Abstract
Although nitroglycerin (NTG) is effective for the acute relief in coronary ischemic diseases, its long-term benefits in mortality and morbidity have been questioned. The possibility has been raised that NTG may increase the activity of matrix metalloproteinases (MMP), which could lead to disruption and dislodging of atherosclerotic plaques. This study examined the broad effects of acute NTG exposure on the expression and activity of genes encoding MMP-9, as well as an array of ECM and adhesion molecules in THP-1 human macrophages. Gene array studies identified that while NTG exposure (100 µM, 48 hours) did not significantly increase MMP-9 gene expression, genes encoding testican-1, integrin α-1, thrombospondin-3, fibronectin-1 and MMP-26 were significantly down-regulated. On the other hand, genes encoding catenin β-1 and vascular cell adhesion molecule-1 were up-regulated. Real-time PCR studies confirmed significant down-regulation of testican-1 gene expression, but its protein expression was not significantly altered. NTG exposure, caused a significant increase in total MMP-9 protein expression (1.96-fold) and active MMP-9 (3.7-fold) concentrations. Recombinant MMP-9 was significantly activated by NTG and its dinitrate metabolites, indicating post-translation modification of this protein by organic nitrates. These results indicate that NTG exposure could broadly affect the gene expression and activity of proteases that govern the ECM cascade, thereby potentially altering atherosclerotic plaque stability.
Keywords: Nitroglycerin, Matrix Metalloproteinase-9, Testican-1, Microarrays, Extracellular Matrix Proteases and Cell Adhesion Molecules
INTRODUCTION
Nitroglycerin (NTG), a prototypical organic nitrate, has been widely used for over 130 years to treat patients with stable and unstable coronary syndromes [1]. Bioactivation of NTG is believed to release nitric oxide (NO) and/or related species, activating soluble guanylyl cyclase to produce cyclic guanosine monophosphate in vivo and resulting in vasodilation. The clinical benefits of short-term use of nitrates is undisputable [2]. However, continuous prolonged use of NTG produces pharmacological tolerance (see reviews [2; 3]), as well neutral [4; 5; 6; 7] or even negative clinical effects in patients with stable coronary artery disease [8; 9; 10; 11]. A suggested mechanism for these negative outcomes was attributed to plaque destabilization through the activation of matrix metalloproteinases (MMPs) by macrophages, thus counterbalancing the beneficial effects of vasodilatation by these drugs [9; 12].
MMPs are zinc-endopeptidases that regulate cell-matrix interactions and are critical for the breakdown and remodeling of the extracellular matrix, and are involved in many physiological processes such as development, wound healing, embryogenesis, repair of blood vessels, as well as pathological processes such as atherosclerotic plaque destabilization, tumor metastasis, infections, inflammation, arthritis, angiogenesis [13; 14]. The activity of these tissue destructive enzymes is tightly regulated by gene expression, producing the latent forms of these enzymes and co-secretion of tissue inhibitors [13; 15; 16]. A balance between synthesis and degradation of the matrix components is critical for the stability of the atherosclerotic plaque. An increase in macrophage density increases the concentrations of MMPs, which weakens the fibrous cap of the plaque and may lead to plaque rupture [17].
MMP-9, the major MMP secreted by the macrophages in the vulnerable plaque region [14; 18], has been linked to plaque rupture [19; 20]. Structurally, MMP-9 consists of a prodomain, catalytic domain, hinge region and hemopexin domain. The conserved sequence, PRCGVPD, containing the so-called “cysteine switch” in the prodomain and the zinc binding motif in the catalytic domain, is the signature of MMP family [21]. This cysteine residue, at position-99, binds to a zinc atom to maintain the enzyme in its latent state [22]. Activation of proMMPs can be effected by several enzymatic/non-enzymatic mechanisms that reacts with this cysteine residue, leading to dissociation of the cysteine switch from the active zinc atom [21].
We have shown recently that NTG can cause significant cellular protein thiol oxidation, including S-glutathionylation [23]. Because of this pro-oxidant property, we hypothesize that NTG may alter the expression of MMPs and other ECM proteases and adhesion molecules. Here, we investigated the broad in vitro transcriptional effects of NTG on MMP-9 and other ECM proteases and adhesion molecules. Follow up studies to verify the mRNA changes of selected genes with real-time quantitative reverse transcriptase real-time PCR reactions (RTQ RT-PCR) were carried out. Further, the post-transcriptional effects of NTG on protein expression and activity of MMP-9 were examined.
MATERIALS AND METHODS
Materials
THP-1 cells were obtained from the American Type Culture Collection (Manassas, VA). RPMI medium with supplemented glutamine, fetal bovine serum (FBS), 0.05 mM 2-mercaptoethanol, nitrocellulose membranes, sterile phosphate buffered saline (PBS), 100 U/ml penicillin, 100 µg/ml streptomycin and TOPO cloning products were purchased from Invitrogen Corporation (Carlsbad, CA). NTG solution was obtained from American Reagent Laboratories Inc. (Shirley, NY). The sources of other reagents were: RNA extraction kit and SV Total RNA® isolation system from Promega (Madison, WI); Oligo GEArray® (OHS-013) from SABiosciences (Frederick, MD); PCR primers of β-actin, GAPDH, cyclophilin, MMP-9, MMP-2 and testican-1 from Operon (Huntsville, AL); DNA purification system from Promega (Madison, WI); recombinant human MMP-9 and testican-1, anti-human testican-1 and anti-mouse IgG-HRP antibody, and quantikine human MMP-9 immunoassay kit from R&D system (Minneapolis, MN); Biotrak MMP-9 activity assay system from GE Healthcare (Piscataway, NJ ); glyceryl 1,2- or 1,3-dinitrate (GDN) from Cerilliant (Round Rock, TX); ECL-enhanced chemiluminiscence system from Pierce (Rockford, IL). All other chemicals were obtained from Sigma (St. Louis, MA).
THP-1 cell culture and differentiation into macrophages
THP-1 (human monocytic leukemia) cells were cultured in RPMI-1640 cell medium supplemented with glutamine 10% FBS, 0.05 mM 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in an incubator with 5% CO2. The cells were counted using trypan blue staining after centrifugation (2000×g for 10 minutes at 4°C), and aliquots of 1 ml (1×106 cells) cell suspension were placed into six well plates. Cell viability was ≥95%, as determined by trypan blue staining. Cells were differentiated using phorbol 12-myristate 13-acetate (PMA) at a final concentration of 100 ng/ml over 24 hours [24].
Nitroglycerin incubation
The medium was removed and each well was washed with sterile PBS. Fresh cell medium (1 ml) containing RPMI-1640 cell medium supplemented with glutamine, 0.2% FBS, 0.05 mM 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin was added. NTG (final concentration 100 µM) or vehicle (30% propylene glycol v/v and 30% ethanol v/v) was added in respective wells (10 µl) and the plates were incubated at 37°C for 48 hours. The medium then was collected, centrifuged (2000×g for 5 minutes at 4°C) and stored at −80°C for MMP-9 activity and protein determinations. The cells were then washed twice with 1 ml of cold and sterile PBS and lysed using a lysis buffer for total RNA extraction.
Total RNA isolation and gene array analysis
Total RNA isolation was carried out using SV Total RNA® isolation system, according to the recommended protocol. The integrity of the extracted RNA was determined by formaldehyde-gel electrophoresis and quantified spectrophotometrically at 260 nm. The samples were stored at −80°C until the microarray assay by Oligo GEArray® (OHS-013) which contained 118 genes encoding proteins that play key roles in mediating cell-cell, cell-tissue and cell-extracellular matrix interactions, housekeeping genes including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-2-microglobulin, β-heat shock 90 kDa protein-1 and β-actin and biotinylated artificial sequence-2 control for chemiluminescent detection. Hybridizations of labeled cRNAs to arrays were conducted according to the manufacturer’s protocols. Briefly, the total RNA from each treatment was first converted to cDNA, followed by cRNA synthesis, labeling and amplification (True Labeling-AMP™ 2.0). The arrays were hybridized overnight and a chemiluminiscent detection kit was used to detect the gene signal. Image acquisition was performed using a Kodak Imager 2000MM (Carestream Health Molecular Imaging, New Haven, CT) and analyzed with software Kodak ID (ver. 3.6.3).
Microarray Data Analysis
A gene stability index [25] was used to determine the expression stability of the housekeeping genes. The principle behind this index is that ideally the expression levels of control genes should remain unchanged irrespective of the exposure or type of cell. The stability of total array intensity and the geometric mean of the four housekeeping genes, as a normalizing factor, was also tested using this index. The internal control gene-stability index is defined as the “average pairwise variation of a particular gene with all other control genes” [25]. Thus, a lower gene stability index indicates more stable expression. Unpaired t-test was not used because of its low statistical power and assumption of normality and equal variance. In addition, to assess the false discovery rate, a nonparametric approach based on resampling techniques, the permutation adjusted t-test (PATS), was applied to the gene expression data [26]. The resampling method is a statistical approach that identifies all possible outcomes within the same empirical data set via repeated sampling. The observed test statistics are then compared against the test statistics from all possible data sets. This approach has been used by several groups [26; 27; 28; 29]. In this study, there were 252 possible permutations of the expression data using 5 replicates for each treatment group (10!/5!*5! = 252). However, only 126 unique permutations of the data sets were considered since the other 126 permutations are mirror permutations with equal magnitude but with a negative sign. The changes in gene expression were considered significant if the given t-statistic from the data set was the highest value amongst the t-statistic distribution from all possible permutated data sets.
Real-Time PCR
RTQ RT-PCR was performed on the house keeping genes; β-actin, GAPDH, cyclophilin and the genes of interest; MMP-9, MMP-2 and testican-1, to verify the results of the gene array study. The PCR primers and reaction conditions are listed in Table 1. Total RNA was reverse-transcribed at 65°C for 5 minutes, at room temperature for 10 minutes, 42°C for 60 minutes and 90°C for 5 minutes. RTQ RT-PCR was performed using Stratagene’s MX4000 quantitative PCR system. Each PCR reaction contained 5 µl of cDNA and 45 µl of master mix containing 0.5 µl each of SYBR green (1/500 dilution) and rhodamine X (1/750 dilution). Amplification was carried out at 95°C for 4 minutes, 94°C for 30 sec, 60°C for 30 sec and 72°C for 1 minute by repeating the complete cycle 40 times. PCR products of all genes were cloned using pCR®2.1-TOPO® vector, into one shot®E.Coli cells. Plasmids with PCR inserts were extracted using Wizard plus SV minipreps DNA purification system, quantified spectrometrically and used to prepare the standard curves, which was run in triplicate concurrently on the same plate with unknown samples. Threshold cycle value was converted into number of DNA molecules using the standard curves. The amount of total RNA was used as the normalizing factor.
Table 1.
RTQ RT-PCR primers and conditions (MgCl2 at 1.5 mM).
| Forward Primer (5’-3’) | Reverse Primer (3’–5’) | Primer Concentration (µM) |
|
|---|---|---|---|
| Testican-1 | atgacaacgtgtctgtccaggtct | tacagcgtgggtcaagggtaacaa | 0.375 |
| MMP-2 | actgctggctgccttagaaccttt | actatgtgggctgagatgcactgt | 0.375 |
| MMP-9 | atttctgccaggaccgcttctact | ttgtatccggcaaactggctcctt | 0.25 |
| β-Actin | ctggccgggacctgact | tccttaatgtcacgcacgattt | 0.375 |
| Cyclophilin | ccaaacacaaatggttcccagtt | tgccttctttcaccttcccaaa | 0.375 |
| GAPDH | tgcacagtcagccgccatct | gcgcccaatacgaccaaat | 0.50 |
MMP-9 protein expression and activity
MMP-9 protein expression (total MMP-9; pro and active forms) was determined in the conditioned media samples using Quantikine human MMP-9 immunoassay kit. MMP-9 concentration was determined using a standard curve, constructed with standard solutions ranging from 0.312–10 ng/ml of MMP-9 and normalized by total protein concentration as determined by the Lowry assay. MMP-9 activity was determined using a Biotrak activity assay system using the manufacturer’s protocol. The concentration of endogenous levels of free active MMP-9, proMMP-9 and total free MMP-9 was determined from the standard curve and normalized by total protein concentration.
In vitro activation of recombinant human proMMP-9 by NTG
Chemical activation of human recombinant MMP-9 was examined by exposure to NTG, 1,2- or 1,3-GDN, APMA (all at a final concentration of 200 µM) or vehicle control at 37°C for 1.5 hours. The concentration of activated MMP-9 was determined by assaying the cleavage rate of a fluorogenic peptide substrate after capturing the active MMP-9, using the specific antibodies coated onto a microwell plate (AnaSpec, San Jose, CA).
Testican-1 Western blot
Medium samples containing equal amounts of protein were denatured in sodium dodecyl sulfate (SDS) loading buffer (1.75% SDS, 65 mM Tris-HCl, pH 6.8, 5% v/v glycerol, and 0.3% bromophenol blue) at 90°C for 5 minutes, separated using 12% SDS-PAGE gel, and electro-transferred to nitrocellulose membrane, using a Mini-PROTEAN II Cell system (Bio-RAD, Hercules, CA). The membrane was then blocked with 1% BSA in PBS containing 0.05% Tween-20 at room temperature with shaking for 1 hour, followed by incubation with monoclonal anti-human testican-1 antibody (1:500 dilution) for 2 hours. After washing, the membrane was incubated with anti-mouse IgG-HRP antibody (1:1000 dilution) for 1 hour. The blot was visualized by ECL-enhanced chemiluminiscence system. Recombinant testican-1 (200 ng) served as a positive control.
Statistical analysis
All data are reported as mean ± standard deviation unless otherwise stated. Statistical analyses were performed on the untransformed data using Student’s t-test or ANOVA with p<0.05 set as the significance level. Gene array data was analyzed using permutation adjusted t-test as indicated earlier.
RESULTS
NTG effects on the gene expression of matrix and adhesion molecules in human THP-1 macrophages
The total gene intensity on each array was used as the normalizing factor as determined by the lowest gene stability index. The expression of housekeeping genes though unaltered in the two groups was less stable than total gene intensity. MMP-9 gene expression after NTG exposure at 100 µM for 48 hours showed an upward trend, but was not significantly increased (NTG/control=1.363, p=0.642). However, this treatment resulted in a significant down-regulation of cysteine proteases (testican-1, MMP-26), transmembrane molecule (integrin-α-1), adhesion molecules (thrombospondin-3, fibronectin-1) and significant up-regulation of adhesion molecules (VCAM-1 and catenin-β-1) (p<0.05; PATS; Table 2). Out of the 118 genes, undetectable signal was observed in the NTG group for the gene encoding neural cell-adhesion molecule-1 and in vehicle group for the following genes: catenin-β-1, MMP-16 and vitronectin. Although statistical significance could not be demonstrated in the present study, several genes exhibited dramatic alterations (>10-fold) in average expression after NTG exposure, including laminin-β-1 ( 10.2 fold increase, p=0.3), integrin-β-6 (11.6 fold increase, p=0.3), MMP-17 (13.6 fold increase, p=0.4), spastic paraplegia-7 (50 fold decrease, p=0.4); collagen type-XI α-2 (12.5 fold decrease, p=0.4); TIMP-2 (12.5 fold decrease, p=0.4); osteonectin (11.1 fold decrease, p=0.2); thrombospondin-4 (10 fold decrease, p=0.4), and sarcoglycan (10 fold decrease, p=0.4).
Table 2.
Genes significantly (p<0.05) altered by NTG treatment (100 µM, 48 hrs) versus vehicle control as shown by permutation adjusted t-statistic (n=5).
| Gene Accession# |
Gene Title/ Symbol | Ratio NTG/control |
|
|---|---|---|---|
| Down- regulated |
NM004598 | Testican-1 | 0.11 |
| NM181501 | Integrin-α-1(ITGA1) | 0.11 | |
| NM007112 | Thrombospondin-3(THBS3) | 0.11 | |
| NM002026 | Fibronectin-1(FN1) | 0.18 | |
| NM021801 | Matrix metalloproteinase-26(MMP-26) | 0.13 | |
| Up- regulated |
NM001904 | Catenin-β-1(CTNNB1) | * |
| NM001078 | Vascular cell adhesion molecule-1 (VCAM-1) | 5.10 |
No signal was observed in control for CTNNB1
Confirmation of gene array results for MMP-9, MMP-2 and testican-1 by RTQ RT PCR
Gene expression changes of MMP-9, MMP-2 and the novel ECM protein, testican-1 were confirmed by RTQ RT-PCR with SYBR green detection. Expression of housekeeping genes; β-actin, GAPDH and cyclophilin were not altered. NTG exposure led to a significant down-regulation of testican-1 expression (control: 2.23 ± 1.54 × 105 versus NTG: 5.23 ± 0.75 × 104 molecules of DNA/µg of total RNA, n=5 each, p<0.05). Expression levels of MMP-2 trended downward (control: 8.92 ± 9.5 × 104 versus NTG: 2.38 ± 1.4 × 105 molecules of DNA/µg of Total RNA, n=5 each, p=0.128), while MMP-9 showed an upward trend (control: 7.63 ± 2.75 × 106 versus NTG: 10.9 ± 4.1 × 106 molecules of DNA/µg of Total RNA, n=5 each, p=0.154), but both changes were not statistically significant. These results are similar to those determined by the microarray technique.
NTG effects on the protein expression of MMP-9 and testican-1 in human THP-1 macrophages
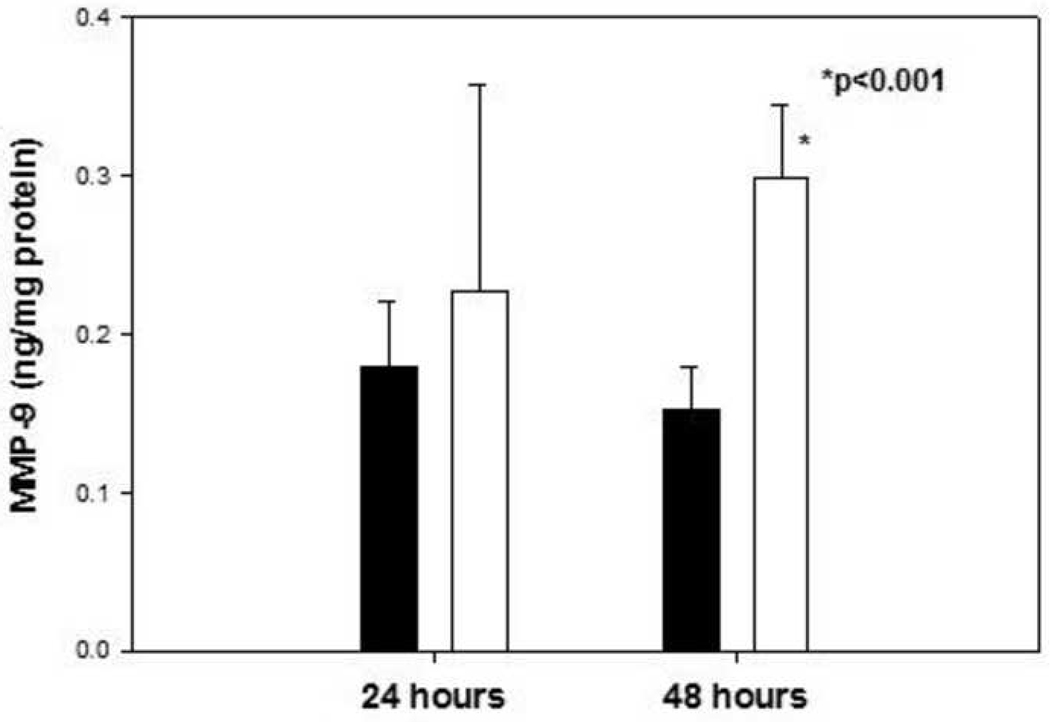
NTG exposure 100 µM led to an approximately 1.96-fold increase in total (pro and active) MMP-9 protein concentration after 48 hours (p<0.001; Fig 1). After 24 hours exposure, an upward trend was observed but the change was not statistically significant (Fig. 1). The protein expression of testican-1 was not significantly altered by NTG exposure (Fig, 2; NTG/control =0.89; p=0.57; n=6).
Fig.1.
Total MMP-9 concentration (pro and active) in medium samples after exposure to 100 µM NTG (unfilled bars) for 24 hours/48 hours versus their respective vehicle controls (filled bars), n=4–5 each. * p<0.001
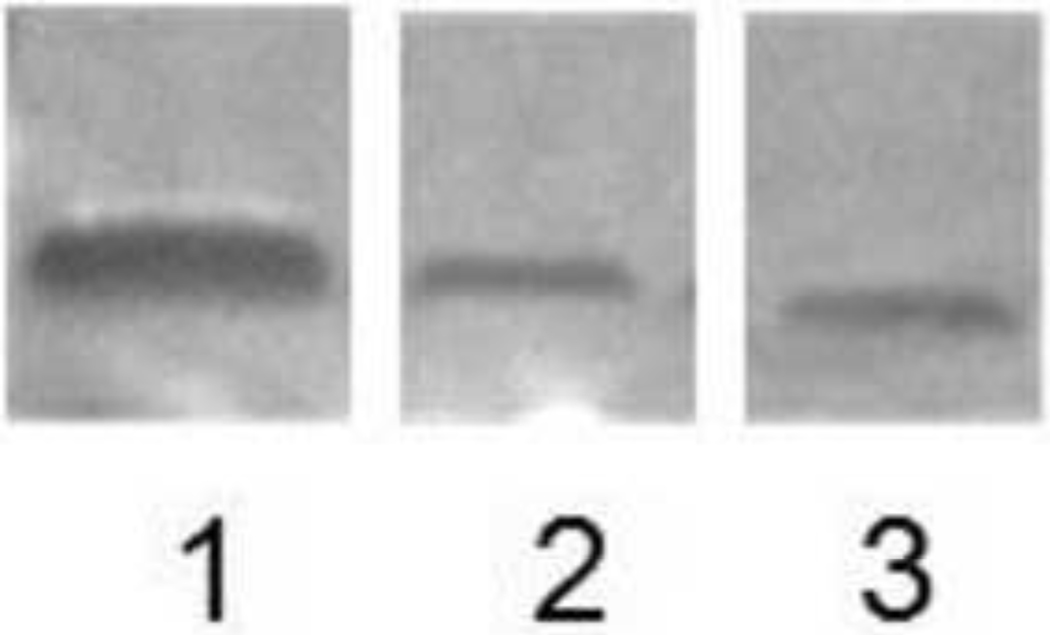
Fig. 2.
Representative Western blots of testican-1. Lane 1: Positive control (recombinant human testican-1, 200 ng); Lane 2: Conditioned media sample from THP-1 macrophages exposed to vehicle control, and Lane 3: Conditioned media sample from THP-1 macrophages exposed to NTG (100 µM, 48 hours).
NTG effects on the activity of MMP-9 in human THP-1 macrophages
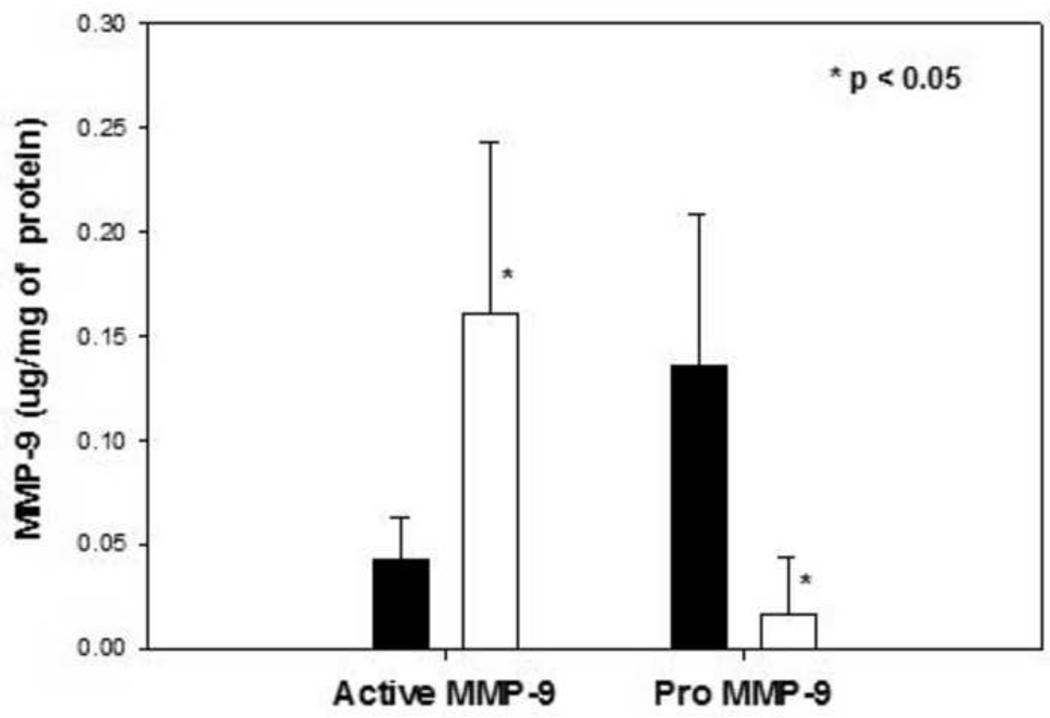
NTG exposure led to 3.7-fold increase (Fig. 3; p<0.05) in active MMP-9 concentrations in human THP-1 macrophages and a concomitant decrease in proMMP-9 concentrations (8.4 fold higher versus control samples; Fig. 3; p<0.05).
Fig. 3.
Active free MMP-9 and proMMP-9 concentration in medium samples after exposure to 100 µM NTG (unfilled bars) for 48 hours versus vehicle control (filled bars), n=5–6 each. * p<0.05
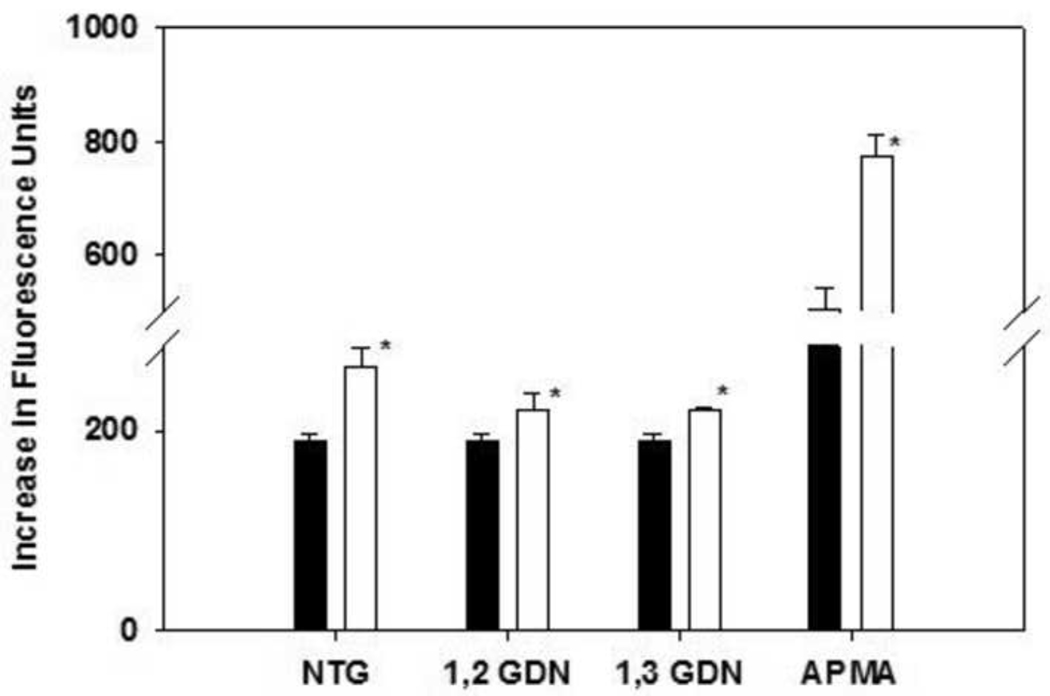
NTG effects on the activation of recombinant human MMP-9
A significant activation of proMMP-9 by NTG and 1,2 and 1,3 GDNs exposure was observed in a cell free medium, compared to its respective control. As expected, the positive control APMA significantly activated MMP-9 (Fig. 4).
Fig. 4.
Activation of recombinant proMMP-9 (100 ng/ml) treated with 200 µM of NTG, 1,2-GDN, 1,3-GDN or APMA (unfilled bars) versus their respective vehicle controls (filled bars) for 1.5 hours, n=3–4, each. *p<0.05.
DISCUSSION
NTG, a well know organic nitrate, was introduced in 1800s for the treatment of angina pectoris. It is still widely used because of its safe and excellent therapeutic profile [1]. Several observational studies [6; 7; 8; 9; 10; 11] and two large randomized trials [4; 5] have raised the possibility that NTG may increase, or may have no beneficial effects, on the mortality and morbidity of patients with unstable coronary disease. This conclusion is quite surprising, considering the excellent vasodilatory effects of organic nitrates. A study published by Death et al. [12] raised the possibility that NTG may activate MMP-9, leading to an unstable plaque.
The present study tests a broader hypothesis that NTG may have matrix degrading effects by altering the gene and protein expression of other extracellular matrix proteases besides that of MMP-9. Currently, there is no standard model for testing plaque vulnerability due to the complexity of the disease and plaque structure [14; 30]. A human derived monocyte cell line, THP-1 [31], differentiated by PMA to macrophages (THP-1 macrophages) has been used as a cellular model to investigate plaque vulnerability via MMP-9 activity changes [24; 32].
We used oligo GEarrays that contained 118 human genes that are relevant to (1) the functions of cell-cell and cell-matrix adhesion, basement membrane constituents, collagens, transmembrane molecules, (2) ECM structure, and (3) proteases involved in ECM degradation and their inhibitors. Overall, the gene array study revealed that NTG caused substantial and broad changes in the gene expression of a number of ECM proteases and adhesion molecules (Tables 2–4). The ability of NTG to cause extensive cellular gene regulation is consistent with our previous observation [26], confirmed by others [33], that wide-spread changes in vascular gene expression occurred in rats after chronic exposure and tolerance development, to NTG in vivo.
In the present study, NTG was shown to up-regulate VCAM-1 significantly, a cell surface glycoprotein adhesive for certain blood leukocytes, which is expressed by activated endothelium in pathologic conditions like atherosclerosis. VCAM-1 is a well known cell-adhesion molecule, which plays a dominant role in the initiation of atherosclerosis by up-regulating monocyte accumulation in the arterial intima [34; 35]. MMP-2 and MMP-9 induction in T-lymphocytes can be mediated by VCAM-1 [36]. In addition, NTG up-regulated another cell-adhesion molecule, catenin-β-1. The role of catenin-β-1 in regulating MMPs was demonstrated in colorectal carcinogenesis, which showed elevated concentrations of a MT1-MMP (membrane-type MMP) in more than 90% of colon carcinomas [37]. MT1-MMP has been reported to induce MMP-2 expression. Deletion of catenin-β-1 gene led to a down-regulation of MT1-MMP [37; 38].
Interestingly, NTG significantly down-regulated testican-1, a multidomain proteoglycan that was first isolated from human seminal plasma [39], and expressed differentially in the human prostate, heart, cartilage and blood with the highest concentrations in the brain [40]. Testican contains calcium ion binding and three potential inhibitory domains (N-terminal region, thyroglobulin and follistatin-like domain), which are targeted towards three different classes of proteolytic enzymes, viz., metalloproteinase, cysteine and serine proteases. Multiple in vitro functions of testican including inhibition of proMMP-2 activation, inhibition of cathepsin-L, anti-adhesive action on neuronal cells and calcium ion binding have been reported [41; 42]. Testican may also inhibit protease in the blood and contribute to the cell-matrix interactions via the characteristic Cys-Trp-Cys-Val peptide protein core. Expression of testican in human vascular endothelial cells suggests possible involvement in vascular wall functions [39].
The present study showed that NTG significantly down-regulated integrin-α-1. An elevated level of MMP-9 in integrin-α-1 null mice has been reported [43]. We also observed that thrombospondin-3 was significantly down-regulated by NTG. The function of this gene is not entirely known at this point, but it is suggested that thrombospondin-3 may play a unique role in cell-matrix interactions [44].
Gene array experiments serve primarily as a screening tool and quantitative conclusions regarding differential gene expression should be selectively validated with RTQ RT-PCR [45; 46]. Our RTQ RT-PCR results, obtained for MMP-2, MMP-9 and testican-1, were consistent with those obtained by microarray: MMP-9 gene expression showed an upward trend, and MMP-2 gene expression showed a downward trend but the changes were not statistically different. Testican-1 was significantly down-regulated.
Changes at the gene expression level may not produce similar changes in protein expression, as post-translational and translational processes may also be affected. We show here that NTG exposure led to 1.96-fold increase (Fig. 1, p<0.0001) in MMP-9 protein concentrations versus control human macrophages, suggesting that NTG also acted at the post-transcriptional level. A two-fold increase in MMP-9 protein concentrations has been clinically shown in patients with unstable coronary artery diseases [20]. The molecular mechanism for this increase in MMP-9 protein expression by NTG can be mediated via nuclear factor-κB, activator protein-1 or wilms tumor-1 dependent pathway [16; 47; 48; 49; 50; 51; 52]. Further studies are required to understand the mechanisms underlying the MMP-9 expression changes caused by NTG expsoure.
Testican-1 contains a cysteine rich domain, which could potentially be oxidized by NTG [39]. We showed that while significant down-regulation of gene expression of testican-1 was observed, its protein expression was not altered under the present experimental conditions. The lack of significant changes in testican -1 protein expression despite substantial gene upregulation can be due to many factors, including post-translational regulatory mechanisms, feedback regulation or delayed effects. Further studies relating to the interaction between testican-1 and NTG (and possibly other NO donors) appear warranted.
Using the apoE knockout mice model of atherosclerosis, Gough et al. [19] demonstrated that an increase in active MMP-9 significantly raised the incidence of plaque rupture. Hence, we examined concentrations of active MMP-9 on NTG exposure in human THP-1 macrophages. In the medium bathing human macrophages, we observed 3.7-fold increase in active MMP-9 after NTG exposure (Fig. 3). Since NTG has been shown to be a pervasive oxidant of protein cysteine groups [23], we examined whether NTG, as well as its dinitrate metabolites, can activate MMP-9 through direct chemical cysteine oxidation, in the absence of cellular bioactivating enzymes. Results in Fig. 4 showed that NTG caused a significant activation of MMP-9 versus control (38.9 % increase in active MMP-9 concentration). Both dinitrate metabolites, 1,2 and 1,3-GDN, also activated proMMP-9 significantly (15.9 % increase with 1,2-GDN and 15.4 % increase with 1,3-GDN). Our findings indicated that these organic nitrates can activate proMMP-9 to MMP-9, without the presence of cellular bioactivating enzymes. This activation could result from the oxidation of the cysteine switch by reactive nitrogen species released by NTG, resulting in ,S-nitrosylation [53]. In cellular systems, MMP-9 activation by NTG may also be effected via S-glutathionylation [54] or S-guanylation [55; 56].
In summary, results in this study indicate that in vitro NTG exposure could broadly modify the mRNA expression of cellular proteases and adhesion molecules that may affect the ECM cascade. In particular, MMP-9 was activated after in vitro NTG exposure, both in THP-1 macrophages and in cell-free medium. The mechanism of activation is likely due to increased protein expression and/or post-translational oxidation of the cysteine switch of proMMP-9. Overall, these results support the hypothesis that NTG may have plaque destabilizing effects in vivo.
Although the concentration of NTG used in the present study (100 or 200 µM) was supra-pharmacologic, it was similar to that used by Gu et al. [53] who first demonstrated the activation of MMP-9 by the NO-donor S-nitrosocysteine at 200 µM. McCarthy et al. [57] recently used a concentration range of 100–500µM of various NO donors, including NONOates and S-nitrosothiols, to examine the role of S-nitrosylation in the NO-mediated regulation of MMP-9 activity. Thus, the concentrations used in the present study are consistent with those reported in the literature. In addition, the resultant concentrations of NO produced by NTG may represent those found in the regions of activated macrophages or in inflammatory conditions [58; 59]. The applicability of these findings to therapeutically relevant concentrations of NTG is being examined.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grant HL81580. We thank Dr. Doanh C. Tran for assistance in the gene microarray studies and Arna Katewa for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nitroglycerin: a blast from the past remains a trusted standby. Nitrates offer quick, effective relief from a common heart disease symptom, often for just pennies per dose. Harv Heart Lett. 2005;15:3. [PubMed] [Google Scholar]
- 2.Munzel T, Wenzel P, Daiber A. Do we still need organic nitrates? J Am Coll Cardiol. 2007;49:1296–1298. doi: 10.1016/j.jacc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- 4.Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Anon, GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 5.ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Anon, ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 6.Nagao K, Kanmatsuse K, Ooiwa K, Satou K, Watanabe I, Yamasita M, Kikushima K. The effects of long-acting nitrates on 5-year cardiac events of patients with coronary thrombolytic therapy for acute myocardial infarction. Intern Med. 2000;39:877–884. doi: 10.2169/internalmedicine.39.877. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Hagiwara N, Kasanuki H, Koyanagi R, Ogawa H the Heart Institute of Japan, Department of Cardiology nitrate evaluation program. Long-term nitrate use in acute myocardial infarction. Cardiovasc Drugs Ther. 2008;22:177–184. doi: 10.1007/s10557-008-6089-8. [DOI] [PubMed] [Google Scholar]
- 8.Kanamasa K, Hayashi T, Kimura A, Ikeda A, Ishikawa K. Long-term, continuous treatment with both oral and transdermal nitrates increases cardiac events in healed myocardial infarction patients. Angiology. 2002;53:399–408. doi: 10.1177/000331970205300405. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C Multicenter Myocardial Ischemia Research Group. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Am Heart J. 1999;138:577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa K, Kanamasa K, Ogawa I, Takenaka T, Naito T, Kamata N, Yamamoto T, Nakai S, Hama J, Oyaizu M, Kimura A, Yamamoto K, Aso N, Arai M, Yabushita H, Katori Y Secondary Prevention Group. Long-term nitrate treatment increases cardiac events in patients with healed myocardial infarction. Jpn Circ J. 1996;60:779–788. doi: 10.1253/jcj.60.779. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa K, Yamamoto T, Kanamasa K, Hayashi T, Takenaka T, Kimura A, Miyataka M, Yabushita H, Kitayama K. Intermittent nitrate therapy for prior myocardial infaraction does not induce rebound angina nor reduce cardiac events. Intern Med. 2000;39:1020–1026. doi: 10.2169/internalmedicine.39.1020. [DOI] [PubMed] [Google Scholar]
- 12.Death AK, Nakhla S, McGrath KC, Martell S, Yue DK, Jessup W, Celermajer DS. Nitroglycerin upregulates matrix metalloproteinase expression by human macrophages. J Am Coll Cardiol. 2002;39:1943–1950. doi: 10.1016/s0735-1097(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 13.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 14.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 16.Ram M, Sherer Y, Shoenfeld Y. Matrix Metalloproteinase-9 and Autoimmune Diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 17.Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- 18.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 21.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkins PA, Ho YS, Smith WW, Janson CA, D'Alessio KJ, McQueney MS, Cummings MD, Romanic AM. Structure of the C-terminally truncated human ProMMP9, a gelatin-binding matrix metalloproteinase. Acta Crystallogr D Biol Crystallogr. 2002;58:1182–1192. doi: 10.1107/s0907444902007849. [DOI] [PubMed] [Google Scholar]
- 23.Tsou PS, Addanki V, Haas JA, Page NA, Fung HL. Role of Glutaredoxin-Mediated Protein S-Glutathionylation in Cellular Nitroglycerin Tolerance. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.149997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rival Y, Beneteau N, Chapuis V, Taillandier T, Lestienne F, Dupont-Passelaigue E, Patoiseau JF, Colpaert FC, Junquero D. Cardiovascular drugs inhibit MMP-9 activity from human THP-1 macrophages. DNA Cell Biol. 2004;23:283–292. doi: 10.1089/104454904323090912. [DOI] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang EQ, Lee WI, Brazeau D, Fung HL. cDNA microarray analysis of vascular gene expression after nitric oxide donor infusions in rats: implications for nitrate tolerance mechanisms. AAPS PharmSci. 2002;4:E10. doi: 10.1208/ps040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Zharkikh A, Li WH. Estimation of confidence in phylogeny: the complete-and-partial bootstrap technique. Mol Phylogenet Evol. 1995;4:44–63. doi: 10.1006/mpev.1995.1005. [DOI] [PubMed] [Google Scholar]
- 30.Rekhter M. Vulnerable atherosclerotic plaque: emerging challenge for animal models. Curr Opin Cardiol. 2002;17:626–632. doi: 10.1097/00001573-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 33.Pi X, Yan C, Kim D, Chen J, Berk BC. Differential expression of genes from nitrate-tolerant rat aorta. J Vasc Res. 2002;39:304–310. doi: 10.1159/000065542. [DOI] [PubMed] [Google Scholar]
- 34.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- 36.Yakubenko VP, Lobb RR, Plow EF, Ugarova TP. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res. 2000;260:73–84. doi: 10.1006/excr.2000.5002. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- 38.Nishida Y, Miyamori H, Thompson EW, Takino T, Endo Y, Sato H. Activation of matrix metalloproteinase-2 (MMP-2) by membrane type 1 matrix metalloproteinase through an artificial receptor for proMMP-2 generates active MMP-2. Cancer Res. 2008;68:9096–9104. doi: 10.1158/0008-5472.CAN-08-2522. [DOI] [PubMed] [Google Scholar]
- 39.Edgell CJ, BaSalamah MA, Marr HS. Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol. 2004;236:101–122. doi: 10.1016/S0074-7696(04)36003-1. [DOI] [PubMed] [Google Scholar]
- 40.Roll S, Seul J, Paulsson M, Hartmann U. Testican-1 is dispensable for mouse development. Matrix Biol. 2006 doi: 10.1016/j.matbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Bocock JP, Edgell CJ, Marr HS, Erickson AH. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–4015. doi: 10.1046/j.1432-1033.2003.03789.x. [DOI] [PubMed] [Google Scholar]
- 42.Hausser HJ, Decking R, Brenner RE. Testican-1, an inhibitor of pro-MMP-2 activation, is expressed in cartilage. Osteoarthritis Cartilage. 2004;12:870–877. doi: 10.1016/j.joca.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, Pozzi A. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer. 2005;116:52–61. doi: 10.1002/ijc.20997. [DOI] [PubMed] [Google Scholar]
- 44.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irizarry RA, Warren D, Spencer F, Kim IF, Biswal S, Frank E, Gabrielson JG, Garcia J, Geoghegan G, Germino C, Griffin SC, Hilmer E, Hoffman AE, Jedlicka E, Kawasaki F, Martinez-Murillo L, Morsberger H, Lee D, Petersen J, Quackenbush A, Scott A, Wilson M, Yang Y, Ye SQ, Yu W. Multiple-laboratory comparison of microarray platforms. Nat Methods. 2005;2:345–350. doi: 10.1038/nmeth756. [DOI] [PubMed] [Google Scholar]
- 46.Snider JV, Wechser MA, Lossos IS. Human disease characterization: real-time quantitative PCR analysis of gene expression. Drug Discov Today. 2001;6:1062–1067. doi: 10.1016/s1359-6446(01)01988-2. [DOI] [PubMed] [Google Scholar]
- 47.Marcet-Palacios M, Ulanova M, Duta F, Puttagunta L, Munoz S, Gibbings D, Radomski M, Cameron L, Mayers I, Befus AD. The transcription factor Wilms tumor 1 regulates matrix metalloproteinase-9 through a nitric oxide-mediated pathway. J Immunol. 2007;179:256–265. doi: 10.4049/jimmunol.179.1.256. [DOI] [PubMed] [Google Scholar]
- 48.Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Knipp BS, Ailawadi G, Ford JW, Peterson DA, Eagleton MJ, Roelofs KJ, Hannawa KK, Deogracias MP, Ji B, Logsdon C, Graziano KD, Simeone DM, Thompson RW, Henke PK, Stanley JC, Upchurch GR., Jr Increased MMP-9 expression and activity by aortic smooth muscle cells after nitric oxide synthase inhibition is associated with increased nuclear factor-kappaB and activator protein-1 activity. J Surg Res. 2004;116:70–80. doi: 10.1016/s0022-4804(03)00306-8. [DOI] [PubMed] [Google Scholar]
- 50.Kang JL, Lee K, Castranova v. Nitric oxide up-regulates DNA-binding activity of nuclear factor-kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol Cell Biochem. 2000;215:1–9. doi: 10.1023/a:1026581301366. [DOI] [PubMed] [Google Scholar]
- 51.Ho TY, Bagnell CA. Relaxin induces matrix metalloproteinase-9 through activation of nuclear factor kappa B in human THP-1 cells. Ann N Y Acad Sci. 2005;1041:314–316. doi: 10.1196/annals.1282.049. [DOI] [PubMed] [Google Scholar]
- 52.Kim GM, Jin KS, Chung CS. Differential effects of corticosteroids on the expression of cyclooxygenase-2, tumour necrosis factor-alpha and matrix metalloproteinase-9 in an animal model of migraine. Cephalalgia. 2008;28:1179–1187. doi: 10.1111/j.1468-2982.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 53.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 55.Zaki MH, Fujii S, Okamoto T, Islam S, Khan S, Ahmed KA, Sawa T, Akaike T. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J Immunol. 2009;182:3746–3756. doi: 10.4049/jimmunol.0803363. [DOI] [PubMed] [Google Scholar]
- 56.Sawa T, Zaki MH, Okamoto T, Akuta T, Tokutomi Y, Kim-Mitsuyama S, Ihara H, Kobayashi A, Yamamoto M, Fujii S, Arimoto H, Akaike T. Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat Chem Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 57.McCarthy SM, Bove PF, Matthews DE, Akaike T, van der Vliet A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry. 2008;47:5832–5840. doi: 10.1021/bi702496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umansky V, Hehner SP, Dumont A, Hofmann TG, Schirrmacher V, Droge W, Schmitz ML. Co-stimulatory effect of nitric oxide on endothelial NF-kappaB implies a physiological self-amplifying mechanism. Eur J Immunol. 1998;28:2276–2282. doi: 10.1002/(SICI)1521-4141(199808)28:08<2276::AID-IMMU2276>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]