Abstract
BACKGROUND CONTEXT
The therapeutic strategies that have thus far been employed for the treatment of intervertebral disc degeneration (IDD) have focused on relieving the symptoms, while reversal of the degeneration remains an important challenge for the effective treatment of IDD. Growth and differentiation factor-5 (GDF5), of which deficiency leads to early disc degeneration changes, has the potential to increase proliferation of disc cells and expression of extracellular matrix proteins.
PURPOSE
To develop a lumbar disc degeneration model in mice and determine the effect of adenoviral GDF5 gene therapy.
STUDY DESIGNE
Compare the degeneration changes of discs punctured by different size needles to develop a mice lumbar disc degeneration model. Evaluate the effects of in vivo gene therapy for the mice disc degeneration model by an adenoviral vector carrying GDF5 gene.
METHODS
A lumbar disc degeneration model was developed by needle punctures to the discs in Balb/c mice. Afterwards, a gene therapy treatment to disc degeneration was evaluated. Two of the mice lumbar discs were randomly chosen to be punctured by a 30- gauge needle and then injected with adenovirus that had been engineered to express either the luciferase gene (Ad-Luc) or the GDF5 gene (Ad-GDF5). Animals were analyzed by bioluminescent imaging, radiographic and MRI scanning, then sacrificed at 1-, 2-, 4-, or 8- week post operation and subjected to histological and biochemical assays.
RESULTS
By the detection of T2-weighted MRI scanning and histological study, the degeneration was found in all of the discs punctured by different size needles. But the development of the degeneration in the discs injured by 30-gauge needle was more reliable and moderate compared with other groups. The detection of luciferase activity by bioluminescent imaging revealed that adenovirus survived and the introduced genes were expressed over 6 weeks after injection. There were no T2-weighted MRI signals in either the Ad-Luc or Ad-GDF5 injected mice up to 4 weeks post operation. At 6 and 8 weeks, T2-weighted signals were detected in the Ad-GDF5 group, but none in the Ad-Luc control group. The percent disc height index (%DHI) was significantly decreased (~ 20%) by 1 week following injury in both groups, indicating the development of disc degeneration. At 2 weeks, the %DHI in the mice injected with Ad-GDF5 increased significantly compared with that of the mice injected with Ad-Luc group; the increase was sustained for the rest of experiment period. The disc histology treated with Ad-GDF5 was improved compared with that in control group. Glycosaminoglycan (GAG) levels were significantly decreased in the Ad-Luc injection group since 2 weeks after injury, and the DNA content had diminished by 4 weeks after the operation. In contrast, in the discs injected with Ad-GDF5, there was no decrease in the GAG and DNA levels following injury throughout the 8 weeks treatment period.
CONCLUSIONS
Disc degeneration animal model can be developed by using needle puncture to the discs in mice. The adenovirus is an effective vehicle for gene delivery with rapid and prolonged expression of target protein, and resulting improvement in markers of disc degeneration. Ad-GDF5 gene therapy could restore the functions of injured discs and has the potential to be an effective treatment.
Keywords: Intervertebral disc, degeneration, gene therapy, growth factor
Introduction
In Western societies, intervertebral disc degeneration (IDD) related lower back pain affects between 60% and 80% of the population at some point in their lives, making it one of the most important public health issues today. [1,2] IDD is thought to result from multifatorial causes. [3–7] However, regardless of the etiological factors, the major pathological manifestations of IDD are the progressive declining proteoglycan content, disappearance of nucleus pulposus and collapse of annulus fibrosus. [8,9] Finally, the height of the intervertebral discs is reduced and osteophytes appear at the anterior and lateral edges of the disc. [10]
The treatments available for IDD include conservative methods and surgical therapies. The traditional surgical discectomy does not reconstitute the functions of disc or allow the spine to regain its characteristic multi-directional flexibility conferred by unique combination of properties of the annulus and nucleus. The challenge of developing a therapeutic method that is able to restore disc functions has therefore been a long-standing aim of researchers.
Recently, the efficacy of a variety of growth factors for the IDD treatment has been investigated [11–15], which included growth and differentiation factor 5 (GDF5). [12,15] The concept underlying these investigations was that growth factors will improve cells proliferation and extracellular matrix recovery. GDF5, as a member of the BMP family, plays an important role in the growth and development of the skeletal system. In humans, the loss of GDF5 gene causes chondrogenic dysplasias, [16,17] while GDF5 deficient mice showed early disc degeneration changes. [18] In vitro, incubation with GDF5 can augment the proteoglycan and collagen production by chondrocytes or disc cells. [15,18–20] Furthermore, the injection of GDF5 protein into rabbit discs can promote cell proliferation. [15] However, the treatment was found to be dose-dependent. [15] Such dose-dependency increases the challenge of translating such a therapy to larger animals or humans. In an attempt to ensure that a sufficient concentration of GDF5 is maintained, multiple injections of the growth factor have been administered; however, the injury caused by the multiple punctures counteracted the beneficial effects of GDF5 and even worsened the disc degeneration. [12]
As an alternative therapeutic strategy for GDF5 delivery, gene therapy has the potential to provide a long-term in vivo expression of functional protein. In this study, we evaluated the effect of Ad-GDF5 gene therapy in a novel mice disc degeneration model.
Materials and Methods
Preparation of the adenoviral vector
The replication-incompetent adenovirus containing the firefly luciferase reporter gene (Ad-Luc) was a kind gift from Dr. Greg Helm (University of Virginia), and was at a concentration of 2×1011pfu/ml. We generated the replication-incompetent adenovirus that was engineered for expression of the GDF5 gene (Ad-GDF5) using AdEasy System (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. [21] The final concentration of Ad-GDF5 was determined to be 1.8×1011pfu/ml.
Study animals
All of the animal work procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Virginia. A total of 56 Balb/c mice (8 weeks old) from Harlan Laboratories (Harlan Laboratories, Indianapolis, IN) were studied, which included 16 mice for the study of the disc degeneration model and 40 mice for the evaluation of gene therapy. Four mice in model study and 10 mice in gene therapy study were sacrificed at each of the time points (1, 2, 4 and 8 weeks after operation).
Surgical procedures and in vivo transduction
Prior to the procedure, the animals were put under general anesthesia. Using aseptic techniques, the spine was exposed through an anterior midline transperitoneal approach. After separating the hind peritoneum and psoas major muscles, the L4, L5 and L6 vertebral bodies were identified. For the mice model study, the L4/5, L5/6 and L6/S1 discs were punctured by a 27-, 30-, and 33-gauge needle (Hamilton, Reno, NV), respectively. For gene therapy study, the L5/6 and L6/S1 discs were randomly injected with 5µl of Ad-GDF5 (1.8×1011pfu/ml) or Ad-Luc (2×1011pfu/ml) through a 30-gauge needle. After the operation, the incision was closed in a layered fashion and the animals were allowed to move freely around their cages.
In vivo bioluminescence imaging
Four of the animals received Ad-Luc injections were subjected to non-invasive, real-time in vivo imaging of bioluminescence, which was assayed on the first and third day after the injection, and then once a week through until the 8th week following the operation. The bioluminescence was imaged using a cryogenically cooled In Vivo Imaging System (IVIS) (Xenogen Corp., Alameda, CA). Using IGOR analysis software (Xenogen Corp.), defined round region of interest (ROI) of 1.5-mm diameter that included the peak photon count was selected at the same position in each image of the same animal. The images obtained from each animal on the first day were used to normalize the images taken at each subsequent time-point.
Radiographic analyses
Four mice in gene therapy study received the radiographic study at each time point. Animals were put under general anesthesia using a 2% isofluorane/oxygen mixture. Data were then acquired using a small animal Digital X-ray scanner. Lateral radiographs were obtained prior to the operation, and then at 1, 2, 4, and 8 weeks after the operation. The heights of vertebral bodies and discs were measured with imaging software (Image J, NIH) and were expressed as Disc Height Index (DHI) using the method developed by Lu et al. [22] The average measurement of anterior, middle and posterior height of the disc was divided by the average height of the adjacent vertebral body. The change in the DHI was expressed as the percentage DHI (%DHI) and normalized to the DHI that was obtained from the pre-operation radiograph.
Magnetic Resonance Imaging
Four mice in model study and two mice in gene therapy study were subjected to an MRI scan at each of the different time points through until 8 weeks after operation. Imaging was acquired using a Varian Inova 4.7 Telsa (T) MR (Varian Inc., Palo Alto, CA) scanner. T2-weighted sections in the sagittal plane were obtained as a series of multiple two-dimensional slices.
Biochemical assay
At each time point, five mice in gene therapy study were used for the biochemical assay. Animals were sacrificed and the discs were harvested immediately. After removing the endplate, the discs were digested in 200 µL of sterile papain solution (125 g/mL in 1× PBE, pH 6.5) at 60°C for 24 hours. DNA content was determined by Hoechst 33258 dye assay with calf thymus DNA as a standard. The glycosaminoglycan (GAG) content of the disc was detected by the dimethylmethylene blue (DMMB) colorimetric assay, with chondroitin sulfate as a standard.
Histological Evaluations
Four mice in model study and five mice in gene therapy study were sacrificed at each time point. Lumbar spinal column specimens were harvested and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) solution for 48 hours. The specimens were then decalcified with 0.25M ethylenediamine tetraacetic acid (EDTA) in PBS for 2 weeks. After being embedded in paraffin, the specimens were sectioned mid-sagittally at 8µm and stained with hematoxylin and eosin staining, or Safranin-O and fast green staining.
Statistical analysis
An ANOVA test was performed to determine whether there was a significant difference in disc height among the three experimental groups at different time points. We also analyzed variance of the GAG and DNA content within each group at different time points using ANOVA test. Data are presented as means ± S.D. and a p value of less than 0.05 was considered significant.
Results
Mice IDD model evaluation
Magnetic Resonance Imaging
MRI scans of injured mice lumbar spines are represented by T2-weighted, midsagittal plane images obtained 1 and 2 weeks after needle puncture (Fig. 1). Signal in the nucleus pulposus area disappeared in the 30- and 27-gauge injured discs at 1 week after injury, while the signal intensity of 33-gauge needle injured discs did not show appreciable differences at 1 week post injury. However, all injured discs, including discs injured by 33-gauge needle, lost T2 weighted signals from 2 weeks after injury.
Figure 1.
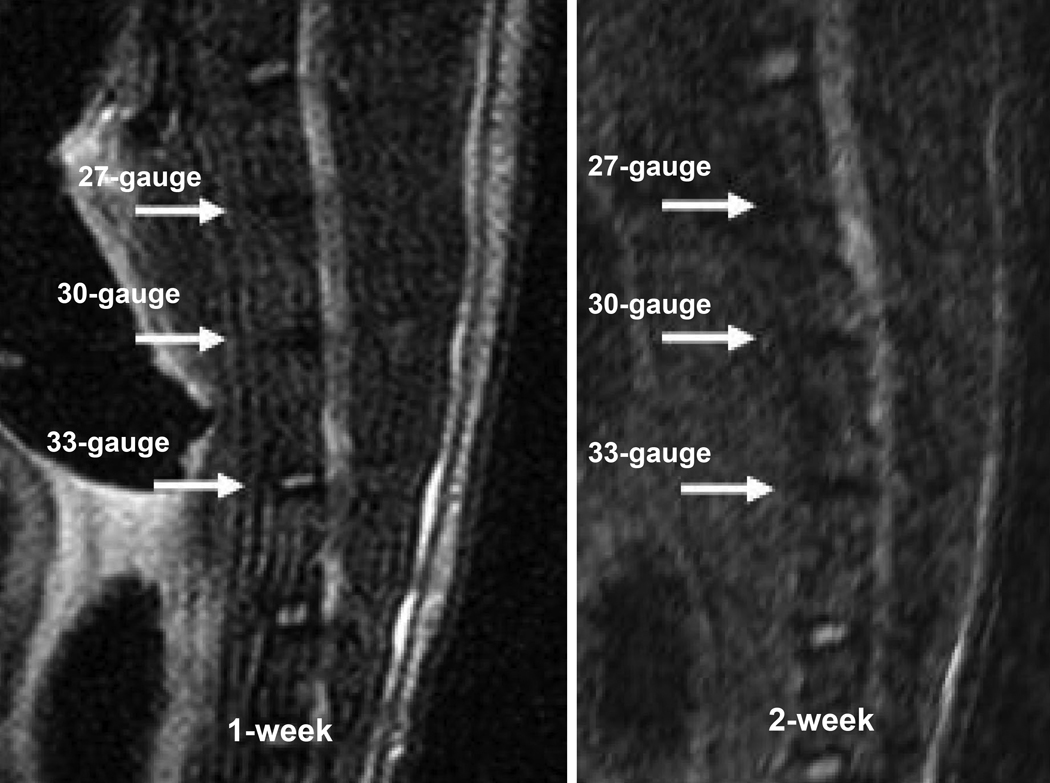
MRI T2 weighted signal of discs punctured by different size needles. When compared with the adjacent intact discs, the signal of 27-gauge (upper arrow pointed) and 30-gauges (middle arrow pointed) needles punctured discs disappeared since the first week post injury. The signal of 33-gauge (lower arrow pointed) needle punctured disc still could be seen at the 1-week time point, but disappeared at 2 weeks post operation.
Histology images of IDD model
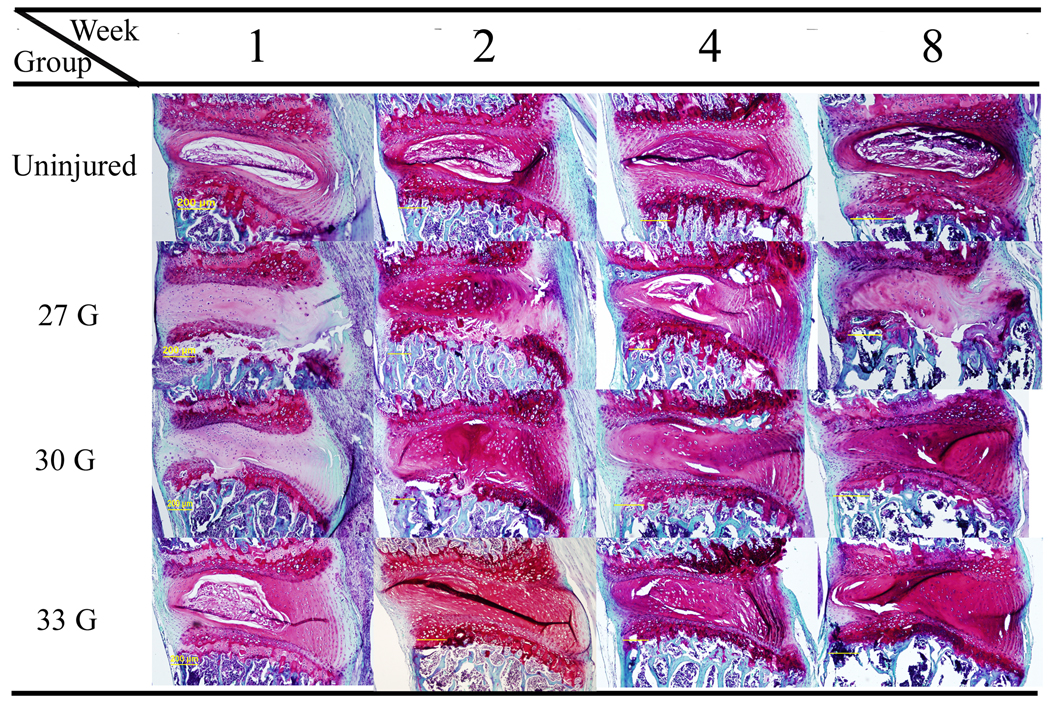
Sections of non-injured and injured discs were stained with Safranin-O at 1, 2, 4 and 8 weeks (Fig. 2). The non-injured discs had distinct nucleus and annulus regions. The nucleus demonstrated a gelatin like structure with scattered cells, while annulus was well organized with its lamellar sheets of collagen. In the 27- and 30-gauge needles injured disc, the nucleus disappearance accompanied with the loss of red staining and the lamellar structure in the annulus since the first week post operation. The reduction of cells and the accumulation of fiber-like tissue aggravated as the degeneration progressing in both groups. In 8 weeks after injury, the 27 gauge needle injured disc almost lost all the red staining and lamellar structure, which was worse than 30-gauge needle injured disc. However, in the 33-gauge needle injured discs, the histological morphology didn’t show obvious changes compared with non-injured disc at the first week. But since 2 weeks post injury, the inner layer of annulus lost the concentric lamellar structure with cracks and fissures appearance. Although the general loss of definition between the annulus and nucleus presented as the evidence of IDD, the nucleus tissue of 33-gauge punctured discs still can be identified by 2 weeks post injury. As the degeneration progressed, the nucleus tissue eventually disappeared at 8 weeks post operation and the space was occupied by disorganized, fibrocartilaginous tissue.
Figure 2.
Safranin-O staining of the sagittal sections of the discs after punctured by different size needles or left intact at different time points. The nucleus pulposus region disappeared in the 27-gauge and 30-gauge needle injured discs and chondroid cells proliferated from inner layer of annulus and endplates at the 1-week time point. Within the following time points of both groups, the cells number decreased and the center of the discs became aggravated morphological derangement with the area occupied by the fiber-like tissue. In the 33-gauge needle punctured discs, the reduction of the nucleus region and the increasing of the cracks and fissures in the annulus regions appeared at the 2 weeks post injury. At 4- and 8- week time points, the pathological progression of 33-gauge needle injured discs was also demonstrated with the loss of nucleus pulposus and the collapse of the annulus and endplates.
Evaluation of gene therapeutic effect of Ad-GDF5
In vivo bioluminescence imaging
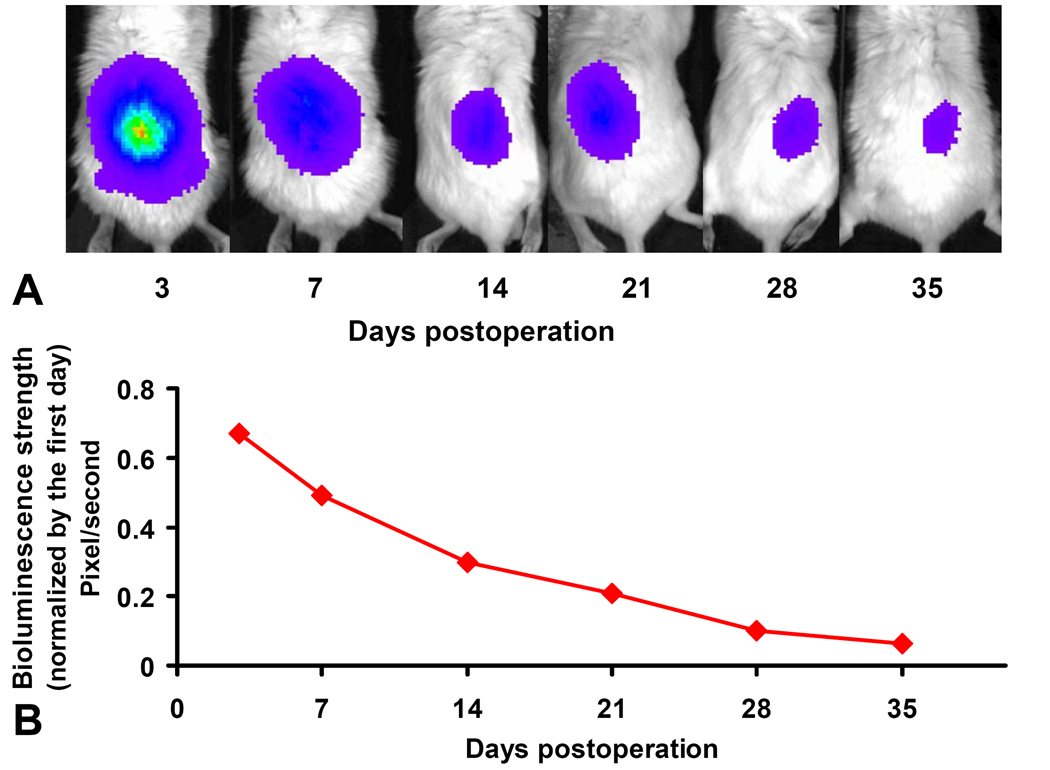
To determine whether mice had been successfully infected with adenovirus competent to express exogenous proteins, we tracked the expression of luciferase in situ in animals transduced with Ad-Luc. Fluorescence was observed on the first day after injection of the Ad-Luc (Fig. 3). In most of the animals it was no longer visible by the 6th week post-operation, but in some mice the expression was sustained through until the mice were sacrificed at 8 weeks. The majority of luciferase activity was confined to the injection site instead of distributed throughout the animal. To determine the expression profile, data were normalized to the fluorescence levels measured on the first day. Quantitative analysis of the peak amplitudes of fluorescence from four mice demonstrated that the activity levels of expressed luciferase steadily declined after the injection of Ad-Luc. At the end of the first week, the fluorescence intensity had decreased to nearly half the peak levels, but after a further 2 weeks the expression was still a quarter of the peak levels, and was still greater than 10% after 28 days.
Figure 3.
In vivo bioluminescence imaging of Ad-Luc injected into vertebral disc at different time points. A: Image of the same animal over the different time points. The signal still could be detected after 5 weeks. B: The trend of the average fluorescence signal strength curve demonstrated that the signal decreased over time. The signal strength initially decreased to nearly 50% within 7 days. But the deamplification subsequently slowed down, and even after 35 days, the signals strength remained approximately 10% of the initial value.
Radiographic analyses
The effects of Ad-GDF5 therapy were determined by assessing changes to the %DHI compared with the pre-operation values (Fig. 4). There were no differences in the %DHI for the control discs, which had been left intact, at any of the time points. However, one week after injection, the %DHIs of discs that had been injected with either Ad-Luc or Ad-GDF5 were remarkably decreased (P<0.05). For the discs that had been injected with Ad-Luc, the %DHI continued to decrease with time. Although %DHI for the discs injected with Ad-GDF5 had initially decreased to a similar extent to those injected with Ad-Luc, the %DHI of these discs recovered by 2 weeks post-operation, and this recovery was maintained for the remaining duration of the experiment, until sacrifice at 8 weeks. Indeed, from the 2-week time point, there was no statistic difference between the %DHIs of the intact and the Ad-GDF5 injected disc, with the difference between them and Ad-Luc injected discs growing more pronounced at each time point (2 and 4 weeks P<0.05; 8 weeks P<0.01).
Figure 4.
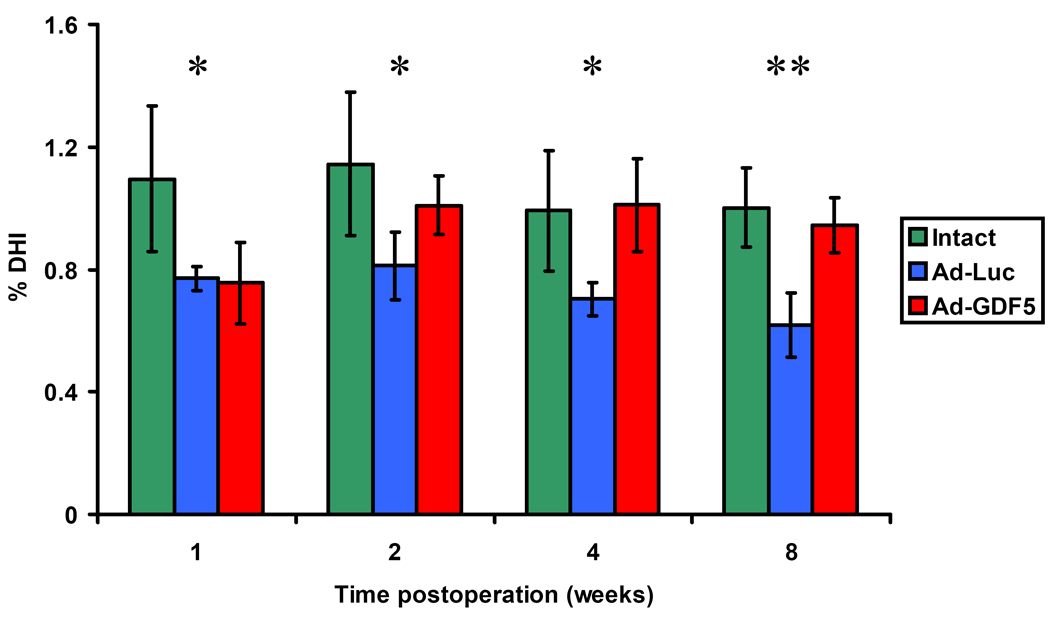
Percentage disc height index (%DHI) of intact discs, Ad-Luc injected discs and Ad-GDF5 injected discs by radiographic analysis. The data was presented as means ± SD. By “Multiple Comparisons” of one-way ANOVA Bonferroni analysis, the intact disc group showed higher mean value than either of other two groups at 1-week and Ad-Luc group showed lower means at 2-, 4-, and 8- week time points compared with either of other two groups. All of the differences reached statistic differences (p<0.05). The means of three groups at each time point were also compared by using ANOVA analysis and the p value was marked as stars in the figure. *: p<0.05; **: p<0.01
Magnetic Resonance Imaging
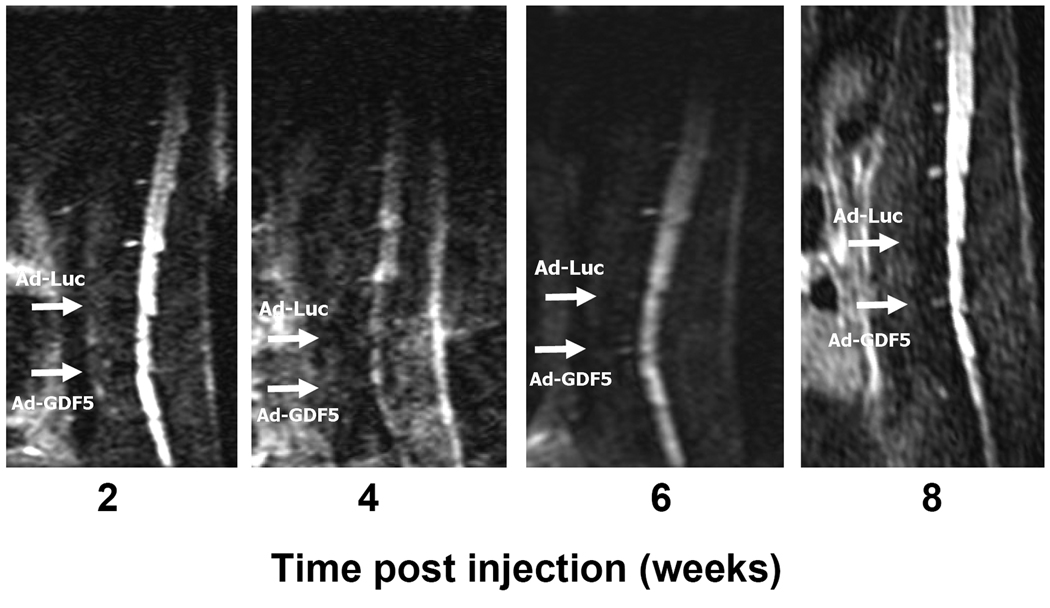
MRI scanning was performed at 2, 4, 6 and 8 weeks following the operation to determine the tissue water content in the discs. In all mice, the intensity of the T2-weighted signal of both the Ad-Luc and Ad-GDF5 injected groups was clearly decreased at both the 2- and 4-week time points (Fig.5). However, the signal from the discs that had been injected with Ad-GDF5 had begun to recover by the 6-week time point. The trend continued to the 8-week time point, when an even higher signal was measured. On the other hand, there were no visible signs of recovery of the signal intensity in the discs that had been injected with Ad-Luc at any of the time points prior to sacrifice at 8 weeks.
Figure 5.
MRI T2 weighted signal images of the same animal at different time points. Upper arrow points to the location where and disc was injected with Ad-Luc, while the lower arrow points to the Ad-GDF5 injected disc. When compared with the adjacent intact discs, both of the discs lost the bright T2 weight signal at the second week post-injection. But the signal of the Ad-GDF5 injected disc began to reappear at 6 weeks, and had become clearer at 8 weeks. In contrast, there were no signs of recovery of the signal of the Ad-Luc injected disc.
Biochemical assay
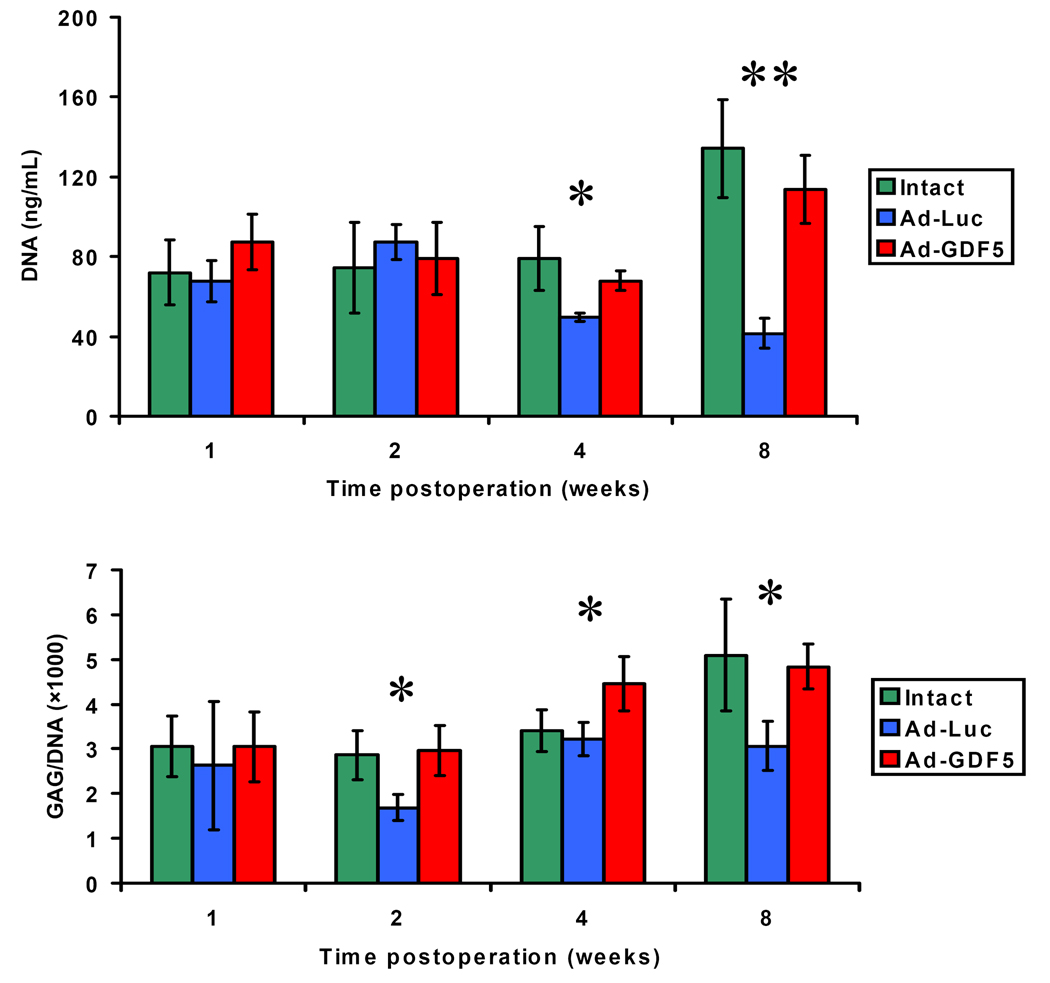
The effect of Ad-GDF5 and Ad-Luc on total DNA, a marker of changes to the overall number of cells in the disc, was determined (Fig. 6). The average DNA content of the disc that had been injected with Ad-Luc had decreased by the 4-week time point, and this was sustained through until the 8-week time point, the differences achieved statistical difference compared with the intact and Ad-GDF5 injection discs (P<0.05). However, the DNA content of the disc that had been injected with Ad-GDF5 did not vary from that of the intact disc at any of the time points.
Figure 6.
Biochemical assays of GAG and DNA content of the discs. The average DNA content in the discs injected with Ad-Luc decreased at the 4- and 8-week time points. However, no differences can be seen between the DNA content of intact disc and disc received Ad-GDF5 injection groups at different time points. The GAG content (demonstrated by GAG/DNA ratio) of Ad-Luc injected disc level decreased at the 2- and 8-week time points post operation compared with other groups. At the same time, there were no statistical differences of the GAG content within Ad-GDF5 group and intact disc group. At the 4-week time point, the GAG/DNA ratio of Ad-GDF5 group was higher than other groups. *: p<0.05; **: p<0.01
The GAG content was normalized by the DNA content of the disc and compare among the groups as GAG/DNA ratios. Compared with the intact disc, the GAG/DNA ratio of Ad-Luc injected disc level decreased at the 2- and 8- week post operation (P<0.05) (Fig. 6). In contrast, the GAG/DNA ratio in Ad-GDF5 group increased at the 4-week compared with other groups and had no statistic difference compared with the intact disc group at 2- and 8-week time points.
Histology image
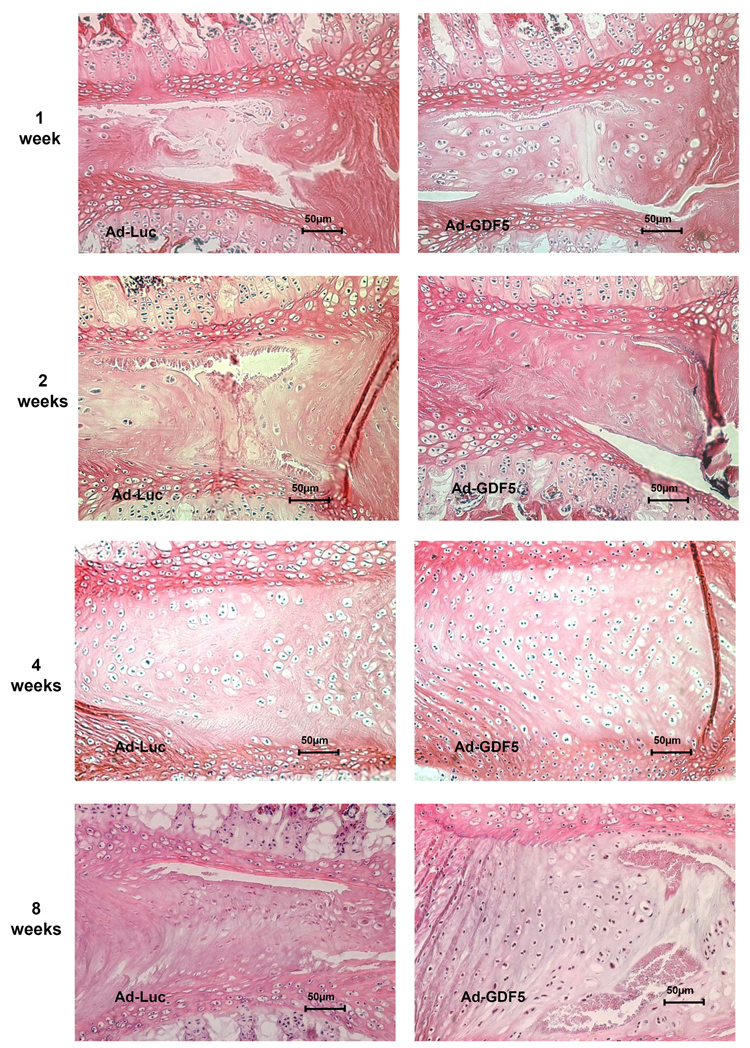
The disc degeneration was studied histologically by staining with hematoxylin and eosin (Fig. 7). We found that discs injected with Ad-Luc and Ad-GDF5 had lost the majority of the nucleus pulposus by the first week following the operation. In addition, by this time point the chondroid cells had begun to proliferate from the annulus towards the center of the disc, an effect that was more pronounced in the Ad-GDF5 injected discs than the Ad-Luc injected discs. The preponderance of cells growing towards the center of the disc after injection with Ad-GDF5 continued throughout the remaining time points. At the 8-week time point, the disc height of the Ad-Luc groups was obviously lower than Ad-GDF5 groups, which confirmed the result of %DHI measurement of radiographic images. Although the Ad-GDF5 discs did not regenerate the typical appearance of the nucleus pulposus, they were nonetheless much more cellular compared with the Ad-Luc injected discs, which were instead filled with fibrous tissue.
Figure 7.
HE staining of Ad-Luc and Ad-GDF5 injected disc at different time points. Histological sections demonstrate the disappearance of the nucleus at the first week. The regeneration of disc began with cells proliferation from the surrounding inner layer of annulus cells, and may include some residual nucleus cells. Cell proliferation increased more quickly in the discs injected with Ad-GDF5 than with Ad-Luc. As the cells proliferation, the appearance of neogenetic cells was similar to that of chondroid cells. In the discs injected with Ad-GDF5, many chondroid cells still can be seen accompanied by abundant expression of the extracellular matrix at 8 weeks post-injection. In the discs injected with Ad-Luc, there was very little sign of chondroid cells and the center of disc was replaced by fiber-like tissue at 8 weeks.
Discussion
In the present research, we investigated the efficacy of Ad-GDF5 gene therapy in an in vivo mouse model of IDD. Several different animal species have been used for in vivo studies of the biology and mechanical characteristics of IDD, including sheep [23], pig [24], dog [25], rabbit [11,26–28], rat [29,30] and mouse. [12,31] Dawn M. et al. made a comparison of the disc geometry parameters of baboons, sheep, rabbits, rat tail, rat lumbar, mouse tail and mouse lumbar with humans, the mouse lumbar discs were found to be the closest representation of the human lumbar discs geometry.[32] This result revealed the advantage of using mouse lumbar discs to mimic the situation of human lumbar IDDs, which related to the changes of anatomy parameters. We thus developed a lumbar needle puncture model of IDD in mice. Similar to the pathological changes in human lumbar IDD [33,34], the collapse of nucleus pulposus tissue followed by the damages of annulus and endplates appeared in this IDD model. The matrix breakdown resulted in the reduction of water content and the decrease of the space in the disc.
Sustained expression of exogenous proteins for the treatment of chronic diseases, such as IDD, is essential to the success of the therapeutic strategy.[35] In a study of transgene expression mediated by an adenoviral vector, the target gene expression in rabbit discs can be found even more than one year post injection.[36] Nishida and Kang et al. reported adenoviral vector carrying TGF-²1 gene can increase proteoglycan synthesis after injected in rabbit discs for one week. [14] In this study, the result of Ad-Luc injection demonstrated that the expression of a target protein after infection of living cells in the disc can be maintained at an assayable level for at least 5 weeks. This results in an extension of the functional exposure of host cells to exogenous proteins, resulting in a long-term effect after a single injection into the disc.
GDF5 is important for the development of skeletal system, especially for the cartilage-like tissue. In our previous study, GDF5 deficient mice showed conspicuous depauperate and degenerated discs. [18] Therefore, it is attractive for using GDF5 in the therapeutic strategy of IDD. We also found that the GDF5 protein can enhance cells proliferation and increase the gene expression for extracellular matrix proteins such as type II collagen and aggrecan in in vitro cultured disc cells. [37] Moreover, GDF5 protein suppressed the expression of matrix metalloproteinase-3 (MMP-3), part of the mechanism underlying the pathological degradation of the extracellular matrix in disc. [37,38] These observations have been confirmed by other research groups.[12,15,20]
In this present study, the cells proliferation of disc influenced by the injection of Ad-GDF5 or Ad-Luc was revealed by DNA content assay and histological study. There was no statistical difference of DNA content at all of the time points between Ad-GDF5 injected and intact discs. We considered that the injection of Ad-GDF5 enhanced the cells proliferation, which compensated the loss of the cell number after the needle puncture induced IDD. Besides the cells proliferation, another effect of GDF5 promotion is the increase of proteoglycan in the extracelluar matrix of discs. There was no reduction of GAG/DNA ratio at any of the time points in the discs that had been injected with Ad-GDF5 compared with intact discs. Moreover, at 4-week time point, the ratio of GAG/DNA in Ad-GDF5 treatment group even higher than that in the intact disc. This indicates that Ad-GDF5 injection not only improved the cells proliferation of the chondriod cells but also promoted proteoglycan production. A previous report described increases in the proteoglycan content of the disc after injection of osteoprotein-1 (OP-1), which were present at the 2-week time point, but not at later time points. [39] It is possible that the stimulating effect of the injected growth factor on the secretion of matrix components was lost after 2 week post injection. However, we found that the expression of GAG content was maintained at the same level throughout the experiment in the Ad-GDF5 group. We conjecture that sustained expression of GDF5 would maintain the continuous secretion of matrix components for long periods of time. The promotion of proteoglycan production after the injection of Ad-GDF5 was also confirmed by MRI studies. [40,41] In Ad-GDF5 treatment group, the T2 signals begin to reappear at the 6-week time point and were more obvious at 8 weeks. We believe this may reflect the appearance of more chondroid cells in the discs injected with Ad-GDF5, which correlated with greater secretion of proteoglycan.
The changes to the number of cells, the expression of matrix components and the water content can all be interpreted in terms of the degeneration and recovery of the disc. These transformations also reflect changes of the height of discs, while the narrowing of disc is considered to be one of the important characteristic features of IDD.[42,43] The height of the discs injected with Ad-GDF5 had recovered and the effect was maintained throughout the rest of the experiment.
Our research reports the curative effects of gene therapy for the treatment of IDD in a novel mice model by adenoviral mediation of human GDF5 gene. The results of this study not only confirmed the long-term expression of the target protein in the disc in vivo, but also highlighted the physiological improvements that occurred to the disc. Our research also confirmed this IDD model developed in mice will be suitable for the study of the physiopathologic changes and the effect of therapeutic intervention.
ACKNOWLEDGEMENT
In support of their research for or preparation of this manuscript, this project was supported by AO Research Fund of the AO Foundation and the National Institute of Health. None of the authors received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Macfarlane GJ, Thomas E, Croft PR, et al. Predictors of early improvement in low back pain amongst consulters to general practice: the influence of pre-morbid and episode-related factors. Pain. 1999;80:113–119. doi: 10.1016/s0304-3959(98)00209-7. [DOI] [PubMed] [Google Scholar]
- 2.Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol.Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natarajan RN, Ke JH, Andersson GB. A model to study the disc degeneration process. Spine. 1994;19:259–265. doi: 10.1097/00007632-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vresilovic EJ, Johannessen W, Elliott DM. Disc mechanics with trans-endplate partial nucleotomy are not fully restored following cyclic compressive loading and unloaded recovery. J.Biomech.Eng. 2006;128:823–829. doi: 10.1115/1.2354210. [DOI] [PubMed] [Google Scholar]
- 5.Battie MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601–2612. [PubMed] [Google Scholar]
- 6.Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol.Biol.(Noisy.-le-grand) 2007;53:4–18. [PubMed] [Google Scholar]
- 7.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- 9.Richardson SM, Mobasheri A, Freemont AJ, et al. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol.Histopathol. 2007;22:1033–1041. doi: 10.14670/HH-22.1033. [DOI] [PubMed] [Google Scholar]
- 10.Pye SR, Reid DM, Lunt M, et al. Lumbar disc degeneration: association between osteophytes, end-plate sclerosis and disc space narrowing. Ann.Rheum.Dis. 2007;66:330–333. doi: 10.1136/ard.2006.052522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 12.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 13.Zhan Z, Shao Z, Xiong X, et al. Ad/CMV- hTGF-beta1 treats rabbit intervertebral discs degeneration in vivo. J.Huazhong.Univ Sci.Technolog.Med.Sci. 2004;24:599–601. 624. doi: 10.1007/BF02911367. [DOI] [PubMed] [Google Scholar]
- 14.Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 16.Polinkovsky A, Robin NH, Thomas JT, et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat.Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- 17.Thomas JT, Lin K, Nandedkar M, et al. A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat.Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Leo BM, Beck G, et al. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 19.Yeh LC, Mallein-Gerin F, Lee JC. Differential effects of osteogenic protein-1 (BMP-7) on gene expression of BMP and GDF family members during differentiation of the mouse MC615 chondrocyte cells. J.Cell Physiol. 2002;191:298–309. doi: 10.1002/jcp.10094. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Kroeber M, Hanke M, et al. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J.Mol.Med. 2004;82:126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 21.Feng G, Wan Y, Balian G, et al. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu DS, Shono Y, Oda I, et al. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine. 1997;22:1828–1834. doi: 10.1097/00007632-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Holm S, Holm AK, Ekstrom L, et al. Experimental disc degeneration due to endplate injury. J.Spinal Disord.Tech. 2004;17:64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Hutton WC, Ganey TM, Elmer WA, et al. Does long-term compressive loading on the intervertebral disc cause degeneration? Spine. 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Yoon ST, Li J, et al. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine. 2005;30:33–37. doi: 10.1097/01.brs.0000149191.02304.9b. [DOI] [PubMed] [Google Scholar]
- 28.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2005;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 29.Lai A, Chow DH, Siu WS, et al. Reliability of radiographic intervertebral disc height measurement for in vivo rat-tail model. Med.Eng Phys. 2007;29:814–819. doi: 10.1016/j.medengphy.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Norcross JP, Lester GE, Weinhold P, et al. An in vivo model of degenerative disc disease. J.Orthop.Res. 2003;21:183–188. doi: 10.1016/S0736-0266(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 31.Lotz JC, Colliou OK, Chin JR, et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 33.Freemont AJ, Watkins A, Le MC, et al. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J.Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 34.Haughton V. Medical imaging of intervertebral disc degeneration: current status of imaging. Spine. 2004;29:2751–2756. doi: 10.1097/01.brs.0000148475.04738.73. [DOI] [PubMed] [Google Scholar]
- 35.Larson JW, III, Levicoff EA, Gilbertson LG, et al. Biologic modification of animal models of intervertebral disc degeneration. J.Bone Joint Surg.Am. 2006;88 Suppl 2:83–87. doi: 10.2106/JBJS.F.00043. [DOI] [PubMed] [Google Scholar]
- 36.Sobajima S, Kim JS, Gilbertson LG, et al. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 37.Cui M, Wan Y, Anderson DG, et al. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Roberts S, Caterson B, Menage J, et al. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 39.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 40.Pearce RH, Thompson JP, Bebault GM, et al. Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human intervertebral disk. J.Rheumatol.Suppl. 1991;27:42–43. [PubMed] [Google Scholar]
- 41.Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur.Spine J. 2005;14:27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfirrmann CW, Metzdorf A, Elfering A, et al. Effect of aging and degeneration on disc volume and shape: A quantitative study in asymptomatic volunteers. J.Orthop.Res. 2006;24:1086–1094. doi: 10.1002/jor.20113. [DOI] [PubMed] [Google Scholar]
- 43.Imai Y, Okuma M, An HS, et al. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197–1205. doi: 10.1097/BRS.0b013e3180574d26. [DOI] [PubMed] [Google Scholar]
- 44.Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603–2611. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]