Abstract
Objective:
Our goal was to test the hypothesis that specific integrin receptors regulate chondrocyte biosynthetic response to dynamic compression at early times in 3D gel culture, during initial evolution of the pericellular matrix, but prior to significant accumulation of further-removed matrix. The study was motivated by increased use of dynamic loading, in vitro, for early stimulation of tissue engineered cartilage, and the need to understand the effects of loading, in vivo, at early times after implantation of constructs.
Methods:
Bovine articular chondrocytes were seeded in 2% agarose gels (15 × 106 cells/mL) and incubated for 18 hours with and without the presence of specific integrin blockers (small-molecule peptidomimetics, function-blocking antibodies, and RGD-containing disintegrins). Samples were then subjected to a 24-hour dynamic compression regime found previously to stimulate chondrocyte biosynthesis in 3D gel as well as cartilage explant culture (1 Hz, 2.5% dynamic strain amplitude, 7% static offset strain). At the end of loading, proteoglycan synthesis (35S-sulfate incorporation), protein synthesis (3H-proline incorporation), DNA content (Hoechst dye 33258) and total GAG content (DMMB dye binding) were assessed.
Results:
Consistent with previous studies, dynamic compression increased proteoglycan synthesis and total GAG accumulation compared to free-swelling controls. Blocking αvβ3 abolished this response, independent of effects on controls, while blocking β1 abolished the relative changes in synthesis when changes in free-swelling synthesis rates were observed.
Conclusions:
This study suggests that both αvβ3 and β1 play a role in pathways that regulate stimulation of proteoglycan synthesis and accumulation by dynamic compression, but through distinct complementary mechanisms.
Keywords: Integrin, Mechanical stimulation, Dynamic Compression, Chondrocyte, Proteoglycan synthesis, Agarose culture, Mechanotransduction
Introduction
In vivo, articular cartilage experiences a combination of compressive, tensile, and shear forces, both dynamic and static in nature1-3. These mechanical forces can induce a variety of macroscopic signals including changes in intratissue pH and osmotic pressure, hydrostatic pressure gradients, fluid flow, streaming potentials and current, and mechanical deformation4-6, which are sensed by chondrocytes and can regulate cell behavior. In vivo, static immobilization or reduced joint-loading leads to a loss of glycosaminoglycan (GAG) content and decreased proteoglycan (PG) synthesis, which can be partially rescued by remobilization7, 8. In vitro, radially unconfined dynamic compression at frequencies greater than 0.001 Hz has been shown to increase chondrocyte biosynthesis of PG and protein in both explant and 3-D agarose gel culture models9-12, while static compressive loading can lead to decreases in biosynthesis13-16. In vitro, static and dynamic loading cause activation of mitogen-activated protein (MAP) kinase pathway and ion channel signaling cascades, which result in time-varying changes in gene transcription of matrix proteins, catabolic enzymes, and transcription factors17-20.
Recent research on the transduction of mechanical signals into changes in cell behavior has begun to elucidate the role of integrin interactions with the extracellular matrix (ECM). Chondrocytes express α1β1, α2β1, α3β1, α5β1, αvβ3 and αvβ5 21-23. This expression can change with local microenvironment, mechanical stimulation, and during osteoarthritis24-26. Integrins play a role in adhesion, cell survival, regulating matrix metabolism, and in chondrocyte response to mechanical stimuli see 27-29 for reviews. β1, α5β1, αvβ5 integrins have been shown to mediate chondrocyte adhesion to cut cartilage surfaces21,30. In monolayer studies, blocking α5β1 integrin interactions led to increased matrix metalloproteinase-13 (MMP-13) activation and cellular apoptosis23,31. Upregulation of aggrecan mRNA and suppression of matrix metalloproteinase-3 (MMP-3) mRNA levels by dynamic stretching of monolayer chondrocytes was shown to involve a β1-integrin-dependent pathway as well as stretch-activated ion channels and autocrine/paracrine stimulation by interleukin-4 (IL-4)32,33. Other studies have also identified a β1-integrin-dependent translocation of protein kinase C alpha (PKCα) to the cell membrane, increased association of intracellular receptor for activated C-kinase1 (RACK1) and PKCα with β1-integrin after mechanical stimulation34, and interactions between the integrin associated protein (CD47/IAP) and α5-integrin35, as possible downstream signaling mechanisms. More recently, monolayer studies demonstrated that blocking with anti-αvβ5 antibody could also abolish chondrocyte responses to dynamic stretching36.
The use of physiologic dynamic compression as a stimulant for cartilage regeneration in tissue engineering has been demonstrated by several groups10,11,19,37-41 and has prompted studies of the mechanisms by which cells in tissue engineered constructs transduce signals under physiological loading conditions. While some studies suggest a role for ion channels, especially calcium channels, in regulating sGAG synthesis in response to dynamic compression42,43, studies in 3-D culture models have suggested that the roles are more complicated than that elucidated in monolayer culture models42. Monolayer studies have provided insights into signaling pathways involved in chondrocyte mechanotransduction, but the role of cell interactions with newly-synthesized and assembled matrix macromolecules in 3-D geometries is less well understood.
The goal of this study was to examine the role of integrins in chondrocyte response to dynamic compression by cells in 3D agarose gel culture at early times in culture, during initial evolution of the pericellular matrix, but prior to significant accumulation of further removed extracellular matrix. Chondrocyte cultures in agarose at early times, with little pericellular matrix present, can respond to dynamic compression with increased sulfate incorporation and sGAG accumulation10,11,39. They also have the added benefit of permitting the use of multiple methods for perturbing integrin-matrix interactions, including the use of blocking antibodies similar to those used in monolayer studies, which have diffusive limitations in native cartilage tissue.
Methods
CELL HARVEST AND CULTURE
Chondrocytes were isolated from the femoral condyle cartilage of 2- to 3- week old bovine calves (Research 87, Marlborough, MA) by sequential digestion in 0.2% pronase (Protease type XIV, Sigma) and 0.025% Collagenase-P (Roche), as described previously44. Cells were seeded in 2% agarose (low melting-temperature, Invitrogen) at concentrations of 15 million cells/mL using a stainless steel casting frame38,45, in a slab geometry approximately 1.6 mm thick. 4 mm diameter disks were cored from the slab using a dermal punch and cultured in 1% ITS-supplemented feed medium (high glucose DMEM, 0.1 mM nonessential amino acids, 0.4 mM proline, 100 U/mL PSA – penicillin, streptomycin, amphoteracin, 10 μg/mL ascorbate).
INTEGRIN-BLOCKING COMPOUNDS
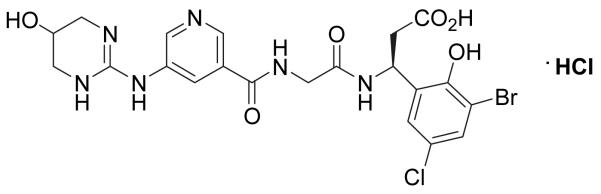
An array of integrin-blocking compounds was used including small-molecule peptidomimetics, function-blocking antibodies, and RGD-containing disintegrins. Small-molecule compounds have the advantage of rapid diffusion times, which allow for more spatial uniformity of treatment. PF001 (previously referenced as S247 46), PF002, PF003 are synthetic peptidomimetics (obtained from Pfizer; Fig. 1) of the conserved amino acid motif RGD (arginine-glycine-aspartic acid) and are potent in vitro antagonists (with IC50s on the order of 1nM) of ligand interaction with specific integrins. Their molecular weights and potencies against selected integrins as measured in integrin-overexpressing cell-adhesion assays are summarized in Table 1. PF001 is a relatively broad spectrum blocker while PF002 and PF003 are more specific blockers of α5β1 and αvβ3 integrins, respectively.
Figure 1.
Chemical structure for PF001 (previously cited as S247 [40]). PF001 is a synthetic RGD peptidomimetic that acts as an integrin binding antagonist with broad specificity to αv and α5 integrins.
Table 1.
Molecular weight and relative specificities for PF001, PF002, PF003. IC50s were measured using specific integrin-transfected HEK 293 cell adhesion assays as in [46]. Data obtained from Pfizer, Inc. PF001 was previously cited as S247 46.
| Mol Wt | IC50 (nM) | |||
|---|---|---|---|---|
| αvβ3 | αvβ5 | α5β1 | ||
|
PF001 (S247) |
569.8 | 0.40 | 1.50 | 64 |
| PF002 | 388.9 | 179 | 1660 | 1.23 |
| PF003 | 681.7 | 0.627 | 1.38 | 8940 |
To test activity and toxicity of the peptidomimetic compounds, a cell-adhesion assay was performed using an RGD-conjugated comb copolymer surface which promotes integrin-mediated adhesion and prevents non-specific adhesion47-49. Prepared surfaces were obtained50 in which cover slips were spin-coated with a poly(methyl methacrylate)-graft-poly(ethylene oxide), PMMA-g-PEO, a comb copolymer with a fraction of PEO side chains functionalized with maleimide groups using N-(p-maleimidophenyl) isocyanate (PMPI), and conjugated with a PHSRN-K-GRGDSP peptide (RGD peptide + fibronectin synergy site). The RGD-containing peptide was presented in dispersed and clustered conformations. Further details of polymer synthesis, peptide synthesis, and surface preparation are described in Kuhlman et al.48. Cover slips were placed at the bottom of a 24-well plate and held in place by silicon rings. After isolation, 25,000 chondrocytes were seeded per well and given 2 hours to attach. PF001, PF002, PF003 were then added at concentrations of 0, 50, 100, or 200 μM. Cells were cultured for 7 days, with medium changes every other day. Cells were imaged daily using an inverted microscope to qualitatively observe adhesion and spreading. After 7 days, cells were stained with FDA/EtBR (live/dead assessment) and imaged using an inverted UV microscope.
Echistatin (Sigma) is a 5.4 kDa disintegrin peptide that non-selectively blocks the activities of several integrins including αvβ3, α5β1, and αIIbβ3 with IC50s on the order of 10nM51. Function blocking antibodies to αvβ3 (MAB1976, clone LM609), αv (MAB1953, clone P3G8), α5 (MAB1956, clone P1D6), and β1 (MAB1965, clone JB1A) integrins were obtained from Chemicon. Cross-reactivity of blocking antibodies MAB1976, MAB1956, and MAB1965 to bovine integrins have been previously demonstrated52-54 and are supported by dot-blot analysis (Figure 1S). Echistatin31 and the blocking antibodies21,23,34,55,56 have been previously used on chondrocytes with no reported cell death. All antibody preps had endotoxin levels below 0.5 EU/mL (LAL method, Lonza QCL-1000 test kit).
MECHANICAL LOADING
On day of casting, 3-4 chondrocyte-seeded agarose disks maintained in free-swelling culture were pre-incubated for 18 hours in one of the following conditions: (1) untreated control (feed medium), (2) 200 μM PF001, PF002, or PF003, (3) 5 μg/mL integrin-blocking antibody (each antibody used in separate experiments), (4) 1 μM echistatin. Parallel sets of disks from treatment conditions (1) – (4) were subjected to dynamic compression. Immediately prior to loading, 5 μCi/mL of 35S-sulfate and 10 μCi/mL of 3H-proline were added to the medium. Disks were then subjected to a 24-hour, 1 Hz continuous, sinusoidal unconfined dynamic compression at 2.5% dynamic strain amplitude (in displacement feedback control) superimposed on a 7% static offset strain, using a non-porous polysulfone loading chamber and platens in an incubator-housed loading apparatus57. Free-swelling cultures over the same 24-hour incubation period served as controls. Throughout the applied compression, the total harmonic distortion of the resulting continuously measured dynamic load signal was < 25%. The applied cyclic displacement waveform and resulting load waveform were similar to that shown in ref. 37. Upon completion of loading, plugs were washed 3 × 20 minutes in PBS with 142 μg/mL sodium sulfate and 50 μg/mL L-proline to remove free radiolabel, and digested in 1mL Proteinase K (0.1 mg/mL, Roche) at 60°C overnight (~16 hours). Experiments were repeated using chondrocytes isolated from 1-6 animals (as noted below).
BIOCHEMICAL MEASUREMENTS
Radiolabel incorporation rates were measured by scintillation counting58,59. sGAG content in the proteinase K digests and collected medium was assayed by DMMB dye binding9. DNA was quantified by Hoechst 33258 dye-binding assay60.
DATA ANALYSIS
Radiolabel incorporation and sGAG content data were normalized to DNA content to account for disk-to-disk variations in cell number. Data were further normalized to averaged free-swell controls for each animal to account for baseline animal-to-animal variability. All data are shown as mean +/− 95% confidence interval (CI). The number of observations varied with treatment, as detailed in the figure legends. A Shapiro-Wilk test was used to test for normality of data. 1-way and 2-way ANOVA was used to test for the effect of treatment (mechanical and/or pharmacological) followed by post-hoc Bonferoni pairwise comparisons to correct for multiple comparisons from multiple treatments. A p-value < 0.05 was considered significant. Statistical analysis was performed using Systat 12 software (Richmond, CA).
Results
ACTIVITY AND TOXICITY OF PF001, PF002, PF003
RGD-conjugated comb copolymer surfaces that can promote binding by the αvβ3 or α5β1 integrins were used to confirm activity of PF001, PF002, and PF003 on chondrocytes. By day 6 in culture, untreated cells seeded onto RGD-conjugated surfaces showed a spread morphology, while cells treated with 100μM PF001, PF002, or PF003 remained rounded on the RGD-conjugated surfaces throughout the 7-day treatment (Supplementary Fig. 2S). Treatment with 1 μM echistatin served as an assay control. At concentrations up to 200μM of each compound over 7 days, there was no qualitative increase in ethidium bromide-stained cells (approximately 10%) (Fig. 2S) compared to untreated controls or quantitative changes in DNA content (Fig. 3S), suggesting no adverse effects on cell viability.
EFFECTS OF SMALL-MOLECULE BLOCKERS ON GAG BIOSYNTHESIS AND ACCUMULATION WITH DYNAMIC COMPRESSION OF AGAROSE GEL CULTURES
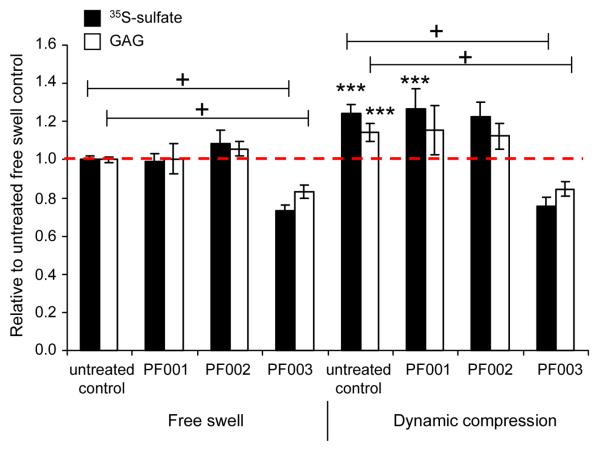
To test the role of integrin-ECM interactions in chondrocyte biosynthetic response to dynamic compression, agarose cultures were incubated with small-molecule peptidomimetics of the RGD binding sequence recognized by integrins such as αvβ3 and α5β1 to block these interactions and subjected to a 24-hour continuous, unconfined dynamic compression. At the end of the loading period, untreated free-swelling cultures incorporated an average of 192 pmol sulfate/μg DNA/hr and accumulated 7.38 μg GAG/μg DNA. Treatment with PF001, PF002, or PF003 did not alter DNA content (Supplementary Fig. 3S). DNA content was used to normalize data for all subsequent analyses. In free-swelling controls, PF001 and PF002 did not significantly change sulfate incorporation or sulfated GAG content, while PF003 (200μM) resulted in a 27% decrease in sulfate incorporation (p<0.0005 compared to untreated control using post-hoc Bonferoni pairwise comparison) and 17% decrease GAG content (p<0.0005 vs. untreated control) (Fig. 2). Free-swell levels for sulfate incorporation and GAG content under all treatment conditions can be found in Supplementary Materials (Figure 5S).
Figure 2.
Biosynthetic response of chondrocytes in agarose culture to 24 hours of dynamic compression (1.0 Hz, 2.5% amplitude) in the presence of small-molecule integrin blockers PF001, PF002, PF003 (200uM). Data were normalized by the averaged untreated free-swell control for each animal. Data shown as mean ± 95% confidence interval (CI), n = 6-19 disks from 2-6 animals (PF001 n=6, PF002 n=6, PF003 n=19). Sulfate incorporation as measured by radiolabel incorporation. Glycosaminoglycan (GAG) accumulation as measured by DMMB dye binding assay. *** p<0.0005 relative to free-swell, + p<0.0005 relative to untreated controls.
Consistent with previous studies10,11,39, low amplitude (<10% strain amplitude) dynamic compression at 1Hz frequency increased sulfate incorporation rates by approximately 25% (to 243 pmol sulfate/μg DNA/hr, p<0.0005 vs. free-swell control) after 24 hours of compression in untreated agarose gel plugs (Fig. 2). GAG loss to the medium was minimal: <10% of the total GAG content (i.e., agarose disk plus medium combined) or approximately 0.7μg GAG/μg DNA, was lost to the medium over 24 hours in free-swell culture. While GAG loss to medium increased by ~75% to 1.2 μg GAG/μg DNA with dynamic compression, possibly due to increased transport, enhanced synthesis caused a net increase in GAG accumulation within the disks after 24 hours of dynamic compression by ~14% (to 8.51 μg GAG/μg DNA, p<0.0005 vs. free-swell control) (Fig. 2). Since the experiment was conducted during early times in culture and GAG loss was minimal, the majority of the GAG content was newly synthesized and GAG accumulation mirrored sulfate incorporation trends.
PF001-treated samples responded to dynamic compression with similar increases in sulfate incorporation (27%, p<0.0005 compared to treated free-swell control in post-hoc Bonferoni comparison) and GAG accumulation (15%, p=0.089 vs. treated free-swell control, p=0.017 uncorrected) (Fig. 2). PF002-treated samples responded to dynamic compression with slight increases in sulfate incorporation (12%, p=0.16 vs. treated free-swell control, p=0.03 uncorrected) and GAG accumulation (6%). In contrast, PF003-treated samples showed no stimulation of sulfate incorporation or GAG accumulation with dynamic compression (Fig. 2). None of the treatments (PF001, PF002, or PF003) affected GAG loss to the medium. Taken together, markers of PG synthesis (sulfate incorporation and GAG content) were responsive to dynamic compression and sensitive to the integrin blocker PF003, the most selective inhibitor of αvβ3 function. A dose response experiment up to the efficacious concentration of 200 μM with PF003 was then conducted: PG synthesis response to dynamic compression was measured at 0, 100, 150, 200 μM PF003. Free-swelling disks showed a graded decrease in sulfate incorporation and GAG accumulation with increasing concentrations of PF003 (Fig. 4SA,B). Stimulation of PG synthesis (sulfate incorporation and GAG accumulation) by dynamic compression relative to free-swell also varied with increasing concentrations of PF003, with a slight (10%, p = 0.8 compared to treated free-swell in Bonferoni post-hoc pairwise comparison, p = 0.15 uncorrected) increase in sulfate incorporation and GAG accumulation at 100μM, and no stimulation by 150μM PF003 (Fig. 4S).
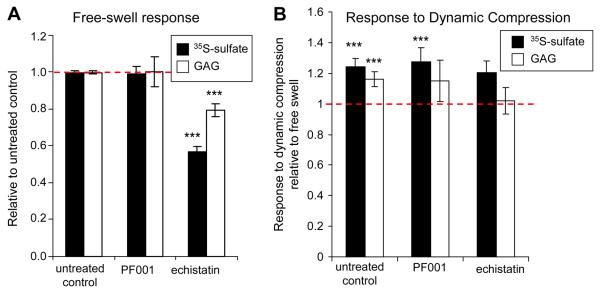
BROAD-SPECTRUM BLOCKERS PF001 AND ECHISTATIN SHOWED LITTLE EFFECT ON RESPONSE TO DYNAMIC COMPRESSION
Echistatin is a disintegrin containing the RGD-motif that has broad specificity to integrins51. In free swelling controls, echistatin decreased sulfate incorporation by 43% (p<0.0005 compared to untreated control in Bonferoni post-hoc pairwise comparison) and GAG content by 21% (p<0.0005 vs. untreated control) (Fig. 3A), and increased GAG loss to medium by 77%. In contrast, PF001 had no effect on radiolabel incorporation or GAG accumulation in free swelling conditions. With PF001 treatment, dynamic compression increased sulfate incorporation by ~20% (p<0.0005 compared to treated free-swell) and slightly increased GAG content (p = 0.071 vs. treated free-swell, p=0.02 uncorrected). In contrast, with echistatin treatment, dynamic compression increased sulfate incorporation in treated samples by ~20% (p=0.125, p = 0.032 uncorrected), and dynamic compression did not stimulate GAG accumulation (Fig. 3B). This may be due to GAG loss to medium, not seen in PF001-treated samples, since the total GAG content (agarose disk + medium) increased by ~9% after 24 hours dynamic compression in the presence of echistatin.
Figure 3.
Effects of broad spectrum integrin antagonists PF001 and echistatin in free-swelling culture (A) and on dynamic compression stimulation of agarose cultures (B). Data shown as mean ± 95%CI. (A) Sulfate incorporation and GAG content in free-swell relative to untreated controls. n = 6-51 samples from 2-11 animals (control n=51, PF001 n=6, echistatin n=7), ***p<0.0005 relative to untreated control. (B) Proteoglycan synthesis as indicated by sulfate incorporation and GAG content with dynamic compression relative to treated free-swell controls. n = 6-13 samples from 2-4 animals (control n=13, PF001 n=6, echistatin n=7), ***p<0.0005 relative to 1 (no change relative to free-swell).
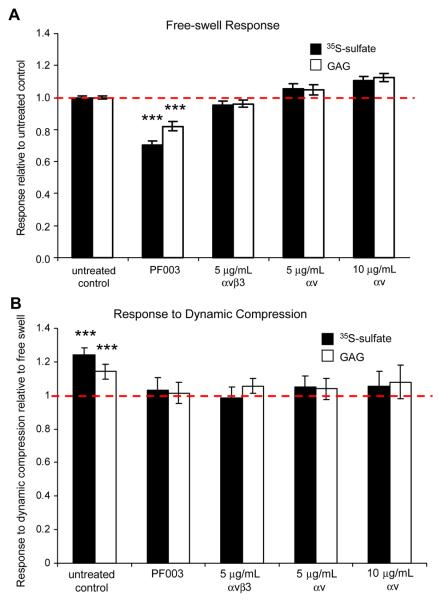
DIFFERENTIAL EFFECTS OF INTEGRIN BLOCKERS IN FREE-SWELLING CULTURE
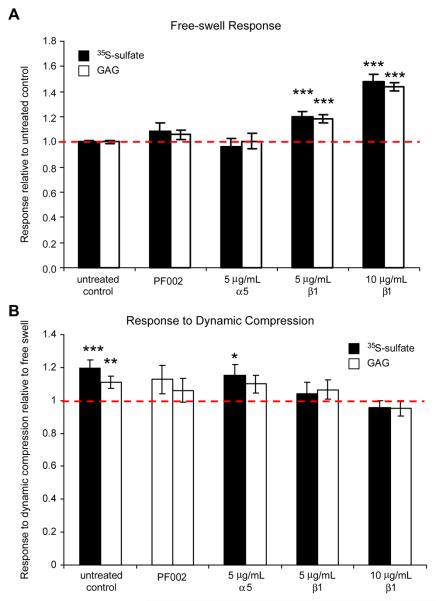
To further test the hypothesis that blocking specific integrin-ECM interactions can disrupt chondrocyte response to dynamic compression, a series of blocking antibodies were used in parallel experiments. Antibodies are larger in size (~150 kDa), but in agarose culture at early times before much accumulation of ECM, antibodies can still diffuse in relatively easily61,62. αvβ3 blocker PF003 decreased sulfate incorporation by approximately 30% (p<0.0005 compared to untreated control by Bonferoni pairwise comparisons) and GAG accumulation by 18% (p<0.0005 vs. untreated control) in free-swelling culture (Fig. 4A). αvβ3 blocking antibody clone LM609 showed a slight but non-significant decrease in sulfate incorporation at 5 μg/mL (Fig. 4A). αv blocking antibodies showed no effects on sulfate incorporation, GAG accumulation, or GAG loss in free-swelling culture (Fig. 4A). In contrast, α5β1 blocker PF002 showed a slight increase in sulfate incorporation (9%) and GAG accumulation (6%) under free swelling conditions (Fig. 5), and blocking antibodies to β1 (5 μg/mL, 10 μg/mL) integrins increased sulfate incorporation rates, and GAG accumulation by 20%-40% (p<0.0005, vs. untreated control) (Fig. 5A). None of the blocking antibodies had significant affects on GAG loss to the medium.
Figure 4.
Effects of blocking αvβ3 integrins on biosynthetic behavior in free-swelling culture and in response to dynamic compression. Data shown as mean ± 95%CI. (A) Sulfate incorporation and GAG content in free-swelling agarose cultures relative to untreated controls. n = 3-51 samples from 1-11 animals (control n=51, PF003 n=29, αvβ3 n=16, 5 μg/mL αv n=12, 10μg/mL αv n=3), ***p<0.0005 relative to untreated control. (B) Proteoglycan synthesis (sulfate incorporation and GAG accumulation) after 24 hours of dynamic compression relative to respective treated free- swell controls. n = 3-19 samples from 1-6 animals (control n=19, PF003 n=19, αvβ3 n=3, 5 μg/mL αv n=9, 10μg/mL αv n=3), ***p<0.0005 relative to 1 (no change compared to free-swell).
Figure 5.
Effects of blocking α5β1 integrins on agarose cultures in free-swelling culture and in response to dynamic compression. Data shown as mean ± 95%CI. (A) Sulfate incorporation and GAG content in free-swelling agarose cultures relative to untreated controls. n = 3-51 samples from 1-11 animals (control n=51, PF002 n=6, α5 n=6, 5 μg/mL β1 n=6, 10μg/mL β1 n=3), ***p<0.0005 relative to untreated controls. (B) Proteoglycan synthesis (sulfate incorporation and GAG content) response after 24 hours of dynamic compression relative to respective treated free-swell controls. n = 3-12 samples from 1-4 animals (control n=12, PF002 n=6, α5 n=6, 5 μg/mL β1 n=6, 10μg/mL β1 n=3). ***p<0.0005, **p=0.001, *p=0.023 relative to 1 (no change compared to free-swell).
αvβ3 AND β1 BLOCKING ANTIBODIES, NOT PF002 OR α5 BLOCKING ANTIBODIES, ABOLISHED PG-SYNTHESIS RESPONSE TO COMPRESSION
In untreated samples, dynamic compression increased sulfate incorporation rates by approximately 20% (p<0.0005 compared to free-swell control by Bonferoni post-hoc comparison) and GAG accumulation by 14% (p<0.0005 vs. free-swell control). Treatment with PF003, αvβ3 blocking antibodies, and αv blocking antibodies abrogated this response to dynamic compression (p<0.0005 by ANOVA) (Fig. 4B). In separate experiments with PF002 and α5 blocking antibody-treated samples, dynamic compression resulted in a 12-15% increase in sulfate incorporation (p=0.079, 0.023, respectively vs. treated free-swell controls, p = 0.011, .006 uncorrected), while GAG accumulation increased by only 5-10% (Fig. 5B). β1 blocking antibody-treated samples showed no stimulation of sulfate incorporation or GAG accumulation by dynamic compression (Fig. 5B). None of these effects described were due to changes in GAG loss to medium. While blockers of αvβ3 and β1 integrins both appear to abrogate the response to dynamic compression, they appear to be acting through distinct pathways considering their opposing effects in free-swelling culture.
Discussion
Dynamic compression and other mechanical stimuli have been increasingly used in tissue engineering to promote development of cartilage constructs through increasing extracellular matrix content and mechanical properties37,38, and the use of immature chondrocytes in tissue engineering and cartilage repair has been shown to be an effective cell source for tissue engineering, with greater activity than adult chondrocytes63-65. Even at early times in culture, when little pericellular matrix is present, chondrocyte cultures in agarose can respond to dynamic compression with increased sulfate incorporation and sGAG accumulation10,11,39. This response to 24 hours of continuous dynamic compression increased with number of days in free-swell culture10. In long-term studies of the effects of dynamic loading, the presence of a pre-elaborated pericellular matrix (either by seeding chondrons initially or culturing for 2 weeks prior to loading) did not alter the stimulatory effects of extended dynamic loading, which increased with loading duration37. This suggests that interactions between the cell and its surrounding matrix developed during dynamic compression may play a greater role than pre-existing interactions. The goal of this study was to examine the role of integrin-ECM interactions in the response of chondrocytes to dynamic compression at early times in culture using a 3D agarose culture of immature bovine chondrocytes as a model system. An added benefit of studying such interactions at early times is the ability to compare multiple integrin blockers, including antibodies, without the complicating issue of diffusion and penetration of antibodies into a dense tissue matrix. The results of this study suggest that multiple integrins (β1, αvβ3) appear to play a role in mechanotransduction and the chondrocyte's ability to sense its local microenvironment; however these integrins appear to play opposing or complementary roles.
In the present study, blocking β1 integrin function with blocking antibodies, or blocking αvβ3 integrins with either small-molecule antagonists or blocking antibodies, abolished proteoglycan stimulation by dynamic compression (as measured by sulfate incorporation or sGAG accumulation). While the concentration of the small-molecule antagonists used in these functional assays were much higher than the IC50s reported in Table 1, previous studies have confirmed the observation that higher concentrations are necessary to see functional response, especially in 3-D culture models 66. Our results using blocking antibodies support the specificity of these small-molecules as well. As previously shown10,11,39, 24 hour continuous unconfined dynamic compression stimulated proteoglycan synthesis at days 1-2 in culture. Measurable amounts of sGAG were accumulated in constructs by the end of culture. Previous studies have also shown that a pericellular matrix begins developing within 4 hours after isolation67 and can be visualized at the cell surface on day 2 in agarose culture68. The β1 integrin subunit can associate with a large number of differentially expressed alpha subunits to form integrins with distinct ligand binding and cell signaling characteristics. Echistatin and the RGD peptidomimetics used in this study are expected to inhibit only the subset of β1-containing integrins that interact with their ligands via the RGD sequence. αvβ3 and α5β1 are both RGD-recognizing integrins. While the main binding partners for αvβ3 and α5β1 are vitronectin and fibronectin, respectively, αvβ3 has also been shown to bind to fibronectin, fibrinogen, osteopontin, and collagen69. Blocking α5β1 specifically using the RGD peptidomimetic PF002 or α5 blocking antibodies may partially decrease proteoglycan stimulation by dynamic compression, but this effect was not significant. Other potential β1 integrins include collagen receptors α1β1 and α2β1, as well as α3β1 and α10β1.
While blocking both αvβ3 and β1 integrins prevented stimulation of proteoglycan synthesis by dynamic compression, these results appeared to be acting through independent mechanisms. In this study, blocking β1 integrins in free-swelling culture with blocking antibodies resulted in a significant upregulation of sulfate incorporation, while blocking αvβ3 integrins with small-molecule compounds resulted in a down regulation. Blocking αvβ3 integrins with antibodies resulted in no detectable change in basal sulfate incorporation. Previous studies have suggested that αvβ3 and α5β1 have modulating roles in articular chondrocytes56. In those studies, treatment with anti-α5β1 antibody JBS5 induced a pro-inflammatory response (upregulation of NO, PGE2, IL-6, IL-8, and IL-1β) in both normal and osteoarthritic cartilage as well as bovine articular chondrocytes, while treatment with αvβ3 blocking antibody LM609 decreased these pro-inflammatory signals and could regulate the α5β1 response in a dominant-negative fashion56. Other studies demonstrated similar responses when blocking with anti-α5β1 antibodies or treating with fibronectin fragments23. In addition, studies have shown that blocking α1β1 and α2β1 with blocking antibodies can stimulate MMP-13 production through the MAP kinase pathway similar to treatment with α5β1 blocking antibodies or fibronectin fragments23,70, although their roles in mechanotransduction had not previously been investigated to our knowledge. These studies demonstrate that the use of blocking antibodies can induce cellular responses by disrupting integrin signaling or causing abnormal signaling. While the stimulation of PG synthesis by blocking β1 integrins in agarose culture might appear to be in contradiction to these previous studies, it is possible that the stimulation observed, here, is part of increased turnover that has been described previously with hyaluronan (HA) oligosaccharide treatment of cartilage71. In addition, only blockers of β1 integrins that induced an increase in biosynthesis in free-swelling controls resulted in abolishment of the response to dynamic compression, and the effects of blocking antibodies were distinct from that of small-molecule antagonists, which likely block integrin interactions through different mechanisms. Taken together, these studies suggest that blocking β1 integrins with blocking antibodies may decrease response to dynamic compression through a pro-inflammatory pathway or an analogous pathway resulting from abnormal integrin signaling.
Consistent with previous studies, we observed that treatment with different types of αvβ3 blockers can result in distinctly different free-swelling responses56. However, all specific blockers of αvβ3 integrins affected stimulation of proteoglycan synthesis by dynamic compression. Thus, blocking αvβ3 appears to be inhibiting a signaling response directly resulting from dynamic compression, independent of effects in free-swelling culture. Finally, our observation that treatment with relatively broad-spectrum blockers (PF001 and echistatin) that target RGD-binding integrins, and which bind to α5β1 and αvβ3 with similar affinities, did not affect stimulation of proteoglycan synthesis by dynamic compression, suggests a modulatory role for α5β1 and αvβ3 as seen in previous studies56.
It is important to note that previous studies of the role of α5β1 integrins in chondrocyte mechanotransduction focused on chondrocyte monolayer cultures23, 31-35. The present study utilizes 3D gel culture, and the results in 3D suggest a more complex view in which multiple integrins may be playing a role in regulating chondrocyte response to compression within the gel construct. This hypothesis is consistent with research on the role of ion channel signaling, where more complex interactions were observed in 3D compared to 2D culture43. A variety of mechanical signals are present during physiological (3D) dynamic compression (e.g., fluid flow, pressure gradients, streaming potentials, deformation)4-6. Even at early times in culture, the presence of proteoglycans would contribute to streaming potentials and osmotic gradients, while cell and pericellular matrix deformation72 and fluid flow may affect receptor- extracellular matrix (ECM) interactions. With the multitude of signals, it is conceivable that individual ligand-surface receptor interactions may be sensing different mechanical signals, mechanisms for which are beginning to be elucidated73. Finally, further studies on downstream signaling events may shed light on how upstream mechanical signals may interact, and allow for better understanding as to how mechanical stimulation may be utilized to optimize ECM development in cartilage tissue engineering applications.
Supplementary Material
Acknowledgements
Funded in part by NIH/NIAMS Grant AR33236 and Pfizer, Inc, and Graduate Student Fellowships from NSF and NDSEG.
Footnotes
Conflict of interest statement
E.C.A. is a former employee and stockholder of Pfizer, Inc.; D.W.G. is an employee and stockholder of Pfizer, Inc.
References
- 1.Li G, Van de Velde SK, Bingham JT. Validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J Biomech. 2008;41(7):1616–22. doi: 10.1016/j.jbiomech.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986 May;83(9):2879–83. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herberhold C, Faber S, Stammberger T, Steinlechner M, Putz R, Englmeier KH, et al. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J Biomech. 1999 Dec;32(12):1287–95. doi: 10.1016/s0021-9290(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 4.Frank EH, Grodzinsky AJ. Cartilage electromechanics--II. A continuum model of cartilage electrokinetics and correlation with experiments. J Biomech. 1987;20(6):629–39. doi: 10.1016/0021-9290(87)90283-1. [DOI] [PubMed] [Google Scholar]
- 5.Mak AF. Unconfined Compression of Hydrated Viscoelastic Tissues - a Biphasic Poroviscoelastic Analysis. Biorheology. 1986;23(4):371–83. doi: 10.3233/bir-1986-23406. [DOI] [PubMed] [Google Scholar]
- 6.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–94. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 7.Jurvelin J, Kiviranta I, Saamanen AM, Tammi M, Helminen HJ. Partial restoration of immobilization-induced softening of canine articular cartilage after remobilization of the knee (stifle) joint. J Orthop Res. 1989;7(3):352–8. doi: 10.1002/jor.1100070307. [DOI] [PubMed] [Google Scholar]
- 8.Behrens F, Kraft EL, Oegema TR., Jr Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7(3):335–43. doi: 10.1002/jor.1100070305. [DOI] [PubMed] [Google Scholar]
- 9.Sah RLY, Kim YJ, Doong JYH, Grodzinsky AJ, Plaas AHK, Sandy JD. Biosynthetic Response of Cartilage Explants to Dynamic Compression. J Orthopaed Res. 1989 SEP;7(5):619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 10.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995 Apr;108(Pt 4):1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 11.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997 Mar;15(2):181–8. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 12.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39(8):1489–97. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Kiviranta I, Jurvelin J, Tammi M, Saamanen AM, Helminen HJ. Weight bearing controls glycosaminoglycan concentration and articular cartilage thickness in the knee joints of young beagle dogs. Arthritis Rheum. 1987 Jul;30(7):801–9. doi: 10.1002/art.1780300710. [DOI] [PubMed] [Google Scholar]
- 14.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6(6):777–92. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 15.Caterson B, Lowther DA. Changes in Metabolism of Proteoglycans from Sheep Articular-Cartilage in Response to Mechanical-Stress. Biochim Biophys Acta. 1978;540(3):412–22. [Google Scholar]
- 16.Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994 May 15;311(1):1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 17.Fanning PJ, Emkey G, Smith RJ, Grodzinsky AJ, Szasz N, Trippel SB. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem. 2003 Dec 19;278(51):50940–8. doi: 10.1074/jbc.M305107200. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004 May 7;279(19):19502–11. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 19.De Croos JN, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006 Aug;25(6):323–31. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ. Shear- and Compression-induced Chondrocyte Transcription Requires MAPK Activation in Cartilage Explants. J Biol Chem. 2008 Mar 14;283(11):6735–43. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 21.Kurtis MS, Schmidt TA, Bugbee WD, Loeser RF, Sah RL. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 2003 Jan;48(1):110–8. doi: 10.1002/art.10704. [DOI] [PubMed] [Google Scholar]
- 22.Salter DM, Hughes DE, Simpson R, Gardner DL. Integrin expression by human articular chondrocytes. Br J Rheumatol. 1992 Apr;31(4):231–4. doi: 10.1093/rheumatology/31.4.231. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002 Sep;46(9):2368–76. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 24.Lucchinetti E, Bhargava MM, Torzilli PA. The effect of mechanical load on integrin subunits alpha5 and beta1 in chondrocytes from mature and immature cartilage explants. Cell Tissue Res. 2004 Mar;315(3):385–91. doi: 10.1007/s00441-003-0836-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Kim EJ, Kim YH, Hahn SB, Lee JW. The modulation of integrin expression by the extracellular matrix in articular chondrocytes. Yonsei Med J. 2003 Jun 30;44(3):493–501. doi: 10.3349/ymj.2003.44.3.493. [DOI] [PubMed] [Google Scholar]
- 26.Lapadula G, Iannone F, Zuccaro C, Grattagliano V, Covelli M, Patella V, et al. Integrin expression on chondrocytes: correlations with the degree of cartilage damage in human osteoarthritis. Clin Exp Rheumatol. 1997 May-Jun;15(3):247–54. [PubMed] [Google Scholar]
- 27.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004 Mar;32(3):435–46. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 28.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002 Jan;59(1):36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26(1):1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- 30.Kurtis MS, Tu BP, Gaya OA, Mollenhauer J, Knudson W, Loeser RF, et al. Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res. 2001 Nov;19(6):1122–30. doi: 10.1016/S0736-0266(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 31.Pulai JI, Del Carlo M, Jr., Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002 Jun;46(6):1528–35. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 32.Millward-Sadler SJ, Wright MO, Lee H, Nishida K, Caldwell H, Nuki G, et al. Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in human articular chondrocytes. J Cell Biol. 1999 Apr 5;145(1):183–9. doi: 10.1083/jcb.145.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millward-Sadler SJ, Wright MO, Davies LW, Nuki G, Salter DM. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000 Sep;43(9):2091–9. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Al-Jamal R, Salter DM. Activation of Integrin-RACK1/PKCalpha signalling in human articular chondrocyte mechanotransduction. Osteoarthritis Cartilage. 2002 Nov;10(11):890–7. doi: 10.1053/joca.2002.0842. [DOI] [PubMed] [Google Scholar]
- 35.Orazizadeh M, Lee HS, Groenendijk B, Sadler SJ, Wright MO, Lindberg FP, et al. CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res Ther. 2008 Jan 10;10(1):R4. doi: 10.1186/ar2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holledge MM, Millward-Sadler SJ, Nuki G, Salter DM. Mechanical regulation of proteoglycan synthesis in normal and osteoarthritic human articular chondrocytes--roles for alpha5 and alphaVbeta5 integrins. Biorheology. 2008;45(34):275–88. [PubMed] [Google Scholar]
- 37.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41(34):223–37. [PubMed] [Google Scholar]
- 38.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004 May;37(5):595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch Biochem Biophys. 2003 Sep 1;417(1):105–11. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 40.Demarteau O, Wendt D, Braccini A, Jakob M, Schafer D, Heberer M, et al. Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem Biophys Res Commun. 2003 Oct 17;310(2):580–8. doi: 10.1016/j.bbrc.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 41.Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of Cartilaginous ECM Gene Transcription by Chondrocytes and MSCs in 3D Culture in Response to Dynamic Loading. Biomech Model Mechanobiol. 2006 May 12; doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 42.Pingguan-Murphy B, Lee DA, Bader DL, Knight MM. Activation of chondrocytes calcium signalling by dynamic compression is independent of number of cycles. Arch Biochem Biophys. 2005 Dec 1;444(1):45–51. doi: 10.1016/j.abb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Mouw JK, Imler SM, Levenston ME. Ion-channel Regulation of Chondrocyte Matrix Synthesis in 3D Culture Under Static and Dynamic Compression. Biomech Model Mechanobiol. 2006 Jun 10; doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- 44.Ragan PM, Chin VI, Hung HH, Masuda K, Thonar EJ, Arner EC, et al. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000 Nov 15;383(2):256–64. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 45.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shannon KE, Keene JL, Settle SL, Duffin TD, Nickols MA, Westlin M, et al. Anti-metastatic properties of RGD-peptidomimetic agents S137 and S247. Clin Exp Metastasis. 2004;21(2):129–38. doi: 10.1023/b:clin.0000024764.93092.5f. [DOI] [PubMed] [Google Scholar]
- 47.Irvine DJ, Mayes AM, Griffith LG. Nanoscale clustering of RGD peptides at surfaces using Comb polymers. 1. Synthesis and characterization of Comb thin films. Biomacromolecules. 2001 Spring;2(1):85–94. doi: 10.1021/bm005584b. [DOI] [PubMed] [Google Scholar]
- 48.Kuhlman W, Taniguchi I, Griffith LG, Mayes AM. Interplay between PEO tether length and ligand spacing governs cell spreading on RGD-modified PMMA-g-PEO comb copolymers. Biomacromolecules. 2007 Oct;8(10):3206–13. doi: 10.1021/bm070237o. [DOI] [PubMed] [Google Scholar]
- 49.Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, Griffith LG. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci. 2002 Apr 1;115(Pt 7):1423–33. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- 50.kindly provided by Maria Ufret from the Griffith Lab at MIT
- 51.Pfaff M, McLane MA, Beviglia L, Niewiarowski S, Timpl R. Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins alpha IIb beta 3, alpha V beta 3 and alpha 5 beta 1 and in cell adhesion inhibition. Cell Adhes Commun. 1994 Dec;2(6):491–501. doi: 10.3109/15419069409014213. [DOI] [PubMed] [Google Scholar]
- 52.Duque H, LaRocco M, Golde WT, Baxt B. Interactions of foot-and-mouth disease virus with soluble bovine alphaVbeta3 and alphaVbeta6 integrins. J Virol. 2004 Sep;78(18):9773–81. doi: 10.1128/JVI.78.18.9773-9781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H936–45. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 54.Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta -induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001 Nov 23;276(47):44173–8. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genes NG, Rowley JA, Mooney DJ, Bonassar LJ. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Archives of Biochemistry and Biophysics. 2004 FEB 15;422(2):161–7. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 56.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J Immunol. 2000 Mar 1;164(5):2684–91. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- 57.Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000 Nov;33(11):1523–7. doi: 10.1016/s0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 58.Hascall VC, Handley CJ, McQuillan DJ, Hascall GK, Robinson HC, Lowther DA. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–23. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 59.Sah RL, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991 Apr;286(1):20–9. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 60.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988 Oct;174(1):168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 61.Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004 Aug 15;70(2):397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- 62.De Rosa E, Urciuolo F, Borselli C, Gerbasio D, Imparato G, Netti PA. Time and space evolution of transport properties in agarose-chondrocyte constructs. Tissue Eng. 2006 Aug;12(8):2193–201. doi: 10.1089/ten.2006.12.2193. [DOI] [PubMed] [Google Scholar]
- 63.Hidaka C, Cheng C, Alexandre D, Bhargava M, Torzilli PA. Maturational differences in superficial and deep zone articular chondrocytes. Cell Tissue Res. 2006 Jan;323(1):127–35. doi: 10.1007/s00441-005-0050-y. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y, Adkisson HD, Bogdanske J, Kalscheur V, Maloney W, Cheung R, et al. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. J Knee Surg. 2005 Jan;18(1):31–42. doi: 10.1055/s-0030-1248155. [DOI] [PubMed] [Google Scholar]
- 65.Feder J, Adkisson H, Kizer N, Hruska K, Cheugn R, Grodzinsky A, et al. The promise of chondral repair using neocartilage. In: Sandell L, Grodzinsky A, editors. Tissue Engineering in Musculoskeletal Clinical Practice. Amer Acad Orthop Surg; Rosemont: 2004. pp. 219–26. [Google Scholar]
- 66.Engleman VW, Nickols GA, Ross FP, Horton MA, Griggs DW, Settle SL, et al. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest. 1997 May 1;99(9):2284–92. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldberg RL, Toole BP. Pericellular coat of chick embryo chondrocytes: structural role of hyaluronate. J Cell Biol. 1984 Dec;99(6):2114–22. doi: 10.1083/jcb.99.6.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimicco MA, Kisiday JD, Gong H, Grodzinsky AJ. Structure of pericellular matrix around agarose-embedded chondrocytes. Osteoarthritis Cartilage. 2007 Oct;15(10):1207–16. doi: 10.1016/j.joca.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 69.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 70.Ronziere MC, Aubert-Foucher E, Gouttenoire J, Bernaud J, Herbage D, Mallein-Gerin F. Integrin alpha1beta1 mediates collagen induction of MMP-13 expression in MC615 chondrocytes. Biochim Biophys Acta. 2005 Oct 30;1746(1):55–64. doi: 10.1016/j.bbamcr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. 2000 May;43(5):1165–74. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 72.Knight MM, Ghori SA, Lee DA, Bader DL. Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Med Eng Phys. 1998 Nov-Dec;20(9):684–8. doi: 10.1016/s1350-4533(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 73.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1042–6. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.