their ability to bind FGF-2 and collagen V and were able to promote FGF-2-dependent cellular proliferation. Collectively, these data highlight the potential role of perlecan oxidation, and consequent deregulation of cell function, in vascular injuries by myeloperoxidase-derived HOCl and HOBr.
Keywords: oxidation, heparan sulfate, perlecan, myeloperoxidase, inflammation
1. Introduction
Oxidative damage within the vessel wall mediated by the leukocyte-derived heme peroxidase enzyme myeloperoxidase (MPO) is implicated in the impairment of vascular cell function during acute inflammatory responses and in the progression of chronic inflammatory diseases such as atherosclerosis (Davies et al., 2008). Neutrophils, monocytes and some macrophages express MPO and release the enzyme extracellularly upon activation, where it catalyzes the production of the potent oxidants hypochlorous acid (HOCl) and hypobromous acid (HOBr) from H2O2 and the corresponding halide ions (Cl− and Br−), with the former predominating (Davies et al., 2008). Production of hypothiocyanous acid (HOSCN) from thiocyanate ions (SCN−) (van Dalen et al., 1997) and the nitrating species nitrogen dioxide (NO2•) from nitrite (NO2−) (Arnhold et al., 2006) are also important reactions catalyzed by MPO. Due to their high reactivity with biomolecules, HOCl and HOBr are predicted to have very limited diffusion radii in biological systems (Pattison and Davies, 2006; Winterbourn, 2008), and extracellular structures with which MPO associates are likely to be important targets for oxidative damage.
The subendothelial matrix (‘basement membrane’), which is synthesized by overlying endothelial cells, has been identified as a key site for deposition of MPO and damage by MPO-derived oxidants in both animal models of inflammation and in human inflammatory disease (Rees et al., 2008). Immunohistochemical studies have demonstrated that MPO co-localizes with HOCl-modified protein, recognized by the monoclonal antibody (mAb) 2D10G9, in subendothelial matrix present in human atherosclerotic lesions (Malle et al., 2006), placental tissue (Hammer et al., 2001) and diseased kidneys (Grone et al., 2002; Malle et al., 1997). A similar association of MPO and 3-nitroTyr in the subendothelial matrix, implicating MPO-dependent protein nitration, has been demonstrated in human atherosclerotic lesions (Baldus et al., 2002). MPO released in the vascular lumen binds avidly to the heparan sulfate chains of proteoglycans present on the endothelial cell surface and subsequently undergoes transcytosis to the subendothelial space (Baldus et al., 2001), with this potentially accounting for targeting of MPO and damage by MPO-derived oxidants to the subendothelial matrix during acute and chronic vascular inflammation. The affinity of MPO for heparan sulfate suggests that heparin sulfate proteoglycans present within the subendothelial matrix could also sequester MPO and thereby localize damage by MPO-derived oxidants to these materials, however their ability to bind MPO has yet to be determined.
The heparan sulfate proteoglycan perlecan is a major proteoglycan of endothelial cell-derived matrix (Whitelock and Iozzo, 2005) and plays key roles in basement membrane assembly and function (Yurchenco et al., 2004). Endothelial cell-derived perlecan consists of a large (470 kDa) multi-domain protein core substituted at its N-terminal domain, domain I, by heparan sulfate (Whitelock et al., 1999; Whitelock et al., 1996); other cell types can also express hybrid forms with chondroitin sulfate, keratin sulfate and dermatan sulfate chains (Knox et al., 2005). The protein core and heparan sulfate chains of perlecan regulate a number of important vascular processes including the maintenance of basement membrane organization (Yurchenco et al., 2004), the control of vascular permeability and the adhesion, proliferation and differentiation of vascular cells (Iozzo, 2005; Kinsella et al., 2003; Morita et al., 2005; Whitelock et al., 2008). For example, the heparan sulfate chains of matrix-bound perlecan act as a reservoir for basic fibroblast growth factor (FGF-2) (Nugent and Iozzo, 2000) and the release of FGF-2 via nzymatic degradation of perlecan is a potentially important mechanism for promoting growth factor bioavailability and activity within the vessel wall (Whitelock et al., 1996). The protein core of perlecan has also been identified as an adhesive molecule for human vascular cells (Whitelock et al., 1999).
Oxidation of endothelial cell-derived matrix by the MPO-H2O2-Cl− system results in the release of perlecan-derived material (Klebanoff et al., 1993) and similar release of proteoglycan-derived material occurs upon exposure of smooth muscle cell-derived matrix to HOCl, HOBr or the MPO-H2O2-Cl− system (McGowan, 1990; Rees et al., 2007; Woods and Davies, 2003). These data indicate that modification and release of perlecan from extracellular matrix may be an important process when MPO-derived HOCl and HOBr are generated within the subendothelial compartment, however the mechanisms underlying this process and its potential biological consequences are unclear. In particular, it is unknown whether damage occurs principally to its protein core or to its heparan sulfate chains. Studies with isolated heparan sulfate show that HOCl and HOBr react rapidly with the free amino groups present on its GlcNH2 residues, resulting in modification and fragmentation of its polysaccharide backbone (Rees and Davies, 2006; Rees et al., 2003; Rees et al., 2007; Rees et al., 2005). It has yet to be established whether HOCl and HOBr can degrade heparan sulfate present on intact proteoglycans. Moreover, the effects of these oxidants on the diverse biological activities of the perlecan protein core and its heparan sulfate chains, such as growth factor binding and cell adhesion, are unknown. In the light of these data, the ability of endothelial cell-derived perlecan to bind MPO, and the structural and functional consequences of its oxidation by HOCl, HOBr and MPO-H2O2-halide systems were determined.
2. Materials and Methods
2.1 Materials
Solutions and media were prepared using water filtered through a four-stage MilliQ system. pH control was achieved using Chelex-treated 0.1 M phosphate buffer or PBS, pH 7.4. HCAECs and HCAEC Growth Medium were from Cell Applications. Medium 199 containing Earles salts were from Gibco BRL. RMPI-1640 medium was from Sigma. Tissue culture plastic ware was from BD Falcon. Sodium hypochlorite (NaOCl) was from Sigma. H2O2 and HOCl solutions were prepared immediately before use and their concentrations were determined spectrophotometrically at pH 12 (H2O2, ε24̃ 43.6 M−1 cm−1; HOCl, ε292 350 M−1 cm−1). Stock solutions of HOBr were prepared by reaction of 20 mM NaOCl with 22.5 mM NaBr in water for 5 min at 22°C and were used immediately. MPO (isolated from human polymorphonuclear leukocytes; purity index A430/A280 0.84) was from Planta Natural Products. Alkaline phosphate substrate (p-nitrophenylphosphate) solution, chondroitin sulfate, fish gelatin, collagen V, fibronectin and heparin were from Sigma. Recombinant human [125I]FGF-2, streptavidin-conjugated horseradish peroxidase, streptavidin-conjugated alkaline phosphatase and ECL Western blotting detection reagents were from Amersham. Heparan sulfate was from Celsus Laboratories. Heparinase III was from IBEX Technologies. Chondroitinase ABC was from Seikagaku. Biotinylated anti-mouse antibody, horseradish peroxidase-conjugated anti-mouse antibody and Oxyblot® protein carbonyl detection kit were from Chemicon. Two-component peroxidase substrate (ABTS) kit was from KPL. NuPAGE® electrophoresis and iBlot® electroblotting consumables were from Invitrogen. CellTiter 96® AQueous One Solution Reagent was from Promega.
2.2 Primary antibodies
Mouse mAbs against heparan sulfate (HepSS-1, JM403, 10E4), Δ-heparan sulfate stubs generated by heparinase III (3G10) and keratan sulfate (5D4) were from Seikagaku. Mouse mAb against chondroitin sulfate (CS56) was from Sigma. Mouse mAbs against perlecan domains I (CSI-076) and V (CSI-074) and against MPO (2C7) were from AbCam. The mouse mAb against perlecan domain III (7B5) (Murdoch et al., 1994) was from Zymed Laboratories. A mouse mAb (clone 2D10G9) recognizing HOCl-modified epitopes (Malle et al., 1995) and HOBr-modified epitopes (Chapman et al., 2000), was generated as described previously (Malle et al., 1995). A mouse mAb against collagen V (1E2/E4) was a gift from Dr J. Werkmeister, CSIRO Molecular Health and Technologies, Australia. The rabbit mAb against 2,4-dinitrophenylhydrazine (DNP) was a component of the Oxyblot® protein carbonyl detection kit from Chemicon.
2.3 Cell culture
Human coronary arterial endothelial cells (HCAECs) were cultured in HCAEC Growth Medium at 37°C in a humidified atmosphere containing 5% CO2. Cell-conditioned medium was aspirated from near confluent flasks of cells and stored at −80°C. For cell adhesion studies, cells were cultured to approximately 90% confluence, the medium removed, the cells washed with PBS containing 1 mM MgCl2/1 mM CaCl2 then incubated with 1% trypsin (3 ml) for 3 min at 37°C. Cell-conditioned medium (8 ml) was added to neutralize trypsin, the cells dislodged by gentle tapping and collected by centrifugation. The cell pellet was twice resuspended in medium-199 containing 1% bovine serum albumin (BSA) and re-centrifuged to remove serum components, then re-suspended in medium-199 containing 1% BSA.
2.4 Purification of perlecan
Perlecan was purified from medium conditioned by HCAECs by DEAE chromatography followed by anti-perlecan (CSI-071) affinity chromatography as described previously (Whitelock et al., 1999). Perlecan protein core concentrations were determined using a Coomassie Plus microplate assay with BSA standards. Perlecan concentrations were calculated using a protein core molecular mass of 470 kDa.
2.5 Surface adsorption of perlecan
Perlecan (10 nM / 4.7 µg/ml, 50 µl) was adsorbed from PBS on to wells of 96-well microtitre plates (high-binding polystyrene, Greiner BioOne; or polyvinyl chloride, BD Biosciences) for 16 h at 22°C.
2.6 Oxidant, endoglycosidase and nitrous acid (HNO2) treatment of surface-adsorbed perlecan
Surface-adsorbed perlecan (prepared as above) was subject to oxidation by reagent HOCl or HOBr (0.5 – 10 µM) or MPO-H2O2-halide systems (10 nM MPO, 100 mM Cl− or 1 mM Br−, 0.5 – 10 µM H2O2; at these halide concentrations, generation of HOCl and HOBr by MPO has been shown to be quantitative with respect to H2O2 (Senthilmohan and Kettle, 2006)) for 4 h at 37°C. Oxidant/perlecan (molar) ratios in these systems were 50 – 1000 (105 – 2100 nmol oxidant/mg protein; surface adsorption of perlecan was assumed to be quantitative). Wells were subsequently incubated with methionine (10 mM, 100 µl, 0.5 h, 22°C) to quench any residual oxidant.
Enzymatic digestion of surface-adsorbed perlecan was carried out with heparinase III (0.01 U/ml, 50 µl) or chondroitinase ABC (0.05 U/ml, 50 µl) in 0.01% BSA or 0.01% fish gelatin (for MPO binding studies) for 2 h at 37°C.
Treatment of surface-adsorbed perlecan with HNO2 was performed at pH 3.9 to cleave heparan sulfate specifically at its GlcNH2 residues (Shively and Conrad, 1976) by incubation with 4.5 M sodium nitrite (50 µl) and 2 M acetic acid (10 µl) for 10 min at 22°C. 2 M sodium carbonate (35 µl) was then added to quench the reaction; control samples were treated with 4.5 M sodium chloride (50 µl) and 2 M acetic acid / sodium acetate, pH 3.9 (10 µl).
2.7 Oxidant and endoglycosidase treatment of soluble perlecan
Perlecan (10 nM or 0.66 – 0.8 µM) was subject to oxidation by reagent HOCl or HOBr at oxidant/perlecan molar ratios of 100 – 1000 for 4 h at 37°C. Samples were then incubated with methionine (1 or 30 mM, 0.5 h, 22°C) to quench any residual oxidant. Enzymatic digestion of perlecan was carried out using heparinase III (0.01 U/ml) or chondroitinase ABC (0.05 U/ml) in PBS containing 0.01% BSA for 2 h at 37°C.
2.8 ELISA
Wells of polystyrene microtitre plates containing surface-adsorbed perlecan were blocked with 0.1% casein in PBS and probed with appropriate mouse mAbs (CSI-076, 3.3 µg/ml; 7B5, 2 µg/ml; CSI-074, 2 mg/ml; HepSS-1, 2 µg/ml; 10E4, 2 µg/ml; JM403, 4 µg/ml; 2D10G9, 1:25). Detection of the immune complexes was performed using a biotinylated anti-mouse antibody with either streptavidin-conjugated horseradish peroxidase/ABTS or streptavidin-conjugated alkaline phosphatase/p-nitrophenylphosphate. ELISA signals were corrected for background values obtained with non-perlecan coated wells unless otherwise stated.
2.9 MPO binding by surface-adsorbed perlecan
Wells of polystyrene microtitre plates containing surface-adsorbed perlecan were blocked with 0.2% fish gelatin in PBS (50 µl, 2 h, 22°C), washed with PBS/0.01% Tween (2 × 200 µl), then incubated with 20 nM MPO in PBS (50 µl, 2 h, 22°C). The wells were then incubated with the mouse anti-MPO mAb 2C7 (2 µg/ml, 50 ml, 2 h, 22°C). To assess the effect of glycosaminoglycans on binding, MPO-treated wells were also incubated with heparin, heparan sulfate or chondroitin sulfate (100 µg/ml, 50 l, 10 min, 22°C) prior to addition of mAb 2C7 against MPO; the effect of glycosaminoglycans on background binding to the gelatin-coated plates was also assessed. Detection of the immune complexes was performed by ELISA using alkaline phosphatase.
2.10 SDS-PAGE and immunoblotting
SDS-PAGE was carried out using 3–8% NuPAGE® Tris acetate gels (ca. 2 µg protein/well) according to the manufacturer’s instructions, along with HiMark® molecular mass standards. Gels were visualized using a combined Stains All / silver staining method (Goldberg and Warner, 1997).
For Western blotting studies, proteins were electroblotted onto nitrocellulose membranes using an iBlot® transfer apparatus. Dot-blotting studies were performed by loading samples (ca. 2 µg protein) directly onto nitrocellulose membranes. Membranes were blocked with 1% casein in PBS/0.1% Tween then probed with appropriate mouse mAbs (CSI-076, 2 µg/ml; 7B5, 1 µg/ml; CSI-074, 1 µg/ml; 10E4, 0.5 µg/ml; 3G10, 2 µg/ml; 2D10G9, 1/500). To quantify protein carbonyls, samples were derivatized with DNP prior to electrophoresis and membranes were probed using a rabbit anti-DNP antibody. Detection of immune complexes was performed using horseradish peroxidase-conjugated anti-mouse/ anti-rabbit Ig antibodies, ECL reagents and a ChemiDoc XRS image acquisition system.
2.11 Cell adhesion, FGF binding and collagen V binding by surface-adsorbed perlecan
Adhesion of endothelial cells (HCAECs, 2.5 × 105 cells/ml in medium-199 containing 1% BSA, 50 µl) to perlecan or fibronectin, each adsorbed from solutions with identical protein contents (4.7 mg/ml protein, 50 µl), was quantified after 2 h incubation at 37°C by staining with Crystal Violet as described previously (Whitelock et al., 1999). Binding of [125I]FGF-2 (50 µl; ca. 70 000 cpm/well) to perlecan was quantified by gamma counting (Whitelock et al., 1999). Binding of collagen V to perlecan was quantified by ELISA using a mouse anti-collagen V mAb 1E2/E4 (1:25) and alkaline phosphatase (Whitelock et al., 1999).
2.12 Promotion of FGF-2-dependent cellular proliferation by perlecan
The ability of perlecan to promote cellular proliferation in response to exogenous FGF-2 was investigated using a heparan sulfate proteoglycan-deficient myeloid cell line (BaF3) expressing the FGFR1c receptor isotype, essentially as described previously (Knox et al., 2002). Briefly, BaF3 cells (105 cells/ml) were incubated in RPMI-1640 medium with perlecan (1.25 nM) or heparin (0.3 – 30nM) in the presence of FGF-2 (0.3 nM) for 72 h at 37°C and proliferation was then determined by the MTS assay using CellTiter 96® AQueous One Solution (10% v/v, 6 h, 37°C).
2.13 Statistical analyses
Statistical analyses were carried out using 1-way ANOVA with Newman Keul’s post-hoc testing, unless otherwise stated. All statistical analyses were performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA, www.graphpad.com), with P <0.05 taken as significant.
3. Results
3.1 Isolation and characterization of endothelial cell-derived perlecan
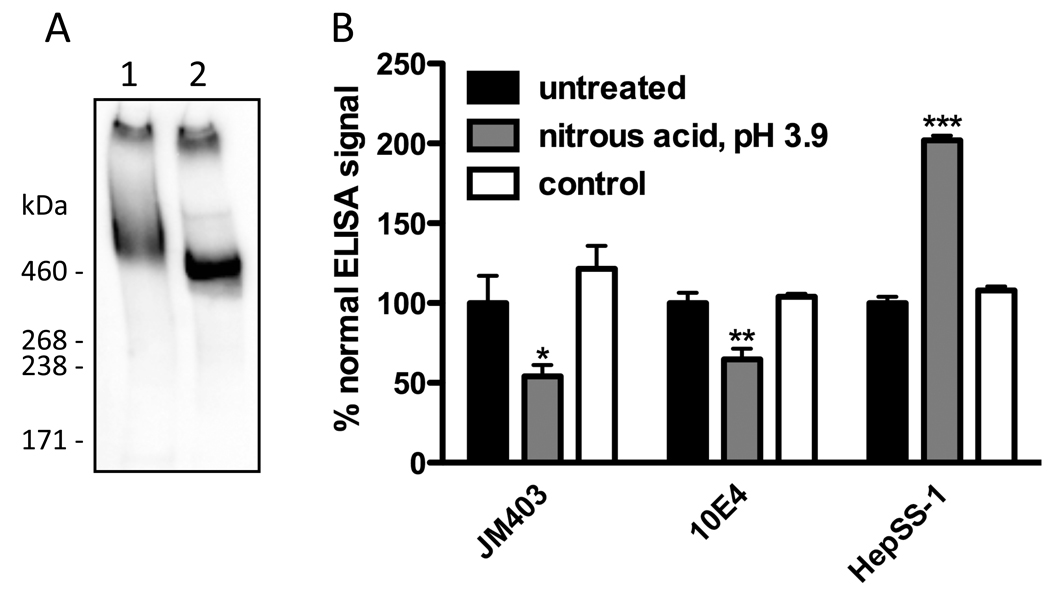
Perlecan was immunopurified from media conditioned by HCAECs as described previously (Whitelock et al., 1999). The purified proteoglycan migrated as a single broad band on 3–8% gradient SDS-PAGE gels, which was recognized by antibodies against the perlecan protein core (CSI-076, against domain I (Fig. 1A; Fig. 4); 7B5, against domain III; CSI-074, against domain V) and against heparan sulfate (10E4) (Fig. 1A; Fig. 4). The perlecan was recognized by the same antibodies in ELISAs and by other antibodies against heparan sulfate (HepSS-1 and JM403) (Fig. 1B and Fig. 2). After removal of heparin sulfate by digestion with heparinase III, the perlecan was resolved as a narrow band at a molecular mass consistent with the presence of the full-length, unsubstituted protein core (ca. 470 kDa) and was recognized by CSI-076 (Fig. 1A) and by 3G10, a mAb against heparinase III-derived heparan sulfate stubs. Digestion with chondroitinase ABC and keratanase I did not affect the migration of perlecan on gels and no recognition was observed with antibodies against chondroitin sulfate (CS56) and keratin sulfate (5D4) in ELISAs. These data establish that the purified proteoglycan consisted of a full-length perlecan protein core substituted by heparan sulfate.
Fig. 1.
Characterization of endothelial cell-derived perlecan. (A) Native perlecan (lane 1) and heparinase III-treated perlecan (lane 2) were electrophoresed in a 3–8% SDS-PAGE gel under non-reducing conditions, electroblotted to nitrocellulose and probed with antibody CSI-076 against perlecan domain I. (B) Surface-adsorbed perlecan was treated with HNO2 at pH 3.9 (3.75 M sodium nitrite, 0.33 M acetic acid) or with a control solution (3.75 M sodium chloride, 0.33 M acetic acid / sodium acetate, pH 3.9), then probed by ELISA using antibodies JM403, 10E4 and HepSS-1 against heparan sulfate. Data are means ± SEM (triplicate determinations from a representative experiment) and are expressed as % normal ELISA signal. * = P <0.05, ** = P <0.01 and *** = P <0.001 compared to untreated perlecan.
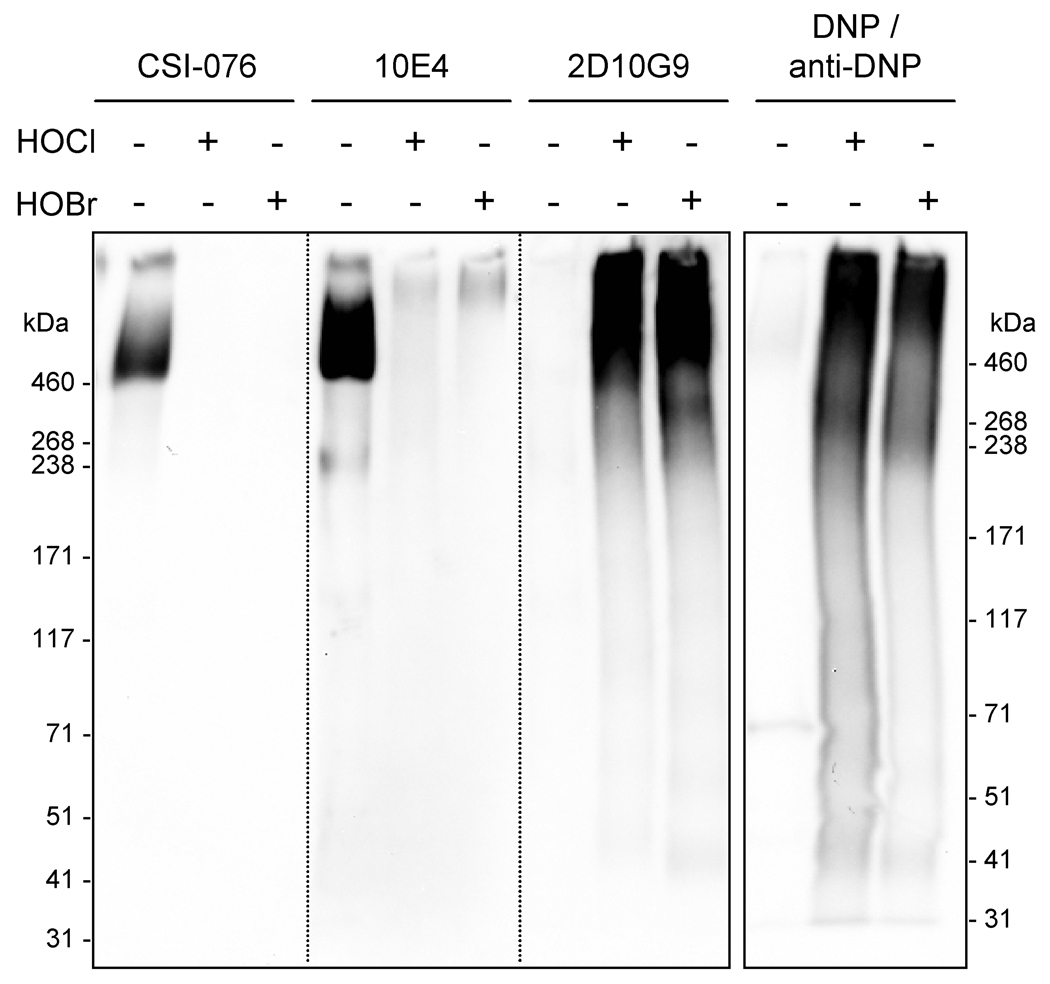
Fig. 4.
Western blotting of perlecan modified by HOCl and HOBr. Perlecan (0.66 µM) was incubated with HOCl or HOBr (660 µM; oxidant/perlecan molar ratio of 1000) at 37°C for 4 h. Samples (ca. 2 µg protein/well) were electrophoresed in a 3–8% SDS-PAGE gel under non-reducing conditions, electroblotted to nitrocellulose and probed with antibody CSI-076 against perlecan domain I, antibody 10E4 against heparan sulfate and antibody 2D10G9 against HOCl/HOBr-modified protein. To examine the formation of protein carbonyls, identical samples were derivatized with 2,4-dinitrophenyl hydrazine (DNP) prior to electrophoresis and probed with an antibody against the corresponding DNP hydrazone adduct.
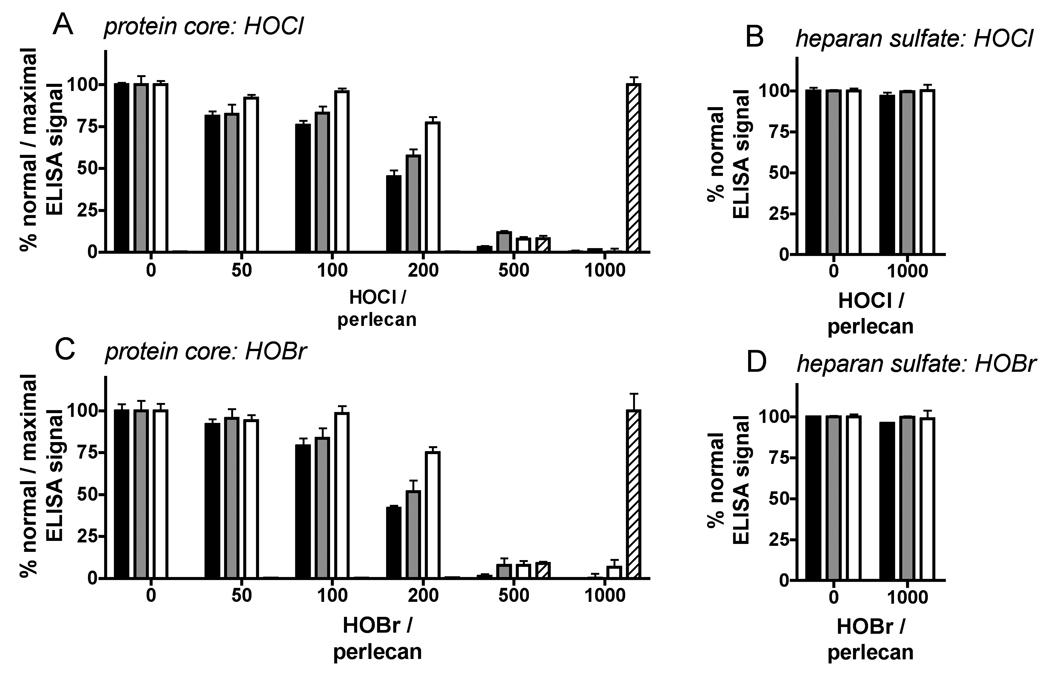
Fig. 2.
ELISA of perlecan modified by HOCl and HOBr. Surface-adsorbed perlecan was treated with HOCl or HOBr (oxidant/perlecan molar ratios of 50 – 1000) at 37°C for 4 h, then probed by ELISA using antibodies CSI-076, 7B5 and CSI074 against perlecan protein core domains I, III and V, antibody 2D10G9 against HOCl/HOBr-modified protein and antibodies HepSS1, 10E4 and JM403 against heparan sulfate. Effect of HOCl (A) and effect of HOBr (C) on recognition of protein epitopes: perlecan domain I (CSI-076, black bars); perlecan domain III (7B5, gray bars); perlecan domain V (CSI-074, white bars); and HOCl/HOBr-modified protein (2D10G9, hatched bars). Effect of HOCl (B) and effect of HOBr (D) on recognition of heparan sulfate (HepSS-1, black bars; 10E4, gray bars; JM403, white bars). Data are means ± SEM (triplicate determinations from a representative experiment) and are expressed as % normal ELISA signal (CSI-076, 7B5, CSI-074, HepSS-1, 10E4 and JM403) or % maximal ELISA signal (2D10G9, oxidant/perlecan molar ratio of 1000).
GlcNH2 residues are potential sites for reaction with HOCl and HOBr (Rees et al., 2007; Rees et al., 2005) and their presence in the heparan sulfate chains of perlecan was indicated by immunological recognition by JM403, whose binding to heparan sulfate is critically dependent on GlcNH2 residues (van den Born et al., 2005). In further support for the presence of these residues, recognition of surface-bound perlecan by JM403 and 10E4 in ELISAs was decreased upon GlcNH2-specific chemical cleavage of its heparan sulfate chains by HNO2 treatment at pH 3.9 (Fig. 1B); this treatment destroys the free amino group recognized by JM403 and truncates the polymer within structures that bind 10E4 (van den Born et al., 2005). Notably, recognition by HepSS-1, which binds to heparan sulfate sequences that do not contain GlcNH2 residues (van den Born et al., 2005), was not only maintained, but was increased in this treatment (Fig. 1B). This is consistent with proteoglycan-bound heparan sulfate stubs generated via GlcNH2-specific cleavage being retained at the surface and bearing structures recognized by HepSS-1; the increase in recognition by HepSS-1 could reflect improved access to these structures following loss of the distal (protein-free) portion of the chain from the surface. Modification of the surface-bound protein core also occurred during the HNO2 treatment, reflected by a loss of recognition by CSI-076 (ca. 90% loss; data not shown).
3.2 HOCl and HOBr preferentially modify the perlecan protein core
The ability of HOCl and HOBr to modify the protein core and heparan sulfate chains of surface-adsorbed perlecan was assessed by an ELISA-based assay. Reaction of surface-adsorbed perlecan (from a 10 nM solution) with HOCl and HOBr (0.5 – 10 µM) resulted in dose-dependent losses in recognition of the perlecan protein core by antibodies against epitopes present in domains I, III and V (CSI-076, 7B5 and CSI-074) (HOCl, Fig. 2A; HOBr, Fig. 2C), with significant losses of recognition at oxidant/perlecan molar ratios of at least 200 and as low as 50 (CSI-076 and 7B5, HOCl). The loss of recognition of native protein structures was accompanied by the generation of oxidatively-modified epitopes recognized by 2D10G9 (HOCl, Fig. 2A; HOBr, Fig. 2C), with this significant (P <0.05, one-tailed t-tests compared to untreated perlecan) at oxidant/perlecan molar ratios of 500 (1064 nmol oxidant/mg protein) or greater. The effects of HOCl and HOBr on immunological recognition of the perlecan protein core were strikingly similar, both in the loss of recognition by native protein epitopes and gain in recognition by 2D10G9. The demonstration that 2D10G9 recognizes HOBr-modified perlecan confirms that this antibody recognizes HOBr-modified protein as well as HOCl-modified protein (Chapman et al., 2000). Similar doses of HOCl and HOBr were required to induce significant recognition of BSA by 2D10G9 (1064 nmol oxidant/mg protein; not shown).
No change in recognition of heparan sulfate by HepSS-1, 10E4 and JM403 was observed under the same conditions (HOCl, Fig. 2B; HOBr, Fig. 2D). HOCl and HOBr readily induce site-specific cleavage of isolated heparan sulfate via reaction with GlcNH2 residues (Rees et al., 2007; Rees et al., 2005), which were shown to be present in perlecan-derived heparan sulfate (cf. Fig. 1B). As the ELISA assay used was sensitive to GlcNH2-specific cleavage of perlecan-derived heparan sulfate (HNO2 treatment at pH 3.9; Fig. 1B), the lack of change in immunological recognition of perlecan-derived heparan sulfate indicates that this material, unlike isolated heparan sulfate, is resistant to modification and fragmentation by HOCl and HOBr.
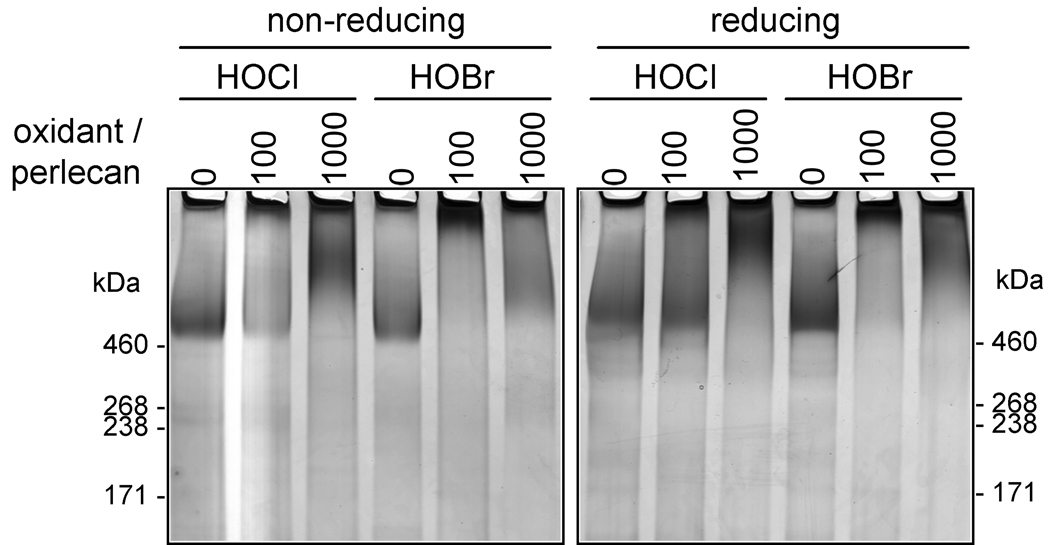
To further examine the effect of HOCl and HOBr on the structure of perlecan, its migration on 3–8% SDS-PAGE gels under non-reducing and reducing conditions was assessed. At an oxidant/perlecan molar ratio of 100, HOCl and HOBr induced aggregation of perlecan protein core (Fig. 3), with the aggregated material barely migrating into gels under non-reducing conditions; under reducing conditions partially reversal of the aggregation induced by HOCl, but not by HOBr, was observed, consistent with HOBr generating a higher yield of non-reducible protein-protein cross-links than HOCl. At a higher doses (oxidant/perlecan molar ratio of 1000) both oxidants generated material with a broader range of molecular masses, consistent with fragmentation as well as aggregation of the protein core (Fig. 3).
Fig. 3.
Gradient SDS-PAGE of perlecan modified by HOCl and HOBr. Perlecan was treated with HOCl or HOBr (oxidant/perlecan molar ratios of 100 and 1000) at 37°C for 4 h. Samples were electrophoresed in 3–8% gels under non-reducing or reducing conditions and stained by a combined Stains-All / silver staining method.
Western blotting studies showed that high-molecular mass aggregates and fragmented materials were generated at an oxidant/perlecan molar ratio of 1000 that contained HOCl/HOBr-modified epitopes recognized by 2D10G9 (Fig. 4). These materials also contained protein carbonyls, a protein modification induced by hypohalous acids (Chapman et al., 2000), as assessed by recognition by an anti-DNP antibody after derivatization of the carbonyls with DNP (Fig. 4). In line with data obtained by ELISA (Fig. 2), analysis of these materials by dot blotting demonstrated that HOCl and HOBr induced a complete loss of recognition of the perlecan protein core (CSI-076) but did not alter recognition of the heparan sulfate chains by 10E4 at an oxidant/perlecan molar ratio of 1000 (Supplementary Fig. 1A). The impaired recognition of HOCl/HOBr-modified perlecan by 10E4 on Western blots (Fig. 4) is attributed to poor transfer of the highly aggregated material (cf. Fig. 3) to the blotting membrane.
3.3 MPO binds to the heparan sulfate chains of perlecan
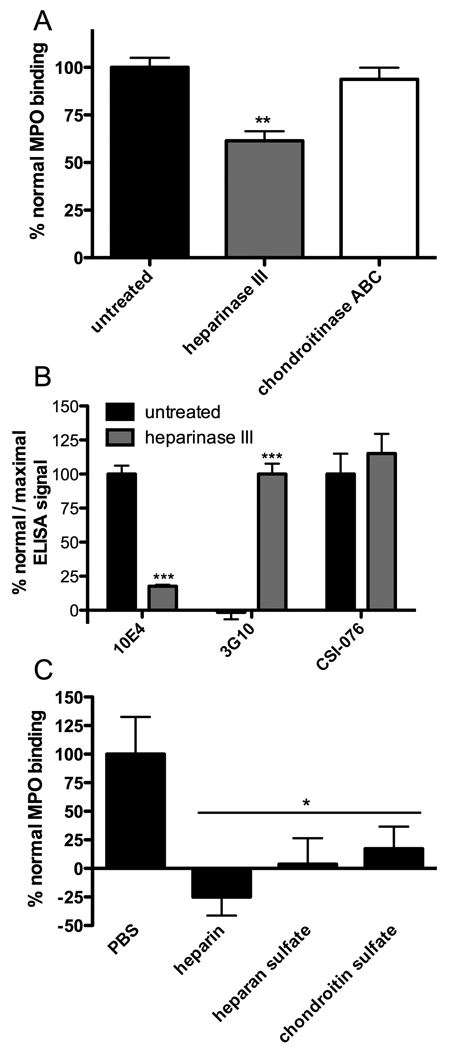
Surface-adsorbed perlecan was found to bind MPO and this binding was partially inhibited by prior removal of the heparan sulfate chains by treatment with heparinase III (Fig. 5A); removal of heparin sulfate by heparinase III was confirmed by ELISA (Fig. 5B). These data demonstrate that MPO binds to the heparan sulfate chains of perlecan. As loss of heparan sulfate (Fig. 5B) exceeded the loss in MPO binding (Fig. 5A), the protein core may also support binding of MPO. Consistent with the absence of chondroitin sulfate chains, chondroitinase ABC treatment had no effect on binding (Fig. 5A). Binding was completely abolished by treatment of the bound MPO with heparin, heparan sulfate and chondroitin sulfate (100 µg/ml) (Fig. 5C).
Fig. 5.
Binding of MPO by perlecan. Surface-bound perlecan, in gelatin-blocked microplate wells, was incubated with MPO and probed by ELISA using antibody 2C7 against MPO. (A) Binding of MPO to perlecan and effect of prior treatment of perlecan with heparinase III and chondrotinase ABC. (B) Confirmation of heparan sulfate removal by heparinase III by loss of recognition by 10E4 and gain in recognition by 3G10 against heparinase III-derived heparan sulfate stubs. (C) Effect of heparin, heparin sulfate and chondroitin sulfate (100 µg/ml, 10 min, 22°C) on binding of MPO by perlecan. Data are means ± SEM (triplicate determinations from a representative experiment) and are expressed as % normal MPO binding, % normal ELISA signal (10E4 and CSI-076) or % maximal ELISA signal (3G10). In (A) and (B), ELISA signals were corrected for values obtained with non-perlecan coated wells; in (B), ELISA signals were corrected for values obtained with non-perlecan coated wells (blocked with fish gelatin) subject to identical treatments. Treatment with glycosaminoglycans did not significantly reverse background binding of MPO to non-perlecan coated wells (data not shown). * = P <0.05, ** = P <0.01 and *** = P <0.001 and compared to binding to untreated perlecan.
3.4 MPO-H2O2-halide systems preferentially modify the perlecan protein core
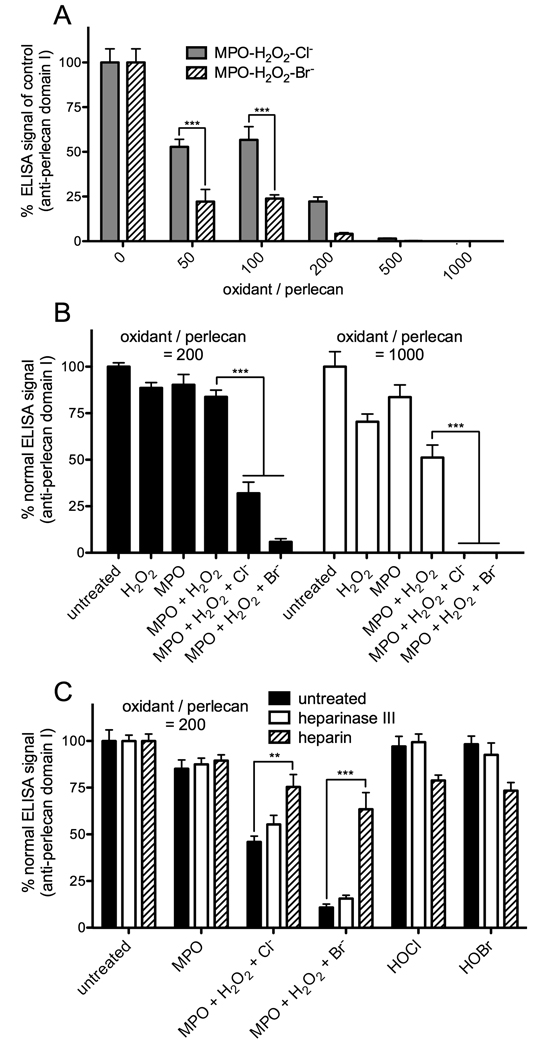
As with reagent HOCl and HOBr, reaction of the surface-adsorbed perlecan (from a 10 nM solution) with MPO-H2O2-Cl− and Br− systems (10 nM MPO, 0.05 – 10 µM H2O2, 100 mM Cl− or 1 mM Br−) resulted in dose-dependent losses in immunological recognition of the protein core (CSI-076) (Fig. 6A). These losses were significant at all oxidant doses examined (oxidant/perlecan molar ratios of 50 – 1000) and were dependent on the generation of HOCl and HOBr (Fig. 6B). The losses induced by enzymatically-generated HOCl (MPO-H2O2-Cl− system) and HOBr (MPO-H2O2-Br− system) were more marked than those observed with reagent HOCl and HOBr at identical oxidant doses. In addition, the MPO-H2O2-Br− system induced significantly greater losses in recognition of the protein core (CSI-076) than the MPO-H2O2-Cl− system at oxidant/perlecan molar ratios of 50 and 100 (Fig. 6A); this contrasts with the effects of reagent HOCl and HOBr, which were very similar at all oxidant doses examined (HOCl, Fig. 2A; HOBr, Fig. 2C). As with reagent HOCl and HOBr, no modification of heparan sulfate (10E4) was detected with either of the MPO-H2O2-halide systems (oxidant/perlecan molar ratios of 50 – 1000; not shown).
Fig. 6.
ELISA of perlecan modified by MPO-H2O2-Cl− and MPO-H2O2-Br− systems. Surface-adsorbed perlecan, in unblocked (A,B) and gelatin-blocked (C) microplate wells, was treated with MPO (10 nM) and H2O2 (0.05 – 10 17 µM; oxidant/perlecan molar ratios of 50 – 1000) in the presence of Cl− (100 mM) or Br− (1 mM) at 37°C for 4 h then probed by ELISA using antibody CSI-076 against perlecan protein core domain I. All data are means ± SEM (triplicate determinations from a representative experiment). (A) Effect of MPO-H2O2-Cl− and MPO-H2O2-Br− systems (oxidant/perlecan molar ratios of 50 – 1000) on recognition of perlecan protein core domain I; no effects were observed on recognition by antibody 10E4 against heparan sulfate (data not shown). Data are expressed as % ELISA signal of control (perlecan + MPO). Statistical differences between the effects of these oxidant systems were determined by two-way ANOVA with Bonferroni post-hoc testing; *** = P <0.001. (B) as for (A) except an oxidant/perlecan molar ratio of 200 was employed and treatments omitting MPO and/or H2O2 were also performed. Data are expressed as % normal ELISA signal (untreated perlecan); *** = P <0.001. (C) Effect of prior treatment of perlecan with heparinase III or inclusion of heparin (100 µg/ml) (cf. Fig. 5) on oxidation of perlecan by MPO-H2O2-halide systems (oxidant/perlecan molar ratio of 200); control reactions with HOCl and HOBr (oxidant/perlecan molar ratio of 200) were also performed. Statistical differences between treatments were determined by two-way ANOVA with Bonferroni post-hoc testing; ** = P <0.01, *** = P <0.001.
To investigate the role of MPO binding in the efficiency of perlecan oxidation by the MPO-H2O2-halide systems, the effect of removal of heparan sulfate with heparinase III and the inclusion of heparin to block binding of MPO to perlecan was examined. In these studies, surface-bound perlecan was treated with heparinase III and then pre-incubated with MPO in the presence or absence of heparin under identical conditions to those employed in Fig. 5 (in gelatin-blocked wells). Oxidation was then initiated by addition of H2O2 without washing of wells, to ensure the presence of identical quantities of MPO in each treatment. Removal of heparan sulfate with heparinase III, which decreased the affinity of MPO binding to perlecan but did not block this interaction (Fig. 5A), did not significantly protect the perlecan protein core against damage by MPO-H2O2-halide systems (oxidant/perlecan molar ratio of 200) (Fig. 6C). However, marked protection was observed in the presence of heparin (100 µg/ml) (Fig. 6C), which completely blocked binding of MPO to perlecan (Fig. 5C), with the extent of damage induced by the MPO-H2O2-Cl− system and the MPO-H2O2-Br− systems being of a similar magnitude to that observed with reagent HOCl and HOBr (the low extents of damage by the reagent oxidants are consistent with gelatin exerting a protective effect by acting as an alternative target for oxidation). The protective effect of heparin did not derive from oxidant scavenging, as it did not protect against damage to perlecan by reagent HOCl and HOBr (oxidant/perlecan molar ratios of 200, in unblocked wells; data not shown). Thus, sequestration of MPO by perlecan, and consequent localization of oxidant production to this target, potentiates oxidative damage to the perlecan protein core.
3.5 HOCl and HOBr impair protein core-dependent adhesion of endothelial cells
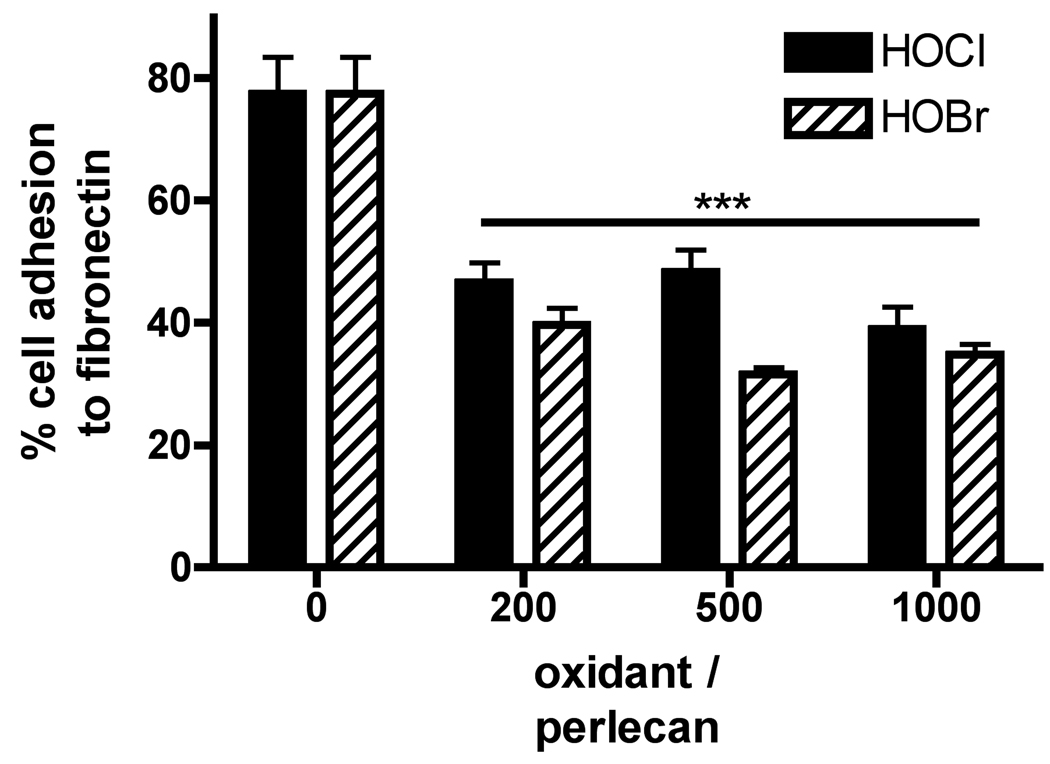
To examine the functional consequences of modification of the perlecan protein core by HOCl and HOBr, the ability of this material to support adhesion of human coronary arterial endothelial cells was assessed. Perlecan supported adhesion of endothelial cells to a similar degree as fibronectin (Fig. 7), and adhesion was unaffected by prior removal of the heparan sulfate chains with heparinase III (data not shown), confirming that adhesion is mediated by the protein core. Oxidation of the surface-adsorbed perlecan by HOCl and HOBr (oxidant/perlecan molar ratio ≥ 200) inhibited endothelial cell adhesion (Fig. 7), reflecting the data obtained by ELISA showing significant losses of native protein structures at these oxidant doses (HOCl, Fig. 2A; HOBr, Fig. 2C).
Fig. 7.
Adhesion of endothelial cells to native and HOCl/HOBr-modified perlecan. Surface-adsorbed perlecan was treated with HOCl or HOBr (oxidant/perlecan molar ratios of 200 – 1000) at 37°C for 4 h and the adhesion of endothelial cells (HCAECs) was measured by staining of bound cells with Crystal Violet and expressed as a percentage of adhesion to native (unoxidized) surface-adsorbed fibronectin. Data are means ± SEM (quadruplicate determinations from a representative experiment). *** = P <0.001 compared to untreated perlecan.
3.6 HOCl and HOBr do not impair heparan sulfate-dependent binding of FGF-2 or collagen V or promotion of FGF-dependent cellular proliferation
To examine the effect of HOCl and HOBr on the function of the heparan sulfate chains of perlecan, the ability of this material bind FGF-2 and collagen V and to promote FGF-dependent cellular proliferation was assessed. The heparan sulfate chains of perlecan act as low-affinity co-receptors for FGF-2, with these interactions stimulating cellular proliferation by promoting interaction of FGF-2 with cell surface FGF receptors (Knox et al., 2002; Nugent, 2000). Perlecan also binds collagen V and other matrix components (Whitelock et al., 1999), with these interactions likely to have a role in matrix organization.
Perlecan binding of FGF-2 and collagen V via its heparan sulfate chains was confirmed and, in line with previous findings (Whitelock et al., 1999; Whitelock et al., 1996), this was reversed by prior treatment of the perlecan with heparinase III (data not shown). Oxidation of surface-adsorbed perlecan by HOCl and HOBr (oxidant/perlecan molar ratio of 1000) did not affect either FGF-2 (Supplementary Fig. 2A) or collagen V binding (Supplementary Fig. 2B), demonstrating that the heparan sulfate sequences that bind these ligands (Guimond et al., 1993; Ricard-Blum et al., 2006) are resistant to modification by these oxidants. As well as maintaining their ability to bind FGF-2, the heparan sulfate chains of HOCl/HOBr-modified perlecan (oxidant/perlecan molar ratios of 100 and 1000) maintained their ability to stimulate proliferation of FGFR1c expressing cells in response to FGF-2 (Supplementary Fig. 3), an activity that requires a dodecasaccharide heparan sulfate sequence encompassing the FGF-2 binding motif (Pye and Kumar, 1998).
4. Discussion
The subendothelial matrix is an important site for deposition of MPO and damage by MPO-derived oxidants in acute inflammatory responses and in chronic inflammatory diseases such as atherosclerosis (Davies et al., 2008; Rees et al., 2008). The heparan sulfate proteoglycan perlecan is a key component of the subendothelial matrix and it is shown here that endothelial cell-derived perlecan can bind MPO via its heparan sulfate chains. Other heparan sulfate proteoglycans present on the endothelial cell surface are important ligands for MPO and facilitate transfer of free MPO from the vascular lumen to the subendothelial space via a transcytotic mechanism (Baldus et al., 2001). Our data indicates that perlecan may be an important ligand for MPO and target for its oxidants, such as HOCl and HOBr, within the subendothelial matrix. The potential biological consequences of damage to perlecan by MPO-derived HOCl and HOBr were examined by determining the effects of these oxidants on the structure and function of its protein core and heparan sulfate chains. The heparan sulfate chains of perlecan displayed resistance to degradation by HOCl and HOBr, despite containing highly reactive GlcNH2 residues, whilst the protein core was extensively modified by these oxidants. Notably, the efficiency of damage to the protein core was greater with oxidants generated by perlecan-bound MPO than with unbound (heparin-associated) MPO or with reagent HOCl and HOBr, highlighting the potential importance of MPO-binding in directing damage to this matrix constituent. Oxidative modification of the protein core by HOCl and HOBr resulted in impairment of its cell-adhesive function, but the ability of perlecan-derived heparan sulfate to bind FGF-2 and collagen V and to promote FGF-dependent cellular proliferation was unimpaired. These data have significant implications for understanding the mechanisms and biological consequences of vascular injuries induced by MPO-derived HOCl and HOBr.
Although HOBr reacts faster with protein components than HOCl, computational kinetic models predict that the distribution of attack of the free oxidants on proteins will be similar (Pattison and Davies, 2001; Pattison and Davies, 2004). Our data obtained with reagent HOCl and HOBr accord with this prediction, with these oxidants modifying structures throughout the perlecan protein core, and inducing covalent protein-protein cross-linking and protein fragmentation, in a similar manner; the higher yield of non-reducible protein-protein cross-links obtained with HOBr may reflect preferential modification of Tyr and Trp residues by this oxidant (Pattison and Davies, 2001; Pattison and Davies, 2004). Notably, oxidants generated by MPO when bound to the proteoglycan surface exerted different effects, with MPO-derived HOBr being more efficient than MPO-derived HOCl in inducing damage to the protein core. We propose that this may reflect faster and therefore more efficient consumption of HOBr by the protein core, with this effect limiting competitive reactions with the MPO protein itself or with other available protein targets (e.g. gelatin).
Antibody 2D10G9 was originally raised against HOCl-modified low-density lipoproteins (Malle et al., 1995) and has been shown here and previously (Chapman et al., 2000) to cross-react with epitopes generated on other proteins (perlecan and BSA) modified by HOCl and HOBr in vitro. The doses of HOCl and HOBr required to generate material recognized by 2D10G9 from perlecan and BSA were >425 nmol oxidant/mg protein, which augments previous data that proteins generally require exposure HOCl or HOBr at these doses (expressed relative to protein by mass) to generate material recognized by this antibody (Chapman et al., 2000; Malle et al., 2006). The detection of 2D10G9-reactive material in the subendothelial matrix of human atherosclerotic lesions and other diseased human tissue (Grone et al., 2002; Hammer et al., 2001; Malle et al., 2006; Malle et al., 1997) indicates that perlecan and other extracellular proteins are exposed to HOCl and/or HOBr in vivo at doses of a similar magnitude to those employed in these in vitro studies. In further support of the potential pathological relevance of the oxidant doses employed, the content of 3-chloroTyr (a specific marker of HOCl-mediated protein oxidation) in low-density lipoprotein isolated from human atherosclerotic lesions indicates an in vivo exposure to HOCl of >200 nmol oxidant/mg protein (oxidant/ low-density lipoprotein molar ratio of 100) (Hazen and Heinecke, 1997).
Impairment of cell adhesion to the protein core occurred at oxidant/perlecan molar ratios of 200 (425 nmol oxidant/mg protein) and greater, correlating with ELISA data showing significant loss of recognition of the protein core at these oxidant doses. Importantly, this functional impairment occurred at oxidant doses that may be pathologically-relevant, i.e. below those required to generate HOCl/HOBr-modified epitopes recognized by 2D10G9 (>425 nmol oxidant/mg protein). The MPO-H2O2-Cl− system has previously been shown to disrupt the adhesive properties of endothelial cell matrix towards endothelial cells (Vissers and Thomas, 1997) and degradation of perlecan by HOCl could contribute to this effect. HOCl-mediated impairment of the reverse cholesterol and lipid binding activities of apoAI, another proposed target for MPO-derived HOCl within the subendothelial space, occurs at similar total oxidant doses (100 – 250 nmol oxidant/mg protein; oxidant/apoAI molar ratios of 2.8 – 7) (Peng et al., 2005; Zheng et al., 2004).
The heparan sulfate chains of proteoglycans were previously identified as potential targets for modification and fragmentation by HOCl and HOBr on the basis of their high rate constants for their reaction with the GlcNH2 residues present in this polysaccharide (Rees et al., 2007; Rees et al., 2005). With isolated heparan sulfate, decomposition of the N-halogenated species formed in these reactions (R-NX-H and R-NX2, X = Cl, Br) has been shown to precede cleavage of the polysaccharide backbone (Rees et al., 2007; Rees et al., 2005). A key finding of this study is that perlecan-derived heparan sulfate, although containing GlcNH2 residues, is resistant to degradation by HOCl and HOBr. As the heparin sulfate chains of perlecan are predicted to be initial targets for HOCl and HOBr, it is likely the protein core acts to prevent subsequent degradation of the chains by quenching the intermediate N-halogenated species (heparan sulfate-NX-H + protein → heparan sulfate-NH2 + protein-X, X = Cl or Br). The presence of transition metal ions and superoxide radicals (O2•−), which stimulate heparan sulfate degradation by decomposing N-halogenated heparan sulfate species to nitrogen-centered radicals (Rees and Davies, 2006; Rees et al., 2007), could give rise to a different selectivity of damage, however these reactions were not examined here.
Glomerular injury in rats induced by renal infusion of MPO and H2O2 induces rapid proteinuria and oxidation of the glomerular basement membrane (Johnson et al., 1987). Furthermore, the permeability of isolated glomeruli to albumin is rapidly increased, in a HOCl-dependent manner, by exposure to the MPO-H2O2-Cl− system or stimulated neutrophils (Li et al., 1994). The heparan sulfate chains of perlecan play an important role in the glomerular filtration barrier (Morita et al., 2005), along with other basement membrane proteoglycans and extracellular proteins (Iozzo, 2005; Morita et al., 2008). The release of perlecan and other proteoglycans from extracellular matrix by HOCl (Klebanoff et al., 1993) is likely to contribute to impairment of the glomerular filtration barrier in this experimental model and our data indicates that this process involves protein modification and/or fragmentation and not direct degradation of heparan sulfate.
In a related carotid artery injury model in rats, infusion of MPO and H2O2 induces neointimal hyperplasia via proliferation of smooth muscle cells (Yang et al., 2006), which is a characteristic feature of atherosclerotic lesions. The proliferative response in this vascular injury could derive, at least in part, from HOCl/HOBr-mediated release of perlecan from subendothelial matrix, where the proteoglycan normally sequesters growth factors and restrains their mitogenic activity (Nugent and Iozzo, 2000). The potential pro-proliferative activity of HOCl/HOBr-oxidized perlecan is established by our demonstration that is can promote FGF-2-dependent cellular proliferation, at least in heparan sulfate-deficient cells. Thus, disruption of the integration of perlecan into extracellular matrix by HOCl and HOBr, via modification of its protein core, may facilitate smooth muscle cell proliferation by impairing growth factor sequestration within the subendothelial matrix and by generating material that promotes growth factor signaling.
In conclusion, our data indicates that oxidation of the perlecan protein core may be an important process in vascular injuries induced by MPO-derived HOCl and HOBr, with damage to this proteoglycan potentially increasing the permeability of the subendothelial matrix, disrupting cell-matrix interactions and altering the proliferation of vascular cells. These processes may contribute to a loss of vascular function and altered vascular cell behaviour in acute inflammatory responses and inflammatory diseases, such as atherosclerosis, in which MPO-mediated damage has been implicated. The finding that perlecan-bound heparan sulfate is resistant to degradation by HOCl and HOBr is of major significance in advancing understanding of the biological consequences of MPO-mediated extracellular matrix oxidation. The data presented here indicates that release of perlecan from extracellular matrix by HOCl and HOBr may liberate functionally-active heparan sulfate in addition to impairing extracellular matrix function.
Supplementary Material
Acknowledgements
This work was supported in part by the Australian Research Council, through the Centres of Excellence and Discovery programs, the National Health and Medical Research Council, and the Austrian Science Fund (FWF, P19074-B05) and by National Institutes of Health grants RO1 CA39481 and CA47282.
Abbreviations
- DNP
2,4-dinitrophenylhydrazine
- GlcNH2
N-unsubstituted glucosamine
- HOBr
the physiological mixture of hypobromous acid and its anion
- HOCl
the physiological mixture of hypochlorous acid and its anion
- HOSCN
the physiological mixture of hypothiocyanous acid and its anion
- MPO
myeloperoxidase
- NO2−
nitrite
- SCN−
thiocyanate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnhold J, Monzani E, Furtmuller PG, Zederbauer M, Casella L, Obinger C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur. J. Inorg. Chem. 2006:3801–3811. [Google Scholar]
- Baldus S, Eiserich JP, Brennan ML, Jackson RM, Alexander CB, Freeman BA. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic Biol Med. 2002;33:1010–1019. doi: 10.1016/s0891-5849(02)00993-0. [DOI] [PubMed] [Google Scholar]
- Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J. Clin. Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ALP, Senthilmohan R, Winterbourn CC, Kettle AJ. Comparison of mono- and dichlorinated tyrosines with carbonyls for detection of hypochlorous acid modified proteins. Arch Biochem Biophys. 2000;377:95–100. doi: 10.1006/abbi.2000.1744. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- Goldberg HA, Warner KJ. The staining of acidic proteins on polyacrylamide gels: enhanced sensitivity and stability of "stains-all" staining in combination with silver nitrate. Anal. Biochem. 1997;251:227–233. doi: 10.1006/abio.1997.2252. [DOI] [PubMed] [Google Scholar]
- Grone HJ, Grone EF, Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab Invest. 2002;82:5–14. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and inhibitory heparin sequences for FGF-2 (basic FGF) - Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993;268:23906–23914. [PubMed] [Google Scholar]
- Hammer A, Desoye G, Dohr G, Sattler WG, Malle E. Myeloperoxidase-dependent generation of hypochlorite-modified proteins in human placental tissues during normal pregnancy. Lab Invest. 2001;81:543–554. doi: 10.1038/labinvest.3780263. [DOI] [PubMed] [Google Scholar]
- Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: From cellar to ceiling. Nat. Rev. Mol. Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ. New mechanism for glomerular injury: myeloperoxidase-hydrogen peroxide-halide system. J. Clin. Invest. 1987;79:1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella MG, Tran PK, Weiser-Evans MCM, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury - Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler Thromb Vasc Biol. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Kinsella MG, Wight TN. Degradation of endothelial cell matrix heparin sulfate proteoglycan by elastase and the myeloperoxidase-H2O2-chloride system. Am J Pathol. 1993;143:907–917. [PMC free article] [PubMed] [Google Scholar]
- Knox S, Fosang AJ, Last K, Melrose J, Whitelock J. Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan. FEBS Lett. 2005;579:5019–5023. doi: 10.1016/j.febslet.2005.07.090. [DOI] [PubMed] [Google Scholar]
- Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal - Interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J Biol Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- Li JZ, Sharma R, Dileepan KN, Savin VJ. Polymorphonuclear leukocytes increase glomerular albumin permeability via hypohalous acid. Kidney Int. 1994;46:1025–1030. doi: 10.1038/ki.1994.363. [DOI] [PubMed] [Google Scholar]
- Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunological detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: Fingerprints of newly recognized potential proatherogenic lipoproteins. Arch Biochem Biophys. 2006;445:245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Malle E, Woenckhaus C, Waeg G, Esterbauer H, Grone EF, Grone HJ. Immunological evidence for hypochlorite-modified proteins in human kidney. Am J Pathol. 1997;150:603–615. [PMC free article] [PubMed] [Google Scholar]
- McGowan SE. Mechanisms of extracellular matrix proteoglycan degradation by human neutrophils. Am. J. Respir. Cell Mol. Biol. 1990;2:271–279. doi: 10.1165/ajrcmb/2.3.271. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshimura A, Inui K, Lodeura T, Watanabe H, Wang L, Soininen R, Tryggvason K. Heparan sulfate of perlecan is involved in glomerular filtration. J. Am. Soc. Nephrol. 2005;16:1703–1710. doi: 10.1681/ASN.2004050387. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshimura A, Kimata K. The role of heparan sulfate in the glomerular basement membrane. Kidney Int. 2008;73:247–248. doi: 10.1038/sj.ki.5002659. [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Liu BA, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain-III and by in situ hybridization. J. Histochem. Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- Nugent MA. Heparin sequencing brings structure to the function of complex oligosaccharides. Proc Natl Acad Sci USA. 2000;97:10301–10303. doi: 10.1073/pnas.97.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J. Biochem. Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side-chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and the use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry. 2004;43:4799–4809. doi: 10.1021/bi035946a. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- Peng DQ, Wu ZP, Brubaker G, Zheng LM, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- Pye DA, Kumar S. Endothelial and fibroblast cell-derived heparan sulphate bind with differing affinity to basic fibroblast growth factor. Biochem Biophys Res Commun. 1998;248:889–895. doi: 10.1006/bbrc.1998.9081. [DOI] [PubMed] [Google Scholar]
- Rees MD, Davies MJ. Heparan sulfate degradation via reductive homolysis of its N-chloro derivatives. J Am Chem Soc. 2006;128:3085–3097. doi: 10.1021/ja0577239. [DOI] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, Davies MJ. Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glycosamines: Evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation. J Am Chem Soc. 2003;125:13719–13733. doi: 10.1021/ja0370591. [DOI] [PubMed] [Google Scholar]
- Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Rees MD, McNiven TN, Davies MJ. Degradation of extracellular matrix and its components by hypobromous acid. Biochem J. 2007;401:587–596. doi: 10.1042/BJ20061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees MD, Pattison DI, Davies MJ. Oxidation of heparan sulphate by hypochlorite: role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem J. 2005;391:125–134. doi: 10.1042/BJ20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S, Beraud M, Raynal N, Farndale RW, Ruggiero F. Structural requirements for heparin/heparan sulfate binding to type V collagen. J Biol Chem. 2006;281:25195–25204. doi: 10.1074/jbc.M603096200. [DOI] [PubMed] [Google Scholar]
- Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Shively JE, Conrad HE. Formation of anhydrosugars in chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem J. 1997;327:487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Born J, Salmivirta K, Henttinen T, Ostman N, Ishimaru T, Miyaura S, Yoshida K, Salmivirta M. Novel heparan sulfate structures revealed by monoclonal antibodies. J Biol Chem. 2005;280:20516–20523. doi: 10.1074/jbc.M502065200. [DOI] [PubMed] [Google Scholar]
- Vissers MCM, Thomas C. Hypochlorous acid disrupts the adhesive properties of subendothelial matrix. Free Radic Biol Med. 1997;23:401–411. doi: 10.1016/s0891-5849(96)00619-3. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Iozzo RV. Heparan sulfate: A complex polymer charged with biological activity. Chem. Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Woods AA, Davies MJ. Fragmentation of extracellular matrix by hypochlorous acid. Biochem J. 2003;376:219–227. doi: 10.1042/BJ20030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cheng YH, Ji RR, Zhang CX. Novel model of inflammatory neointima formation reveals a potential role of myeloperoxidase in neointimal hyperplasia. Am. J. Physiol.-Heart Circul. Physiol. 2006;291:H3087–H3093. doi: 10.1152/ajpheart.00412.2006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Zheng LM, Nukuna B, Brennan ML, Sun MJ, Goormastic M, Settle M, Schmitt D, Fu XM, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.