Abstract
Background/Objectives
Monitoring changes in total fat mass and abdominal adiposity are important in understanding the impact of different types of weight loss interventions on health risks. Our objective was to assess the usefulness of anthropometry and bioelectrical impedance analysis (BIA) in predicting fat mass changes during moderate weight loss.
Subjects/Methods
Fat mass changes were assessed in 34 overweight adults (24 females, 10 males) after a 12-week supervised weight loss induced by caloric restriction (−30% of requirement) using BIA and DXA. Agreement between BIA and DXA measurements were assessed by Bland-Altman plots. Linear regression modeling was used to predict body and truncal fat mass from anthropometric measures.
Results
Diet intervention resulted in a significant decrease in body weight (− 7.86 ± 2.87 kg), body mass index (BMI − 2.69 ± 0.98 kg/m2), total body fat (− 5.22 ± 2.32 kg), truncal fat (− 2.80 ± 1.94 kg) and waist circumference (− 5.52 ± 3.57 cm). BMI and body weight were highly correlated with body fat (0.83 and 0.92 in females and 0.94 and 0.92 in males respectively) and truncal fat (0.75 and 0.87 in females; 0.90 and 0.84 in males respectively) during weight loss. Waist circumference was more correlated with truncal fat in males than females (0.94 vs. 0.85 in females). Compared to DXA, BIA underestimated total body fat changes in males (− 8.8 kg, p<0.001) and overestimated total body fat changes in females (+ 2.1 kg, p< 0.001).
Conclusions
Body mass index, body weight, and waist circumference provide simple and more accurate than BIA estimates of relative changes in total and truncal fat during moderate weight loss in adults.
Keywords: Energy Restriction, Anthropometry, Body Composition, Abdominal Obesity
Introduction
Monitoring changes in body fat and abdominal adiposity are important in understanding the impact of different types of weight loss interventions on health benefits and risks. There is substantive evidence that abdominal excess fat is more correlated with obesity associated complications than excess total body fat [1–10], and waist circumference (WC) has been included among the diagnostic criteria for the metabolic syndrome [11].
The need for having a simple, inexpensive, noninvasive, and universally applicable technique of measuring changes in body and truncal fat during weight loss is important in the context of popularity of weight loss programs and obesity pandemic[12,13]. Accurate measurement of body composition using reference standards is either cumbersome, as in the 4-compartment model and underwater weighing, expensive as in magnetic resonance imaging (MRI) and computerized tomography (CT), or involving a small dose of radiation as with dual energy x-ray absorptiometry (DXA) and CT [14,15]. Additional limitations that include lack of portability and scanner size that may be too small for some obese individuals are shared by MRI, CT, and DXA [16]. Although bioelectrical impedance analysis (BIA) has emerged as a popular method of assessing changes in body composition [17–19] [20,21], its accuracy to identify these changes in overweight patients and during weight loss is not certain [22–25].
The use of BMI and WC in predicting health risks has been well recognized. Health risk has been shown to increase in a graded fashion when moving from normal weight to obese BMI categories and within each BMI category, individuals with high WC values are at a greater health risk than those with normal WC values [26,27]. Further, recent studies testing anthropometric parameters with non-invasive body fat estimates indicate that anthropometric measures particularly WC may offer simple and practical method for assessing changes in abdominal fat content [30–32]. However, the task of better characterization of anthropometric parameters to gauge visceral adiposity is inherently complex due to multiple variables including race and ethnicity, affecting body fat and its distribution [28,29].
Few studies have systematically examined anthropometric, total body fat, and abdominal fat changes during moderate weight loss. Thus, the principal aim of the present study was to compare the usefulness of simple anthropometric parameters and BIA in predicting changes in body fat during moderate weight loss using DXA as a reference standard.
Materials/Subjects and Methods
Participants
Volunteers were participants of a 12-week study on the effect of energy restriction and calcium on weight loss. Forty subjects were enrolled and 34, ten male and twenty four female, mean age 38 ± 7 (range 25–47) years and BMI 36.1 ± 4.2 (range 28.9–45.5) kg/m2 (Table 1), completed the study (i.e. ate the provided food during the entire study and returned for the final 12-week data collection visit). Volunteers were recruited through the Vanderbilt University email system and through flyers placed on bulletin boards, elevators, and other visible places around Medical Center. Exclusion criteria included drug therapy, smoking, special diet, taking dietary supplements for weight loss, regular participation in heavy physical activities, pregnancy and lactation. Those with a history of diabetes, hypertension, renal, liver, or heart disease, those with active thyroid disease or receiving thyroid hormone substitution, volunteers with known or suspected drug or alcohol abuse or with any clinical condition rendering them unfit to participate were also excluded from participation. This study was conducted at the Vanderbilt University’s Clinical Research Center and was approved by the Institutional Review Board. Each participant provided written consent before the study.
Table1.
Participant characteristics and body composition before and after weight loss
| Parameter | Before Weight Loss (n = 34) | After Weight Loss (n = 34) | ||||
|---|---|---|---|---|---|---|
| Females (24) | Males (10) | All (34) | Females (24) | Males (10) | All (34) | |
| Weight (kg) | 101.16 ± 13.4 (74–124.94) |
114.9 ± 12.3 (99.7–131.2) |
105.2 ± 14.4 (74.0–131.2) |
93.81 ± 13.8 (67.9–121.3) |
105.8 ± 12.7 (91.3–128.7) |
98.09 ± 13.9 (67.9–128.7) |
| BMI (kg/m2) | 36.16 ± 4.5 (28.91–45.56) |
35.16 ± 3.4 (29.55–40.56) |
36.14 ± 4.2 (28.91–45.56) |
33.51 ± 4.5 (25.56–41.34) |
32.36 ± 3.4 (27.06–37.6) |
32.42 ± 7.4 (25.56–41.34) |
| WC (cm) | 103.43 ± 9.3 (87–120.4) |
115.8 ± 12.5 (93–134) |
107.42 ± 11.3 (87–134) |
99.56 ± 10.6 (84–126) |
109.7 ± 12.7 (86–127) |
103.22 ± 11.7 (84–127) |
| BIA Body Fat (kg) | 51.68 ± 10.6 (33.2–70.1) |
32.71 ± 6.4 (24.0–41.8) |
45.93 ± 12.9 (24.0–70.1) |
45.93 ± 10.8a (28.5–67.6) |
27.53 ± 6.1 (19.4–37.7) |
40.52 ± 12.79 (19.4–67.6) |
| DXA Truncal Fat (kg) |
24.22 ± 4.7 (17.67–32.93) |
23.25 ± 6.02 (12.28–34.2) |
23.93 ± 5.0 (12.28–32.93) |
21.45 ± 4.9 (13.4–31.89) |
20.35 ± 5.82 (8.19–27.63) |
21.13 ± 5.1 (8.19–31.89) |
| DXA Body Fat (kg) | 49.45 ± 8.5 (34.53–62.15) |
41.6 ± 7.7 (29.34–54.07) |
47.14 ± 8.9 (29.34–62.15) |
41.75 ± 10.5 (26.05–61.12) |
36.2 ± 9.5 (20.35–52.61) |
41.92 ± 10.33 (20.35–61.12) |
| DXA Body Fat (%) | 48.76 ± 3.99 (37.63–53.78) |
36.14 ± 3.87 (29.22–41.91) |
45.05 ± 7.02 (29.22–53.78) |
46.83 ± 5.03 (34.19–54.75) |
33.8 ± 5.63 (22.29–41.14) |
43.0 ± 7.91 (22.29–54.75) |
Values are mean ± SD; values in parentheses are ranges. All values have been rounded to one decimal. All p values are <0.001
data for 23 females
BMI, body mass index; WC, waist circumference; DXA, dual energy x-ray absorptiometry; BIA, bioelectrical impedance analysis.
Weight loss study design
This study had a parallel design with a random assignment to either a dairy or non-dairy diet containing 30% less energy than required. The study consisted of two phases: a 7-day lead in phase and a 12-week intervention period of energy restriction. The individualized diet contained approximately 70% (± 50 kcal) of daily energy requirements, 52–54% of energy from carbohydrates, 26–29% of energy from fat, 17–21% of energy from protein, and 17–21 g of fiber. To assure sufficient micronutrients content of the diet, participants received multivitamin supplement. After 6-weeks energy content of the diet was adjusted to reflect changes in body weight (− 100 to 200 kcal/day). Participants were allowed moderate amounts of calorie-free foods such as water, coffee (without sugar or milk), diet soft drinks, salt, and pepper.
Anthropometric Measurements
The National Health and Nutrition Examination Survey (NHANES) protocols [33] were followed for all anthropometric measurements. All testing took approximately 1-hour and was conducted in the morning after a 12-h fast at the baseline and after a 12 weeks energy restriction diet. Body weight was measured to the nearest 0.05 kg with a monthly calibrated digital scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL) 3 times per week to monitor study compliance. Height was measured using stadiometer (Perspective Enterprises, Portage, MI). Waist circumference was measured in standing position to the nearest 0.1 cm at the midaxillary high point of the iliac crest at minimal respiration at the start and after 4, 8 and 12 weeks of energy restriction diet. The averages of the two readings were used for analysis. All measurers were performed by the same investigator (CAD).
Body composition
All weight measurements were performed with the same calibrated scale following the National Health and Nutrition Examination Survey (NHANES) protocols as explained above. Consequently measurements of body composition were done by feeding in the same weight into both DXA and BIA software and the measurements were performed sequentially with BIA performed after DXA while the volunteer was still on DXA table.
Dual energy X-ray Absorptiometry (DXA)
Body composition was determined by DXA using narrow fan-beam technology (GE Lunar Prodigy™, enCORE™ software version 10.5, Madison, WI) before and after a 12-weeks diet intervention. Fat mass (FM) and fat-free mass (FFM) were determined and FFM was further divided into lean body mass (LBM) and bone mineral content (BMC). Calibration was performed before each scan using standards to simulate lean (0.6% NaCl), fat (stearic acid), and bone (hydroxapatite). Our laboratory’s intra-assay coefficient of variation for %FM using DXA is 0.79 ± 0.49%. All subjects were scanned in the supine position by the same investigator (CAD).
Bioelectrical impedance (BIA)
BIA was measured using the Quantum-II Desktop (RJL Systems, Clinton Township, MI). Briefly, two signaling electrodes were placed on the dorsal surface of the right foot between the second and third toe, as well as the dorsal surface of the right hand between the second and third digits. Two sensor electrodes were placed on the right hand and right ankle. Participants were asked to remain motionless in the supine position with legs and arms slightly abducted so there was no contact between the extremities and torso. The measurement was done promptly after DXA measurement by the same investigator (CAD). Data output included reactance, resistance and impedance. Data were entered into the software program provided by the manufacturer (Cypress, RLJ Systems, Clinton Township, MI). Data output included fat mass (kg), fat free mass (kg), total body water (kg), extracellular water (kg) and intracellular water (kg).
Statistical Analysis
All data are presented as mean ± SD. The primary outcome was the fat mass measured by 2 different methods (DXA and BIA) at baseline and after weight loss for each subject. The changes in the fat mass, weight, BMI, waist circumference and total body water measured by BIA after weight loss from the baseline were tested using random effects models to take into account correlation among the repeated measurements. The models included gender and time interactions to test gender differences in the changes. Bland-Altman plots were used to assess the agreement between the percentage of fat mass measured by DXA and those by BIA. The 95% limits of agreement are presented as the mean difference ± 2 SD of the differences. To test gender differences in the agreements between methodologies (DXA and BIA), random effects models included gender and methodology interactions. As secondary analyses, the differences in the percentage of truncal fat mass between females and males were tested using Wilcoxon rank-sum tests.
To predict fat mass and truncal fat from anthropometric variables, linear regression models were developed. The predictors for fat mass included weight, weeks in a weight loss program, and gender whereas those for truncal fat mass were weight, waist circumference, weeks in a weight loss program and gender. The models allowed all interactions among the predictors. Based on the predictive model equations, nomograms were developed to predict body and truncal fat losses during moderate weight loss. To validate these nomograms, we used an independent data set which consisted of 18 females and 6 males (age 42.9 ± 6.4, BMI 32.1 ± 3.1). The fat mass was predicted using the predictive equation, and Bland-Altman plots were used to assess the agreement between the predicted fat mass and the fat mass measured by DXA.
All tests were two-tailed, and a P-value of <0.05 was considered significant. Analyses were performed with STATA 9.2 (StataCorp, College Station, TX) and R (www.r-project.org).
Results
Weight and body composition changes
The mean body weight decreased significantly by 7.3 kg in females (95% CI: −8.5 to −6.2 kg; p < 0.001) and 9.2 kg in males (95% CI: −10.8 to −7.6 kg; p < 0.001). The mean WC was significantly decreased by 4.5 cm for females (95% CI: −6.4 to −2.5 cm; p < 0.001) and by 6.1 cm for males (95% CI: −8.9 to −3.3 cm; p < 0.001). The BMI also significantly decreased by 2.7 kg/m2 for females (95% CI: −3.1 to −2.2 kg/m2; p < 0.001) and by 2.8 kg/m2 for males (95% CI: −3.3 to −2.3 kg/m2; p < 0.001) (Table 1). Both the weight and WC registered a roughly linear decrease during the study period. There were no differences between weight and fat loss between the diets (dairy vs. no dairy, p > 0.05).
Fat mass, measured by DXA, also decreased significantly by −5.1 kg for females (95% CI: −6.1 to 4.2 kg; p < 0.001) and by −5.4 kg for males (95% CI: −6.8 to −4.0 kg; p < 0.001). The mean %BF loss was 1.94±2.39 in females, 2.34±2.34 in males, and 2.06±2.35 in total study population. The differences in fat mass loss between male and female were not significant (p > 0.05). The truncal fat (%), measured by DXA, was significantly greater in males compared to females both at baseline (medians 49.0% vs. 56.0%, p = 0.049) and after weight loss medians.48.0% vs. 56.2%, p = 0.005). Fat mass measured by BIA was greater than that measured by DXA in females (mean difference of 2.1 kg, p < 0.001) and smaller in males (mean difference of 8.8 kg, p < 0.001).
Weight, BMI, and WC were significantly correlated with body and truncal fat in both sexes at the baseline and more so after weight loss (Table 2).
Table 2.
Correlation between anthropometric parameters and body and truncal fat measured by Dual energy x-ray absorptiometry (DXA).
| Anthropometric Parameter |
Participants | Body Fat Mass [kg] | Truncal Fat Mass [kg] | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After Weight Loss |
Change | Baseline | After Weight Loss |
Change | ||
| Waist circumference (WC) [cm] |
Female | 0.596 | 0.738 | 0.416 | 0.777 | 0.846 | 0.563 |
| Male | 0.864 | 0.937 | 0.577 | 0.766 | 0.939 | 0.509 | |
| Combined | 0.333 | 0.534 | 0.445 | 0.625 | 0.757 | 0.488 | |
| Weight [kg] | Female | 0.891 | 0.916 | 0.441 | 0.812 | 0.871 | 0.636 |
| Male | 0.906 | 0.921 | 0.483 | 0.724 | 0.843 | 0.508 | |
| Combined | 0.552 | 0.650 | 0.447 | 0.652 | 0.749 | 0.541 | |
| Body Mass Index (BMI) [kg/m2] |
Female | 0.766 | 0.829 | 0.445 | 0.660 | 0.751 | 0.625 |
| Male | 0.911 | 0.944 | 0.485 | 0.702 | 0.898 | 0.426 | |
| Combined | 0.765 | 0.832 | 0.455 | 0.656 | 0.776 | 0.514 | |
Validation of prediction equations
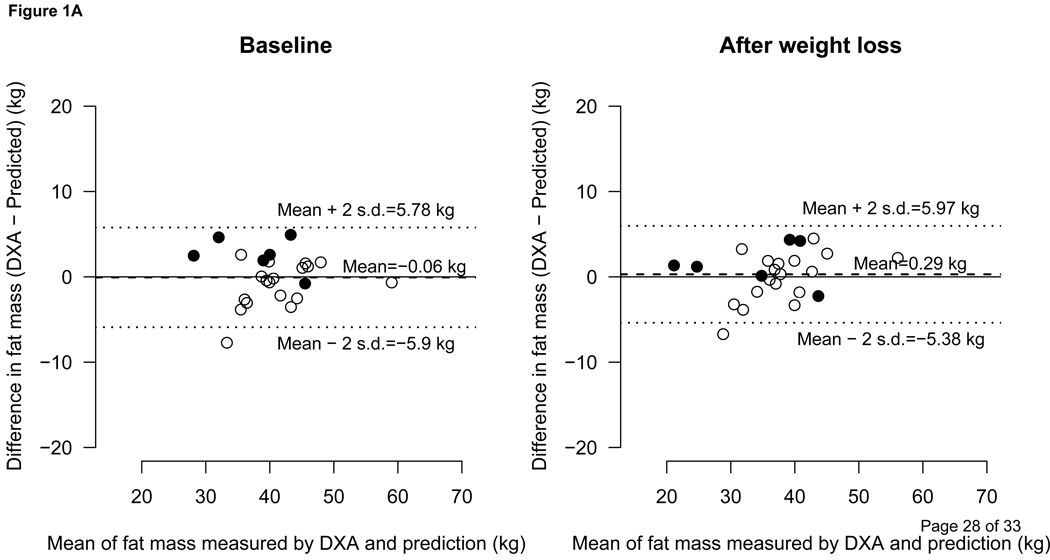
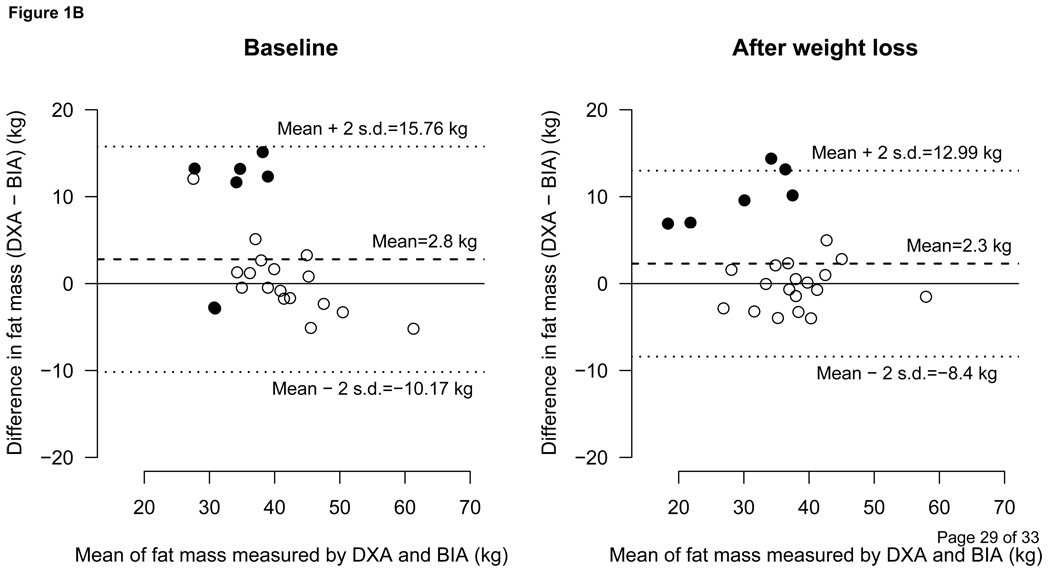
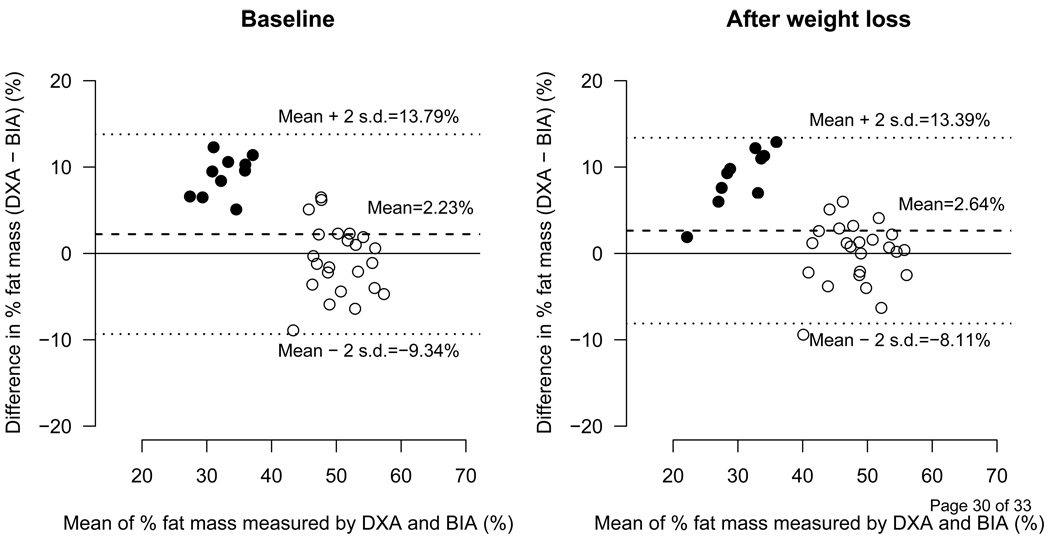
The predicted fat mass was in good agreement with fat mass measured by DXA as shown in Figure 1A. In males and females combined, the differences between the predicted fat mass and the fat mass measured by DXA at the baseline and after weight loss were −0.06 ± 2.92 kg and 0.29 ± 2.84 kg, respectively. The same differences between BIA vs. DXA were 2.8 ± 6.48 and 2.3 ± 5.35 respectively (Figure 1B). Figure 2 depicts the difference of BIA and DXA fat mass in %BF terms.
Figure 1.
1A- Comparison between the Predicted and DXA measured Fat Mass
Bland-Altman plot showing agreement between predicted vs. DXA (dual energy x-ray absorptiometry)-measured fat mass (kg). The dashed line represents the means of difference and upper and lower dotted lines represent ± 2 SD from the mean, i.e., 95% limit of agreement (±1.96 SD). Open circles represent females and filled circles represent males.
1B- Comparison between the BIA and DXA measured Fat Mass
Bland-Altman plot showing comparison between BIA vs. DXA (dual energy x-ray absorptiometry)-measured fat mass (kg). The dashed line represents the means of difference and upper and lower dotted lines represent ± 2 SD from the mean, i.e., 95% limit of agreement (±1.96 SD). Open circles represent females and filled circles represent males.
Figure 2.
Comparison between DXA and BIA Fat Estimates
Bland-Altman Plot showing difference in % Fat mass measured by DXA (dual energy x-ray absorptiometry and BIA (bioelectrical impedance analysis) before (Left) and after (Right) weight loss. The dashed line represents the means of difference and upper and lower dotted lines represent ± 2 SD from the mean, i.e., 95% limit of agreement (±1.96 SD). n=33, data for 1 female participant is missing.
Prediction Nomograms for Body and Truncal fat
In line with correlation data (Table 2), nomograms based on predictive equations, showed that the amount of body and truncal fat mass predicted by weight (Figure 3) and combinations of WC and weight or BMI (Figure 4 and 5) respectively differed between females and males and the relationship was nonlinear. Changes in body fat can be calculated from fat mass predicted from the nomogram (Figure 3) by simply intersecting body weight in kg (x-axis) and time in weeks (y-axis) during the study. Changes in truncal fat can be calculated from fat mass predicted by intersecting body weight or BMI (x-axis) and WC in cm (y-axis) at baseline and after time in weeks of diet intervention (Figure 4 and 5).
Figure 3.
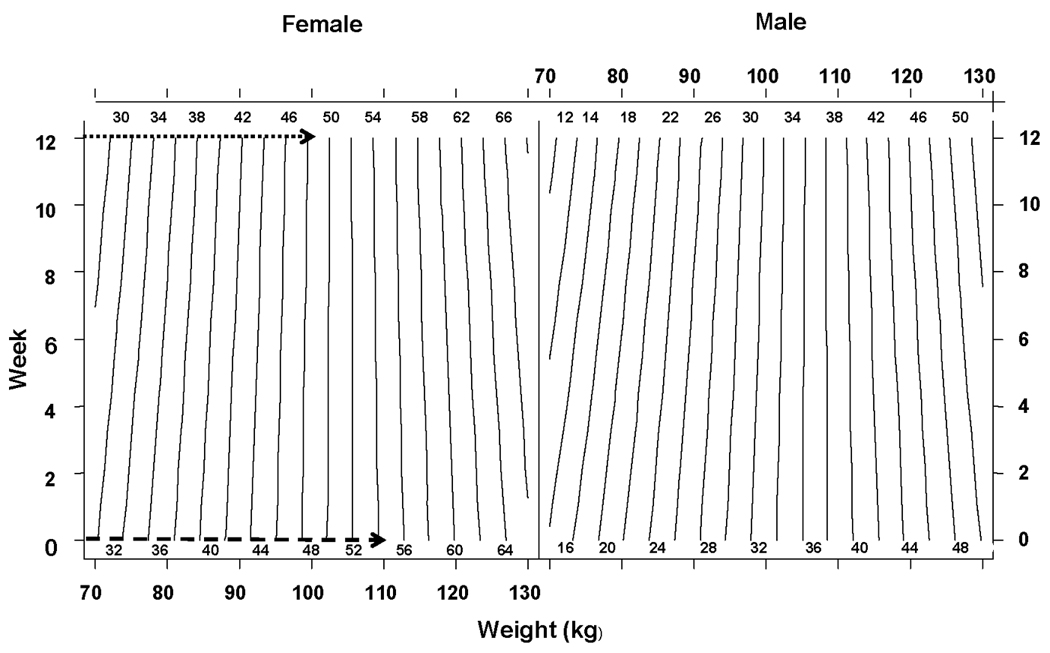
Relationship between Body Weight and Fat Mass
Nomogram showing relationship between Body Weight (kg) and Fat Mass (kg) during weight loss. Difference of dashed (54.2 kg) and dotted (48.2 kg) intersections represents the predicted fat mass loss (6 kg) for a female who lost 10 kg weight after 12 week diet intervention from a baseline weight of 110 kg (week 0).
Figure 4.
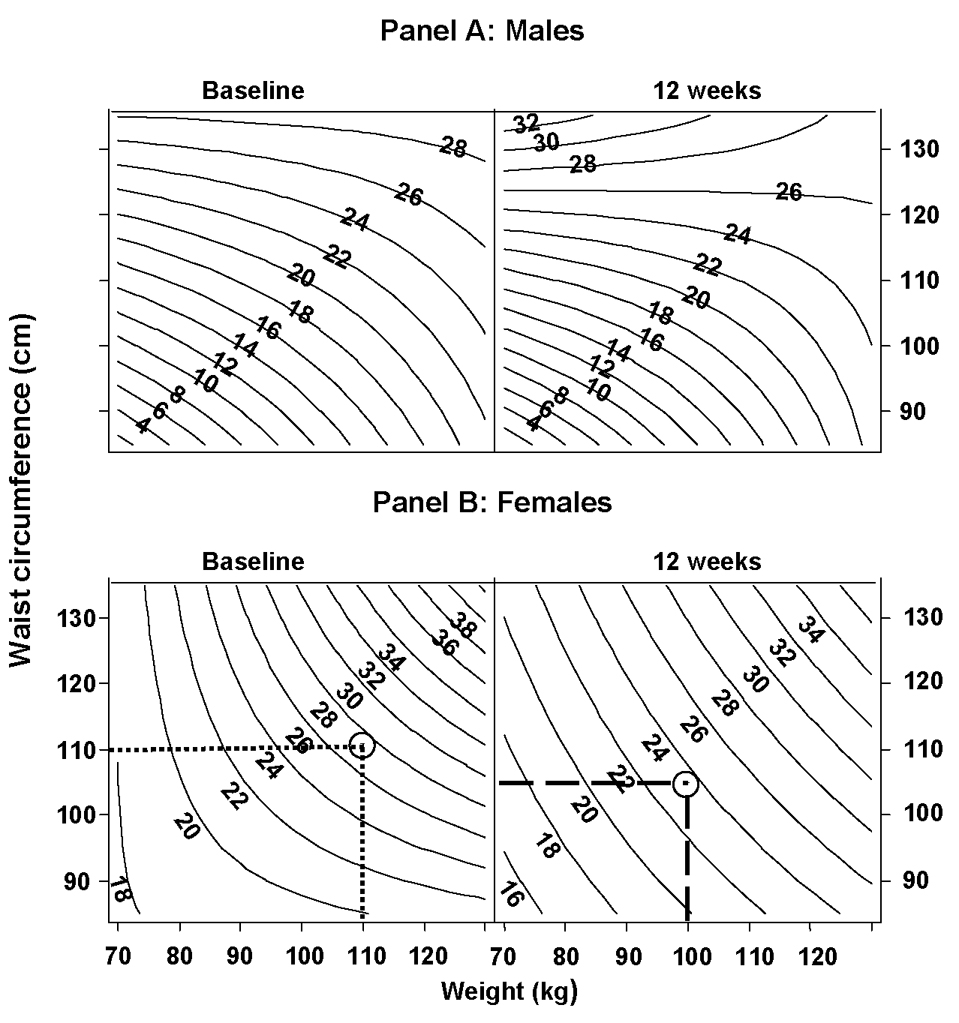
Relationship between Body weight, Waist circumference and Truncal fat
Nomogram showing relationship among body weight (kg), waist circumference (cm) and truncal fat mass (kg) during weight loss in males (panel A) and females (panel B).
Panel B: Difference between dotted (27 kg) and dashed (23.5 kg) intersections (circled) represent predicted truncal fat loss (3.5 kg) for a female who lost 10 kg of body weight and 5 cm of waist circumference from her baseline (week 0) weight 110 kg and waist circumference 110 cm after 12 weeks of diet intervention.
Figure 5.
Relationship between Body mass index, Waist circumference and Truncal fat
Nomogram showing relationship among body mass index, waist circumference (cm) and truncal fat mass (kg) during weight loss in males (panel A) and females (panel B).
Panel B: Difference between dotted (27 kg) and dashed (23.5 kg) intersections represent predicted truncal fat loss (3.5 kg) for a female who lost 5 points of body mass index and 5 cm of waist circumference from her baseline (week 0) body mass index of 45 and waist circumference 110 cm after 12 weeks of diet intervention.
Discussion
In this study we examined the usefulness of simple anthropometric measurements to predict changes in body and truncal fat during moderate weight loss. We found that predicted fat mass based on regression modeling of anthropometric measures, was in closer agreement with DXA measurements than BIA estimated fat mass (Figure 1 and Figure 2). We also found that anthropometric measurements were more strongly correlated with body and truncal fat after than before weight loss. This finding suggests that correlation between anthropometric parameters and fat mass changes with body size. Instead of attempting to estimate fat mass by using population specific equations we have developed simple nomograms relating anthropometric measures with body and truncal fat mass during moderate weight loss in our study population. Once authenticated with bigger studies, such nomograms could be simple, practical, and very helpful in assessing body fat changes during weight loss in clinical practice. They could be equally useful for epidemiological studies.
Our results are in line with past studies in children [34], adults [35], and elderly [36] reporting the usefulness of anthropometric parameters as measures of obesity and visceral adiposity. Although there is no consensus about the most sensitive and specific obesity indicator associated with metabolic risk factors, recent data indicate that simple anthropometric parameters were more strongly related to those risks compared to the measurement of body composition [37–41]. Similar to our findings, Stevens et al have recently suggested using validated prediction equations based on multiple anthropometrical measures, along with demographic variables, for assessment of adiposity in large populations , whereas WC can provide a feasible assessment of abdominal adiposity [42]. In clinical practice combination of BMI and WC is used for indirect assessment of adiposity. In our data (Table 2), compared to weight, BMI was better correlated with body and truncal fat in males and in males and females combined but weight was better correlated with body and truncal fat in females. Given its small size, our data is not conducive to point out whether BMI + WC or weight + WC would be better for indirect assessment of adiposity.
Limitations with the use of anthropometrical parameters for assessing body and truncal fat seem at least equally workable when contrasted with the limitations of the currently available techniques for measuring body and segmental fat including BIA. These techniques suffer from methodological errors when collecting raw data. Instead of measuring body composition directly, they predict it from measurements of body properties. And errors in assumptions by which raw data are converted to final values [43]. Methodological differences in recording anthropometrical parameter like WC seem to have overall insignificant effects on the recorded values and their correlation with health outcome. The intra- and inter-observer reliability of measuring waist circumference has recently been reported [44]. Even self-reported WC was found to be sufficiently accurate [45,46]. Ross et al have also shown that WC measurement protocol has no substantial influence on the association between WC, all-cause and CVD mortality, CVD and diabetes [47].
Compared to DXA, BIA estimates of fat mass were significantly higher in women and lower in men before and after moderate weight loss. The gender discrepancy in fat mass measured by BIA may be reflective of difference in the regional distribution of fat in men and women as measured with DXA. However, polarity of significant underestimation of body fat in men and overestimation in women with BIA as compared to DXA both before and after weight loss suggests a relative insensitivity of BIA and its ability for detecting segmental changes in body composition in general and truncal fat in particular. It seems that with higher amounts of truncal fat, BIA becomes less accurate in estimating total body fat mass. Our findings are similar to studies which reported that BIA significantly under-predicted body fat content compared with DXA in obese men [20], obese children [48], and abdominally obese women [15]. Additionally, in abdominally obese women BIA underestimated fatness with greater mean bias for truncal than total body fat [15] and in the same population it was shown that BIA has limited validity for detecting larger fat losses [19]. Hemmingsson et al compared the ability of a new BIA device ability to perform segmental body composition analysis and found that BIA data were of lower quality than WC or BMI for detecting metabolic risk factors in abdominally obese middle-aged women [49]. Bracco et al [50] has pointed out that given its larger diameter, trunk contributes minimally to total body impedance as compared to extremities. Thus, BIA may systemically underestimate android obesity.
There were some limitations to our study. First, use of DXA as the reference standard has several well known limitations [43,51,52]. However, given comparability with other reference techniques [52,53], DXA is considered a good reference standard for validation of new techniques for measuring body composition. Second, hydration status might have impacted BIA readings. Total body water after weight loss was 96% of the baseline values for females (95% CI: 94% to 97%; P < 0.001) and 95% of the baseline values for males (95% CI: 93% to 97%; P < 0.001). However, the differences between BIA vs. DXA fat mass measurements at the baseline and after weight loss were not significantly different indicating that impact of body water changes on BIA estimates was not significant in this study. Although relatively small sample size allowed collection of detailed individual-level data during controlled weight loss but did not allow us drawing an equation directly predicting loss in body and truncal fat for a given weight loss. That task would require a much lager sample given the complexity of relationship between weight and body and truncal fat mass and its sensitivity to the race and gender [28,29]. Further, we have not used %BF as the primary outcome since we did not observe a strong association between %BF and weight in our data. The mean weight loss (~ 8kg or 9.3%) in our population was distributed between fat and fat free mass in such a manner that the mean BF% loss was only ~2%. And when %BF = [(BF/ Wt) × 100] was regressed on weight, the strong association between BF and weight was no more there. However, our study goal was limited to test the utility of simple anthropometric parameters for predicting body and truncal fat mass during moderate weight loss without using population-specific equations. Future bigger studies will be more focused on general usefulness of our approach in research and clinical practice.
In view of the results of present study and past reports we suggest that BIA estimation of body fat changes during moderate weight loss in adults should be interpreted and applied to research and clinical use with caution. Our results further indicate that BMI, body weight, and waist circumference (WC) are good predictors of changes in total fat mass and truncal fat mass, respectively. These changes are different in men and women and could be predicted using simple nomograms. However, bigger studies with balanced demographic representation are needed before any generalization of the present findings could be used in clinical practice.
Conclusions
Body mass index, body weight, and waist circumference provide simple and more accurate than BIA estimates of relative changes in total and truncal fat during moderate weight loss in adults.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
Authors have no conflict of interest to declare.
References
- 1.Wahrenberg H, Hertel K, Leijonhufvud B-M, Persson L-G, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330:1363–1364. doi: 10.1136/bmj.38429.473310.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janiszewski PM, Janssen I, Ross R. Does Waist Circumference Predict Diabetes and Cardiovascular Disease Beyond Commonly Evaluated Cardiometabolic Risk Factors? Diabetes Care. 2007;30:3105–3109. doi: 10.2337/dc07-0945. [DOI] [PubMed] [Google Scholar]
- 3.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal Adiposity and Coronary Heart Disease in Women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 4.Rankinen T, Kim S, Pérusse L, Després J, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–809. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 5.Reeder B, Senthilselvan A, Després J, Angel A, Liu L, Wang H, Rabkin S. The association of cardiovascular disease risk factors with abdominal obesity in Canada. Canadian Heart Health Surveys Research Group. CMAJ. 1997;157:S39–S45. [PubMed] [Google Scholar]
- 6.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The Atherogenic Lipoprotein Profile Associated With Obesity and Insulin Resistance Is Largely Attributable to Intra-Abdominal Fat. Diabetes. 2003;52:172–179. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 7.Lebovitz HE, Banerji MA. Point: Visceral Adiposity Is Causally Related to Insulin Resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 8.Galassi A, Reynolds K, He J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. The American Journal of Medicine. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J Clin Endocrinol Metab. 2008;93:s57–s63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MD. Is Visceral Fat Involved in the Pathogenesis of the Metabolic Syndrome? Human Model. Obesity. 2006;14:20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 11.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.James WPT. The epidemiology of obesity: the size of the problem. Journal of Internal Medicine. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 13.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 14.Garaulet M, Hernandez-Morante JJ, Tebar FJ, Zamora S, Canteras M. Two-dimensional Predictive Equation to Classify Visceral Obesity in Clinical Practice. Obesity. 2006;14:1181–1191. doi: 10.1038/oby.2006.135. [DOI] [PubMed] [Google Scholar]
- 15.Neovius M, Hemmingsson E, Freyschuss B, Udden J. Bioelectrical Impedance Underestimates Total and Truncal Fatness in Abdominally Obese Women. Obesity. 2006;14:1731–1738. doi: 10.1038/oby.2006.199. [DOI] [PubMed] [Google Scholar]
- 16.Genton L, Hans D, Kyle UG, Pichard C. Dual-Energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 17.Willett K, Jiang R, Lenart E, Spiegelman D, Willett W. Comparison of Bioelectrical Impedance and BMI in Predicting Obesity-Related Medical Conditions. Obesity. 2006;14:480–490. doi: 10.1038/oby.2006.63. [DOI] [PubMed] [Google Scholar]
- 18.Haapala I, Hirvonen A, Niskanen L, Uusitupa M, Kröger H, Alhava E, Nissinen A. Anthropometry, bioelectrical impedance and dual-energy X-ray absorptiometry in the assessment of body composition in elderly Finnish women. Clin Physiol Funct Imaging. 2002;22:383–391. doi: 10.1046/j.1475-097x.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- 19.Neovius M, Uddén J, Hemmingsson E. Assessment of change in body fat percentage with DXA and eight-electrode BIA in centrally obese women. Med Sci Sports Exerc. 2007;39:2199–2203. doi: 10.1249/mss.0b013e3181579.38a. [DOI] [PubMed] [Google Scholar]
- 20.Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie Y-g, Mathews M, Barron JR, Fitzpatrick DG, Gulliver W, Zhang H. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81:74–78. doi: 10.1093/ajcn/81.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Stewart S, Bramley P, Heighton R, Green J, Horsman A, Losowsky M, Smith M. Estimation of body composition from bioelectrical impedance of body segments: comparison with dual-energy X-ray absorptiometry. Br J Nutr. 1993;69:645–655. doi: 10.1079/bjn19930066. [DOI] [PubMed] [Google Scholar]
- 22.Levenhagen D, Borel M, Welch D, Piasecki J, Piasecki D, Chen K, Flakoll P. A comparison of air displacement plethysmography with three other techniques to determine body fat in healthy adults. JPEN J Parenter Enteral Nutr. 1999 Sep–Oct;23:293–299. doi: 10.1177/0148607199023005293. [DOI] [PubMed] [Google Scholar]
- 23.Weyers A, Mazzetti S, Love D, Gómez A, Kraemer W, Volek J. Comparison of methods for assessing body composition changes during weight loss. Med Sci Sports Exerc. 2002;34:497–502. doi: 10.1097/00005768-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Frisard MI, Greenway FL, DeLany JP. Comparison of Methods to Assess Body Composition Changes during a Period of Weight Loss. Obesity. 2005;13:845–854. doi: 10.1038/oby.2005.97. [DOI] [PubMed] [Google Scholar]
- 25.Buchholz AC, Bartok C, Schoeller DA. The Validity of Bioelectrical Impedance Models in Clinical Populations. Nutr Clin Pract. 2004;19:433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health Bethesda MD. The Practical Guide: Identification, Evaluation and Treatment of Overweight and Obesity in Adults. 2000:1–77. In.
- 27.Health Canada Ottawa Canada. Canadian Guidelines for Body Weight Classification in Adults. 2003. pp. 1–40. In. [Google Scholar]
- 28.Carroll J, Chiapa A, Rodriquez M, Phelps D, Cardarelli K, Vishwanatha J, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and Gender Differences in the Relationships Between Anthropometrics and Abdominal Fat in Youth. Obesity. 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 30.Pouliot M, Després J, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien P. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 31.Mukuddem-Petersen J, Snijder MB, van Dam RM, Dekker JM, Bouter LM, Stehouwer CDA, Heine RJ, Nijpels G, Seidell JC. Sagittal abdominal diameter: no advantage compared with other anthropometric measures as a correlate of components of the metabolic syndrome in elderly from the Hoorn Study. Am J Clin Nutr. 2006;84:995–1002. doi: 10.1093/ajcn/84.5.995. [DOI] [PubMed] [Google Scholar]
- 32.Hess R, Bryce CL, Paone S, Fischer G, McTigue KM, Olshansky E, Zickmund S, Fitzgerald K, Siminerio L. Exploring Challenges and Potentials of Personal Health Records in Diabetes Self-Management: Implementation and Initial Assessment. Telemedicine and e-Health. 2007;13:509–518. doi: 10.1089/tmj.2006.0089. [DOI] [PubMed] [Google Scholar]
- 33.Control CfD . In: Anthropometry Procedures Manual. In Control CfD, editor. 2000. [Google Scholar]
- 34.Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, Peters DM, Barbeau P, Simone MD, Pietrobelli A. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes Relat Metab Disord. 2006;30:23–30. doi: 10.1038/sj.ijo.0803163. [DOI] [PubMed] [Google Scholar]
- 35.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 36.McTigue KM, Hess R, Ziouras J. Obesity in Older Adults: A Systematic Review of the Evidence for Diagnosis and Treatment. Obesity. 2006;14:1485–1497. doi: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Song Y-M, Sung J. Which Obesity Indicators Are Better Predictors of Metabolic Risk: Healthy Twin Study. Obesity. 2008;16:834–840. doi: 10.1038/oby.2007.109. [DOI] [PubMed] [Google Scholar]
- 38.Bosy-Westphal A, Geisler C, Onur S, Korth O, Selberg O, Schrezenmeir J, Muller MJ. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes. 2005;30:475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 39.Santos REd, Aldrighi JM, Lanz JRn, Ferezin PC, Marone MMS. Relationship of body fat distribution by waist circumference, dual-energy X-ray absorptiometry and ultrasonography to insulin resistance by homeostasis model assessment and lipid profile in obese and non-obese postmenopausal women. Gynecological Endocrinology. 2005;21:295–301. doi: 10.1080/09513590500361937. [DOI] [PubMed] [Google Scholar]
- 40.Simpson JA, MacInnis RJ, Peeters A, Hopper JL, Giles GG, English DR. A Comparison of Adiposity Measures as Predictors of All-cause Mortality: The Melbourne Collaborative Cohort Study[ast] Obesity. 2007;15:994–1003. doi: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- 41.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 42.Stevens J, McClain JE, Truesdale KP. Selection of measures in epidemiologic studies of the consequences of obesity. Int J Obes. 2008;32:S60–S66. doi: 10.1038/ijo.2008.88. [DOI] [PubMed] [Google Scholar]
- 43.Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nádas J, Putz Z, Kolev G, Nagy S, Jermendy G. Intraobserver and interobserver variability of measuring waist circumference. Med Sci Monit. 2008;14:CR15–CR18. [PubMed] [Google Scholar]
- 45.McAdams MA, Van Dam RM, Hu FB. Comparison of Self-reported and Measured BMI as Correlates of Disease Markers in U.S. Adults[ast] Obesity. 2007;15:188–188. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 46.Spencer E, Roddam A, Key T. Accuracy of self-reported waist and hip measurements in 4492 EPIC-Oxford participants. Public Health Nutr. 2004;7:723–727. doi: 10.1079/phn2004600. [DOI] [PubMed] [Google Scholar]
- 47.Ross R, Berentzen T, Bradshaw AJ, Janssen I, Kahn HS, Katzmarzyk PT, Kuk JL, Seidell JC, Snijder MB, Sørensen TI, Després JP. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obesity Reviews. 2008;9:312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 48.Eisenkölbl J, Kartasurya M, Widhalm K. Underestimation of percentage fat mass measured by bioelectrical impedance analysis compared to dual energy X-ray absorptiometry method in obese children. Eur J Clin Nutr. 2001;55:423–429. doi: 10.1038/sj.ejcn.1601184. [DOI] [PubMed] [Google Scholar]
- 49.Hemmingsson E, Udden J, Neovius M. No Apparent Progress in Bioelectrical Impedance Accuracy: Validation Against Metabolic Risk and DXA. Obesity. 2008 doi: 10.1038/oby.2008.474. [DOI] [PubMed] [Google Scholar]
- 50.Bracco D, Thiebaud D, Chiolero RL, Landry M, Burckhardt P, Schutz Y. Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J Appl Physiol. 1996;81:2580–2587. doi: 10.1152/jappl.1996.81.6.2580. [DOI] [PubMed] [Google Scholar]
- 51.Valentine RJ, Misic MM, Kessinger RB, Mojtahedi MC, Evans EM. Location of body fat and body size impacts DXA soft tissue measures: a simulation study. Eur J Clin Nutr. 2007;62:553–559. doi: 10.1038/sj.ejcn.1602770. [DOI] [PubMed] [Google Scholar]
- 52.Sopher AB, Thornton JC, Wang J, Pierson RN, Jr, Heymsfield SB, Horlick M. Measurement of Percentage of Body Fat in 411 Children and Adolescents: A Comparison of Dual-Energy X-Ray Absorptiometry With a Four-Compartment Model. Pediatrics. 2004;113:1285–1290. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol. 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]