Abstract
Background
There is no current method to precisely assess pruritus despite its importance as a major symptom in many skin diseases. Pruritus induces scratching that worsens various inflammatory skin diseases.
Objective
The purpose of this study was to determine the effects of scratching on allergic skin reactions using murine contact hypersensitivity (CH) as a model and to assess classical “anti-pruritic” agents using this model.
Methods
We utilized plastic collars which were placed around the necks of mice to prevent them from scratching their ears during the development of CH. This allowed us to assess ear swelling as an index of CH, obviating the effects of scratching that occurs during the development of CH.
Results
Following elicitation, the ear swelling of these “collared” mice was decreased by approximately 50%, compared to control mice in which collars were not used, suggesting that scratching contributes to the ear swelling that is measured as an index of CH. Using this model, we assessed the anti-pruritic effects of antihistamines, corticosteroids, non-steroidal antiinflammatory and sedative agents. All agents decreased CH when collars were not used. When collars were used, all agents, other than the sedatives, appeared to suppress CH, indicating their antiinflammatory effects. Sedative agents did not decrease CH in collared mice, indicating that their inhibitory effects in CH may be entirely due to their sedative effects.
Conclusions
This model enables the dissection of the various elements assessed when measuring CH in mice and may provide a simple tool to assess or screen potential anti-pruritic agents.
Keywords: Scratch, Pruritus, Contact hypersensitivity
1. Introduction
There is no current method to precisely assess pruritus, despite its being a prominent symptom in many skin diseases [1] such as atopic dermatitis, psoriasis, contact dermatitis, prurigo, or in systemic diseases such as diabetes, cirrhosis or chronic renal failure [1]. Pruritus is a symptom that affects quality of life both physically and psychologically and, as a consequence of scratching, aggravates preexisting skin eruptions. Considerable effort has been expended to assess pruritus in order to better understand mechanisms involved as well as to develop better anti-pruritic agents. Since pruritus is a perception, verbal itch score [2] or visual analogue scales that were originally developed for assessing pain sensation [3] have limited value because of their subjectivity. As well, since pruritus is, by definition, almost inexorably associated with scratching, various measures of scratching have been developed such as a sensitive limb movement meter [4], or videotaping the movements of patients during sleep [5]. Assessing scratching behavior is certainly an objective method, but analyzing hours of records along with the limited availability of such expensive automated recoding systems [6,7], remain a challenge.
Many antiinflammatory agents have been developed for clinical use (such as antihistamines, glucocorticosteroids, and others) and their anti-pruritic properties have been objectively evaluated using animal models [8,9]. In this study, we have developed a new, simple method to assess the effect of various agents on the scratching behavior in mice. In this assay, scratching is assessed as a physiological skin response using a murine model of contact hypersensitivity (CH). We utilized light-weight plastic collars which were placed around the necks of mice to prevent them from scratching or grooming their ears during the development of CH. The effects of scratching were assessed on the ear swelling assay of CH. Various agents with known sedative and/of anti-pruritic properties were also assessed using this assay.
2. Materials and methods
2.1. Reagents and monoclonal antibodies
Monoclonal antibodies (mAb) to CD16/CD32 (Fcγ III/II receptor), CD45 and MHC class II (MHC II) either unconjugated or conjugated with fluorescein isothiocyanate or with allophycocyanin were purchased from BD Bioscience Pharmingen (San Diego, CA). Propidium iodide, deoxyribonuclease I (DNase I), ammonium thiocyanate, olive oil, chlorpheniramine, prednisolone, aspirin, and phenobarbital were purchased from SIGMA–Aldrich (St. Louis, MO). Hexadecyl trimethylammonium bromide and o-dianisidine were purchased from Sigma Chemical Co. (St. Louis, MO).
Sodium phosphate and potassium phosphate were purchased from ICN Biomedicals Inc. (Aurora, OH). Acetone was purchased from Mallincrodt Baker Inc. (Paris, KY). Trinitrochlorobenzene (TNCB) was purchased from Polyscience Inc. (Warrington, PA). 1× phosphate buffered saline (PBS) and 1× Hank’s balanced salt solution (HBSS) and fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Woodland, CA). Trypsin was purchased from USB Corporation (Cleveland, OH). 0.5 M EDTA was purchased from Quality Biological Inc. (Gaithersburg, MD).
2.2. Animals and protocols for contact hypersensitivity
BALB/c mice (7–12-week old) were purchased from the National Cancer Institute Frederick Cancer Research and Developmental Center (Frederick, MD). On day 0, the right ear or shaved abdomen of each mouse was sensitized with 3% TNCB solution in a vehicle (1:4 of acetone:olive oil) of 12 µl (ear) or of 100 µl (abdomen), respectively. On day 6, the left ear of the sensitized mouse was painted with a total of 20 µl of 1% TNCB solution (in 4:1 of acetone:olive oil) and ear swelling was measured using a dial thickness gauge at 24 h after elicitation. (Mice were sensitized on the right ears except for those used in experiments where intact unchallenged ears were needed for assays as negative controls.) Non-sensitized mice not painted or painted with vehicle alone served as negative controls. In these experiments, light plastic weigh boats were utilized as collars and were placed around the neck to prevent mice from scratching or grooming their ears at the time of sensitization or elicitation of CH. These types of collars had been used in prior studies that assessed early and late activation events in CH (unpublished data, S. Katz). In some experiments, chlorpheniramine (60 mg/kg), prednisolone (40 mg/kg), aspirin (200 mg/kg) and phenobarbital (90 mg/kg) was administered intraperitoneally at the time of or just after the elicitation phase of CH. The animal protocol has been approved by IRB of NIH.
2.3. Preparation of epidermal single cell suspensions and flow cytometric analysis
Split ears of mice were floated dermal side down on 0.5% trypsinized PBS and incubated for 45 min at 37 °C. The epidermis was then gently detached from the dermis, and put into HBSS with 5% FBS and 0.025% DNase I. Epidermal cell suspensions were obtained by pipetting and filtering the epidermis through 100-µm nylon mesh. Epidermal Langerhans cells (LC) were enriched using a lympholyte M-density gradient and were washed once with PBS containing 5% FBS and 2 mM EDTA before further procedures. Cells prepared as above were Fc-blocked with CD16/CD32 mAb for 10 min and incubated with various antibodies for 20 min at 4 °C. Cells were then washed twice and were analyzed using a Becton Dickinson FACS Calibur flow cytometer and CELLQuest software version 3.3 (San Jose, CA).
2.4. Preparation of epidermal sheets
Epidermis was gently detached from the dermis after incubation of split ears with 0.038% ammonium thiocyanate solution in 0.05 M sodium/potassium phosphate buffer for 20 min at 37 °C. After acetone fixation for 10 min at −20 °C, epidermal sheets were incubated with anti-MHC II mAb for 30 min at room temperature, washed thrice by PBS, then incubated with goat anti-mouse serum conjugated with FITC for 30 min at room temperature. The epidermal sheets were observed through a Nikon Eclipse TE300 fluorescent microscope (Tokyo, Japan), and images were captured by IPLab version 3.6.3 (Scanalytics Inc., Fairfax, VA).
2.5. Assessing myeloperoxidase (MPO) activity in situ
Dermal infiltration with polymorphonuclear cells (PMN) was assessed by tissue MPO activity in situ as previously described [10,11]. A six millimeters punch biopsy was used to punch a hole through both the challenged (elicited) and control ears of each mouse. These tissues were obtained at 6, 12 and 24 h after elicitation and were then weighed. Ear swelling was determined by subtracting the weight of the control ear from that of challenged ear in each mouse. These punched out ears were then homogenized in a 50 mM potassium phosphate, pH 6.0 buffer containing 0.5% hexadecyl trimethylammonium bromide using a FastPrep® homogenizer (GMI Inc., Ramsey, MN), and the collected supernatants were assessed for MPO activity.
2.6. Statistical analysis
Statistical analyses were performed using CA-Cricket Graph III ver. 1.5.3 (Computer Associates Intern. Inc., Islandia, NY) and Microsoft Excel 2001 (Microsoft corp., Seattle, WA), and p-values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. Scratching contributes to the activation of epidermal LC
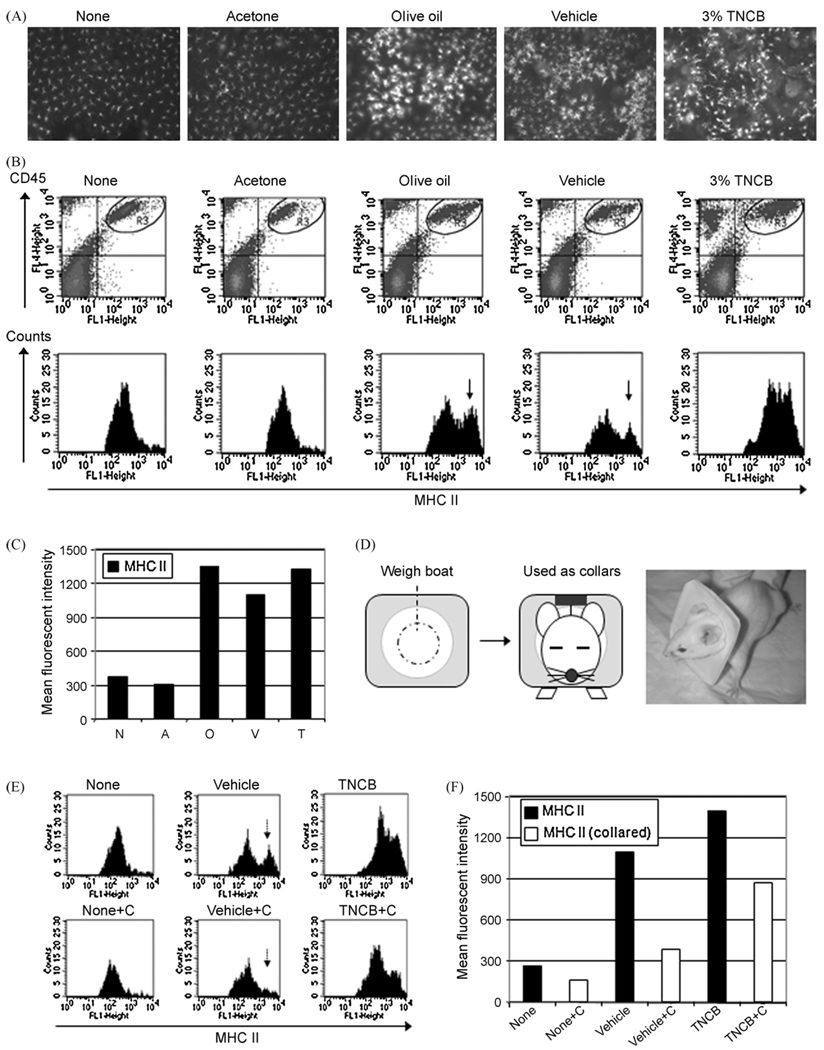
Epidermal sheets were examined 24 h after painting either 3% TNCB solution, vehicle [acetone plus olive oil (1:4)], acetone or olive oil (alone). Clusters of large sheets of MHC IIhigh dendritic cells were evident in mice painted with TNCB, olive oil, and vehicle whereas non painted and acetone (alone) painted ears did not show such MHC II upregulation (Fig. 1A). In flow cytometric analysis using epidermal cell suspensions, CD45+ MHC IIhigh (LC) were identified in mice painted with olive oil, vehicle and 3% TNCB, but not with acetone alone (Fig. 1B and C). Plastic collars were used around the neck to prevent mice from scratching their ears (Fig. 1D). The use of collars completely diminished LC upregulation of MHC II in ears of mice treated with vehicle and partially diminished MHC II expression in TNCB-painted ears (Fig. 1E and F).
Fig. 1.
Scratching behavior contributes to the activation of epidermal LC. Activated MHC IIhigh LC were observed at 24 h after painting in the epidermal sheets from mice painted with olive oil, vehicle (acetone + olive oil) and 3% TNCB, but not with acetone alone (A). Note that the appearance of the MHC IIhigh LC population (B, arrows) and (C) high MHCII-mean fluorescence intensity (MFI) of LC in mice painted with olive oil or vehicle or TNCB. A light-weight plastic collar was placed around the neck to prevent mice from scratching ears (D). LC exhibited high MHC II in mice painted with vehicle (E), but use of collars diminished the LC upregulation of MHC II in vehicle-painted mice (F). MHC II: MHC class II, MFI: mean fluorescent intensity, C: use of collars.
3.2. Scratching contributes to the expression of CH in mice
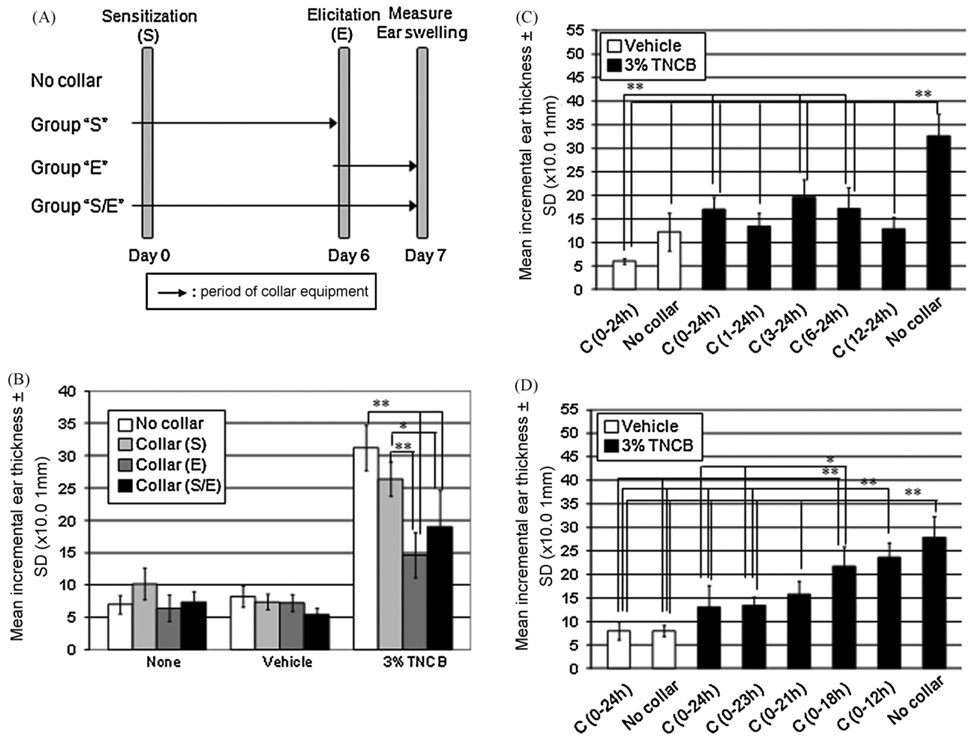
The effects of collars were examined on sensitization and/or elicitation of CH as shown (Fig. 2A). Ear swelling of CH significantly decreased when collars were used during the elicitation phase of CH (Fig. 2B). Use of collars during the last 12 h, but not during the first 12 h, of the 24 h after elicitation appeared more critical to demonstrate the inhibitory effects on ear swelling of CH (Fig. 2C and D).
Fig. 2.
Scratching behavior contributes to the ear swelling of CH. Collars were used during the sensitization and/or elicitation of CH to prevent mice from scratching the ears (A). Ear swelling of CH significantly decreased when collars were used during the elicitation phase (B). Applying the collars during the last 12 h, of the 24 h after elicitation significantly inhibited ear swelling(C). The longer the collars were used in the elicitation phase the greater the suppression of CH (D). S: sensitization, E: elicitation, S/E: both sensitization and elicitation, h: hour(s), C: use of collars, SD: standard deviation. Note that some description of statistical analysis, such as those between vehicle-treated group and sensitized group, were omitted in graph 2B for clarity. **p < 0.01, *p < 0.05.
3.3. Phenobarbital decreases CH in control mice, but not that in collared mice
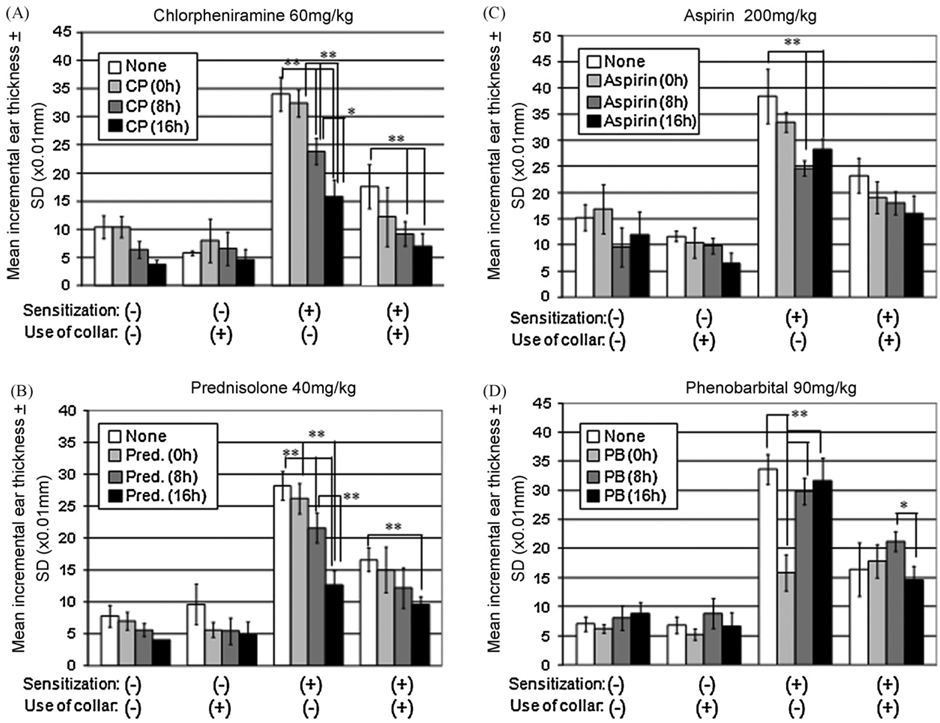
Various agents with possible anti-pruritic properties were assessed for their effects on the ear swelling response using control non-collared sensitized mice and collared mice. Use of each of these agents decreased CH when collars were not used (Fig. 3A–D). In contrast to the effects of other agents, phenobarbital was effective only when used at the time of elicitation (0 h). In addition, when phenobarbital was administered, there was no decrease in ear swelling in the collared mice (Fig. 3A–C).
Fig. 3.
Inhibition of CH by various agents. Various agents were administrated intraperitoneally immediately before (at 0 h), 8 and 16 h after elicitation, and ear swelling were measured at 24 h after elicitation. The administration of chlorpheniramine (A), prednisolone (B), and aspirin (C) significantly decreased CH both in collared- and control mice, and these drug appeared more effective when used in the later phases of elicitation (A–C). The administration of phenobarbital decreased CH when used at 0 h, but not when administered at 8 or 16 h after elicitation. Note that, in contrast to other agents, phenobarbital did not decrease CH in collared mice, as compared to that of non-treated collared mice (D). CP: chlorpheniramine, Pred.: prednisolone, PB: phenobarbital, SD: standard deviation. Note that statistical analysis is described only within each group to show that the effects of agents depends considerably on the timing of its application. **p < 0.01, *p < 0.05.
3.4. Use of collars decreases ear swelling, but does not affect inflammatory infiltration in skin exhibiting CH
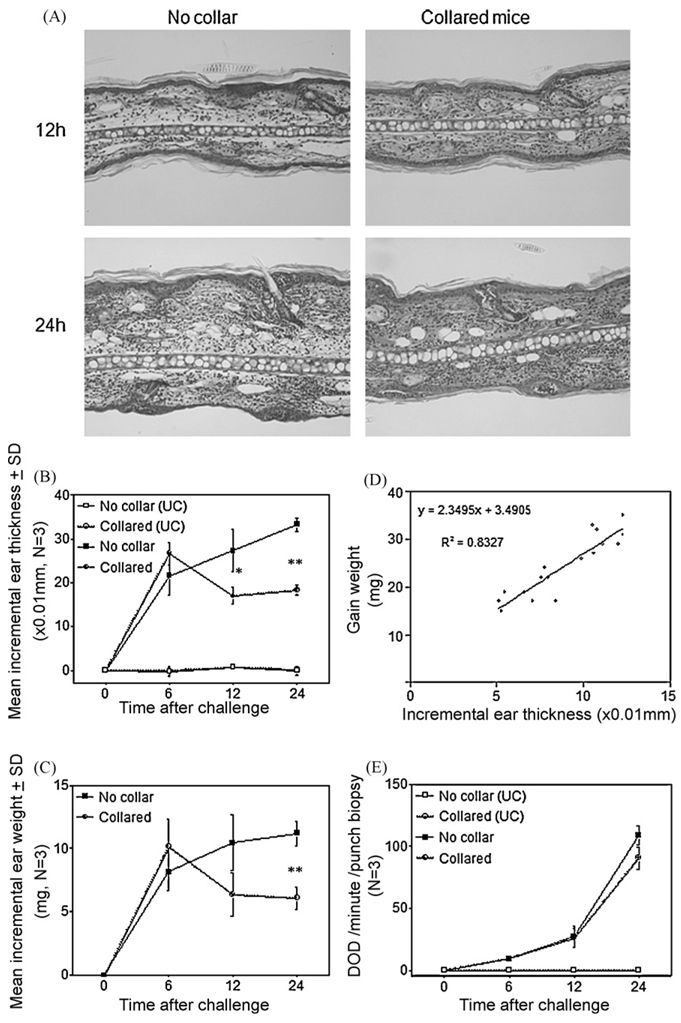
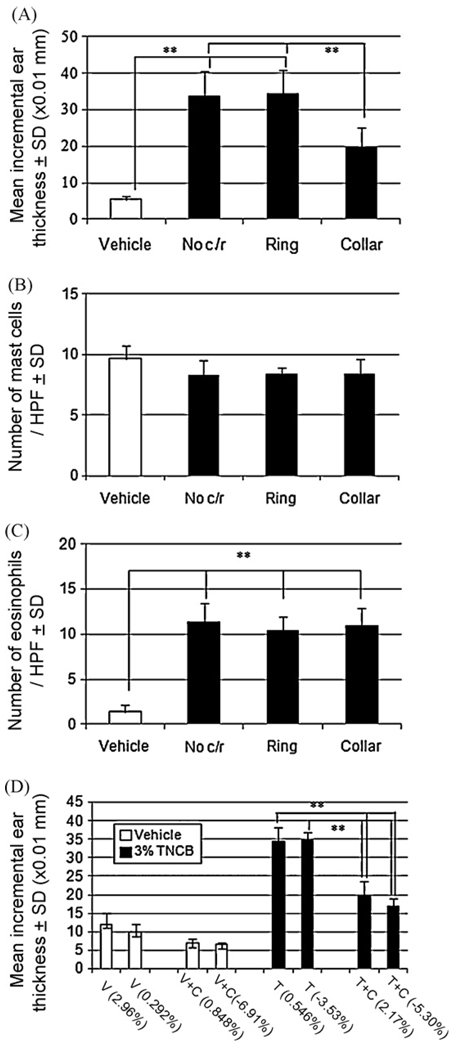
Histological examination revealed that elicited ears of collared mice appeared to be less edematous but had a similar degree of inflammatory infiltration in the dermis as compared to the ears of control mice (Fig. 4A). Ear swelling was assessed by conventional thickness dial gauging (Fig. 4B) and by weighing punched out ears (Fig. 4C). The ear swelling in collared mice did not change at 6 h, but decreased at 12 and 24 h after elicitation, compared to that seen in control mice in which the ears continued to thicken. There was a very high correlation between the two measures for the assessment of ear swelling (Fig. 4D). Use of collars did not decrease the degree of dermal infiltration as a whole, as evidenced by equal MPO activity in situ of challenged ears that were either collared or not collared. We further extended these experiments by using plastic rings (collars without the upper part) to determine whether mice that are stressed by wearing the ring but could scratch their ears fully can develop normal CH reactions. Such “ringed” mice showed as much ear swelling of CH as that of non-collared and non-ringed mice, while collared mice showed a significant decrease of CH (Fig. 5A). The number of mast cells and infiltrating eosinophils was no different among sensitized mice regardless of the use of collars or rings (Fig. 5B and C). We also measured the mean weight change and ear swelling during development of CH elicitation and found that weight change did not seem to affect ear swelling of CH (Fig. 5D), suggesting that the decrease of CH did not merely come from dehydration of mice that wore plastic collars.
Fig. 4.
Use of collars does not decrease inflammatory infiltration in the elicited dermis of CH. Histologically, the dermis of collared mice was less edematous at 12 and 24 h after elicitation compared to that of control mice, but the inflammatory infiltration appeared similar in the both groups (A). Ear swelling of CH decreased in collared mice at 12 and 24 h after elicitation as measured by conventional dial gauging (B) and by weighing punched out ears (C). The two measures for ear swelling showed a very high correlation (D). MPO activity in situ of challenged ears was no different between control- and collared mice (E). UC: unchallenged ears, SD: standard deviation. **p < 0.01, *p < 0.05.
Fig. 5.
Use of collars, but not rings, does not decrease ear swelling of CH. Ear swelling of CH decreased only when collars were used (A). The number of mast cells and infiltrating eosinophils was unchanged among sensitized groups regardless of whether they were wearing collars and/or rings (B and C). Note that mean body weight change did not seem to affect ear swelling in control sensitized mice or the decrease of ear swelling in collared mice (D). HPF: high power field, No c/r: no collars or rings, SD: standard deviation. Note that some description of statistical analysis, such as those between vehicle-treated groups and sensitized groups were omitted in graph 5D for clarity. **p < 0.01.
4. Discussion
The purpose of this study was to determine the effects of scratching on allergic skin reactions using murine contact hypersensitivity (CH) to TNCB as a model and to assess classical “anti-pruritic” agents using this model. Scratching by the mice was found to contribute significantly to ear swelling. This is in keeping with clinical experience wherein scratching exacerbates skin eruptions in pruritic skin diseases such as atopic dermatitis. The contribution of scratching to skin inflammation was first examined using an in vivo LC activation assay. Epidermal LC exhibited increased MHC II expression and elongated dendrites when vehicle (or olive oil) were used, but these LC changes were abolished when collars were used to prevent mice from scratching. LC exhibited these changes (increased MHC II expression and elongated dendrites) when TNCB was used even in the presence of collars. It is not known whether these LC changes in mice painted with vehicle is accompanied by their migration to the regional lymph nodes as occurs in the induction of CH after hapten painting [12,13]. Although we initially suspected that acetone was responsible for LC changes because of its induction of dryness after evaporation [14], we found that olive oil was responsible for the scratching induced by the vehicle. This grooming behavior (scratching) by the hind legs was very rapid. This observation led us to think that the scratching/grooming behavior might possibly contribute to the intensity of skin inflammation. Our hypothesis was that the MHC II increase and elongated dendrites in LC that occurred in olive oil- or vehicle-painted mice might not be due to the chemicals themselves but due to the inflammation caused by scratching/grooming. LC and other dendritic cells (DC) are known to be activated through TNF-α and/or IL-1β [15,16], both of which are upregulated with hapten painting [17]. Although these cytokines are key pro-inflammatory cytokines in the initiation of skin inflammation, we do not know whether the MHC II upregulation and increased dendrites exhibited by LC is caused by cytokine upregulation.
We then hypothesized that the scratching/grooming behavior might possibly contribute to the ear swelling assessed during CH. When collars were used to prevent mice from scratching during the last 12 h of 24 h after elicitation of CH, ear swelling was diminished. Ear swelling in control, non-collared sensitized mice exceeded that of collared mice at 12 and 24 h after elicitation, and the diminished ear swelling appeared to be associated with the degree of tissue edema of the elicited dermis. Interestingly, there was no significant difference between control non-collared mice and collared mice when MPO activity was assessed (as an index of inflammatory cell infiltration) in situ. This, we think, reflected the equivalent numbers of dermal infiltrating polymorphonuclear cells, the major infiltrates in CH in mice when TNCB is used [18]. Thus, scratching may modulate factors that affect tissue edema significantly in the challenged dermis. Vascular permeability-associated factors are also important in the formation of contact hypersensitivity [19–21] and they can be upregulated by TNF-α [22–24], and/or by minor trauma such as gentle cutaneous rubbing [25] which may be equivalent to scratching.
An important point from a methodological standpoint is that the use of collared mice can dissect out the ear swelling induced by scratching. Thus, there are two components of CH that can be dissected: (1) the allergic reaction and (2) the scratching.
All of the agents used in the current study caused diminished ear swelling-the decreased expression of CH could be due either to the anti-pruritic (anti-scratching) effects, or the antiinflammatory effects or both. Phenobarbital appeared to decrease ear swelling in CH and this was probably due to its effect on scratching because phenobarbital could not suppress CH of collared mice where scratch-enhanced ear swelling was disabled. Other agents including chlorpheniramine appear to have some antiinflammatory effects because they could suppress CH in collared mice as well as in control mice.
In conclusion, we established a model to assess the effects of scratching on allergic skin disease using CH as a model. This enables us to assess scratching as a physiological phenomenon and to dissect out the scratching-enhancement on the expression of CH. In addition, CH-suppressing effects of phenobarbital, possibly through their soporific effects, can be identified using the collared mice. Thus, this in vivo model might be useful in the assessment or development of agents with potential anti-pruritic properties.
Acknowledgement
We would like to thank Mr. Jay Linton for his excellent technical help.
Footnotes
Supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Grillo M, Long R, Long D. Habit reversal training for the itch-scratch cycle associated with pruritic skin conditions. Dermatol Nurs. 2007;19(3):243–248. [PubMed] [Google Scholar]
- 2.Kawashima M, Tango T, Noguchi T, Inagi M, Nakagawa H, Harada S. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol. 2003 Jun;148(6):1212–1221. doi: 10.1046/j.1365-2133.2003.05293.x. [DOI] [PubMed] [Google Scholar]
- 3.Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain. 1981;10(2):241–248. doi: 10.1016/0304-3959(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 4.Summerfield JA, Welch ME. The measurement of itch with sensitive limb movement meters. Br J Dermatol. 1980;103(3):275–281. doi: 10.1111/j.1365-2133.1980.tb07244.x. [DOI] [PubMed] [Google Scholar]
- 5.Ebata T, Aizawa H, Kamide R. An infrared video camera system to observe nocturnal scratching in atopic dermatitis patients. J Dermatol. 1996;23(3):153–155. doi: 10.1111/j.1346-8138.1996.tb03990.x. [DOI] [PubMed] [Google Scholar]
- 6.Talbot TL, Schmitt JM, Bergasa NV, Jones EA, Walker EC. Application of piezo film technology for the quantitative assessment of pruritus. Biomed Instrum Technol. 1991;25(5):400–403. [PubMed] [Google Scholar]
- 7.Orito K, Chida Y, Fujisawa C, Arkwright PD, Matsuda H. A new analytical system for quantification scratching behaviour in mice. Br J Dermatol. 2004;150(January 1):33–38. doi: 10.1111/j.1365-2133.2004.05744.x. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki N, Nagao M, Nakamura N, Kawasaki H, Igeta K, Musoh K, et al. Evaluation of anti-scratch properties of oxatomide and epinastine in mice. Eur J Pharmacol. 2000;400(July 14 1):73–79. doi: 10.1016/s0014-2999(00)00349-6. [DOI] [PubMed] [Google Scholar]
- 9.Takano N, Arai I, Hashimoto Y, Kurachi M. Evaluation of antipruritic effects of several agents on scratching behavior by NC/Nga mice. Eur J Pharmacol. 2004;495(July 14 2–4):159–165. doi: 10.1016/j.ejphar.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Stoker TE, Robinette CL, Britt BH, Laws SC, Cooper RL. Prepubertal exposure to compounds that increase prolactin secretion in the male rat: effects on the adult prostate. Biol Reprod. 1999;61(December 6):1636–1643. doi: 10.1095/biolreprod61.6.1636. [DOI] [PubMed] [Google Scholar]
- 11.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(March 3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 12.Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Epidermal Langerhans cell migration and sensitisation to chemical allergens. APMIS. 2003;111(July–August 7–8):797–804. doi: 10.1034/j.1600-0463.2003.11107811.x. Review. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179(November 15 10):6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88(March 3):285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths CE, Dearman RJ, Cumberbatch M, Kimber I. Cytokines and Langerhans cell mobilisation in mouse and man. Cytokine. 2005;32(October 21 2):67–70. doi: 10.1016/j.cyto.2005.07.011. [Epub 2005 September 8. Review]. [DOI] [PubMed] [Google Scholar]
- 16.Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, et al. Migration langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66(September 3):462–470. [PubMed] [Google Scholar]
- 17.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89(February 15 4):1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roupe G, Ridell B. The cellular infiltrate in contact hypersensitivity to picryl chloride in the mouse. Acta Derm Venereol. 1979;59(3):191–195. [PubMed] [Google Scholar]
- 19.Halin C, Fahrngruber H, Meingassner JG, Bold G, Littlewood-Evans A, Stuetz A, Detmar M. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am J Pathol. 2008;173(July 1):265–277. doi: 10.2353/ajpath.2008.071074. [Epub 2008 June 5]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komura K, Hasegawa M, Hamaguchi Y, Saito E, Kaburagi Y, Yanaba K, et al. Ultraviolet light exposure suppresses contact hypersensitivity by abrogating endothelial intercellular adhesion molecule-1 up-regulation at the elicitation site. J Immunol. 2003;171(September 15 6):2855–2862. doi: 10.4049/jimmunol.171.6.2855. [DOI] [PubMed] [Google Scholar]
- 21.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93(January 15 2):580–589. [PubMed] [Google Scholar]
- 22.McHale JF, Harari OA, Marshall D, Haskard DO. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha. J Immunol. 1999;162(February 1 3):1648–1655. [PubMed] [Google Scholar]
- 23.Harari OA, McHale JF, Marshall D, Ahmed S, Brown D, Askenase PW, et al. Endothelial cell E- and P-selectin up-regulation in murine contact sensitivity is prolonged by distinct mechanisms occurring in sequence. J Immunol. 1999;163(December 15 12):6860–6866. [PubMed] [Google Scholar]
- 24.Cordiali-Fei P, Trento E, D’Agosto G, Bordignon V, Mussi A, Ardigó M, et al. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Ann N Y Acad Sci. 2007 September;(1110):578–589. doi: 10.1196/annals.1423.062. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi F, Sterilein RD, Hall RP., 3rd Increased E-selectin, IL-8 and IL-10 gene expression in human skin after minimal trauma. Exp Dermatol. 2003 Dec;12(6):777–783. doi: 10.1111/j.0906-6705.2003.00088.x. [DOI] [PubMed] [Google Scholar]