Abstract
The way in which anti-CD3 monoclonal antibodies (mAbs) modify human immune responses in Type 1 diabetes (T1DM) is not known. We prepared a panel of Class I HLA-A2.1 tetramers with peptides from diabetes-associated antigens and studied the frequency and phenotype of the cells in patients with T1DM and blood donors and in patients treated with anti-CD3 mAb (Teplizumab). More patients with T1DM showed positive staining for at least 1 tetramer using frozen and fresh samples (p<0.05). Three months following treatment with anti-CD3 mAb, the proportion of GAD65- and InsB- peptide reactive CD8+ T cells increased (p<0.05). The phenotype of these cells was modulated from naïve to effector memoryRA+. We conclude: Class I MHC tetramers can identify antigen specific CD8+ T cells in patients with T1DM. The frequency of certain specificities increases after treatment with anti-CD3 mAb. Their modulated phenotype may have functional consequences for their pathogenicity.
Keywords: type 1 diabetes, CD8 T cells, tetramer, antiCD3 monoclonal antibody
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease in which insulin-producing β cells are selectively destroyed by autoreactive T cells[1]. Autoantibodies have been used to predict and even clinically define the disease, but T cells are thought to play a key role in islet destruction[2–7]. CD8+ T cells are the most likely mediators of human disease. Compared to other T cell subsets, they are the most abundant infiltrating cells in human insulitis [8, 9]. Therefore tools that allow an accurate enumeration and characterization of autoreactive CD8+ T cells and discriminate between patients with T1DM and nondiabetic subjects in terms of quantity and functional characteristics may be useful for tracking the autoimmune process and understanding the effects of immune interventions that have been tested to stop disease progression.
A number of groups have developed assays that can identify T cells that are specific for the autoantigens thought to be involved in the disease. These assays have measured proliferative responses to islet antigens, cytokine production that is induced by diabetes-associated antigens, and have identified autoantigen specific T cells with Class I and II MHC tetramers[10][11–14][15]. Using tetramers, both the frequency and phenotype of autoantigen specific T cells can be determined. In setting of islet allotransplants, Class I MHC tetramers have been used to identify an increased number of islet-antigen specific T cells at the time of recurrent autoimmunity[15]. There is no information about the changes in these cells, however, with newer immune therapies that are currently in clinical trials for treatment of T1DM.
One of these therapies is anti-CD3 monoclonal antibody (mAb). Two mAbs have shown favorable results in attenuating the progression of T1DM in published trials[16–18]. The mechanisms of the effects of the anti-CD3 mAbs remain unclear. The experience with other anti-T cell monoclonal antibodies, including OKT3, suggest that there is depletion of T cell subsets, but in preclinical models, cell margination rather than depletion was thought to account for the changes in T cells that were seen after drug administration[19–21]. We therefore studied the effects of anti-CD3 mAb treatment on the circulating diabetes-antigen specific T cells in patients with new onset T1DM who were enrolled in a Phase II/III trial of the humanized anti-human CD3 mAb, teplizumab[22, 23]. We first analyzed the frequency of diabetes-antigen specific CD8+ T cells in patients with T1DM compared to normal control subjects and then studied the changes in these cells following treatment with anti-CD3 mAb.
Materials and methods
Study populations, cell preparation and HLA typing
Peripheral blood mononuclear cells (PBMC) were isolated from 82 subjects (31 control subjects (blood donors) and 51 patients with up to 1 yr duration of T1DM). The median duration was 2.5 months (range 0.5–12 mos). All subjects were positive for at least one biochemical autoantibody (anti-GAD65, anti-ICA512, or anti-insulin if within the first 10 days after commencing insulin therapy). PBMC were frozen for studies at later times or immediately used for analysis. Amongst these subjects were 6 who received a 14 day course of anti-CD3 mAb as part of segment 1 of an open labeled clinical trial (Protégé, Clinicaltrials.gov NCT00385697). The mean age of these subjects was 26.6±5.9 years (range: 19–34 years) and the mean duration of diabetes was 2.4±0.9 mo (range: 1–3.5 mo). PBMC from these subjects were isolated before (day 0) and after treatment (day 14, 28, 91, 182, 210, 273) with anti-CD3 mAb, Teplizumab (Day 0: 51 μg/m2, Day 1: 102 μg/m2, Day 3: 204 μg/m2, Day 4: 408 μg/m2, Days 5–13: μ816 g/m2). Samples were also obtained before and at similar time points in 3 untreated subjects with T1DM enrolled in clinical trials. In addition, PBMC from HLA-A2+ and HLA-A2− blood donors were isolated from material provided by the New York Blood Bank. These samples were provided in a de-identified manner so demographic or medical information was not available. Ethical approval for the study was granted by the Human Investigation Committee at Yale University and from institutions participating in the Protégé study.
Clinical studies
Anti-EBV IgM and IgG and EBV viral loads were measured by Esoterix laboratories. The number of circulating CD4 and CD8+ T cells were determined by multiplying the absolute lymphocyte count, from the complete blood count, by the frequency (in percentage) of CD4+ or CD8+ T cells measured by flow cytometry with a scatter gate placed around the lymphocyte subpopulation.
Staining with monoclonal antibodies and Class I MHC tetramers
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll Paque density gradient centrifugation (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Subjects who were HLA-A2+ were identified by staining with FITC-conjugated mAb BB7.2 (BD Phamingen, San Diego, CA). The frequencies of tetramer+ cells were determined on freshly isolated and frozen PBMC but are considered separately in the analysis. Frozen cells were used for the phenotypic analysis performed only when tetramer staining was positive, so that staining could be minimized to avoid waste of cells. The cells were stored in liquid nitrogen until use. They were thawed, washed, and stained as described.
Allophycocyanin (APC)-conjugated MHC class I (HLA-A0201) tetramers were prepared with peptides from T1DM-associated antigens by the National Institute of Allergy and Infectious Diseases-MHC Tetramer Core Facility (Atlanta, GA) (Table 1). In addition an APC-labeled negative tetramer that does not recognize human CD8+ T cells of any HLA allele was used to assess the level of background fluorescence and nonspecific binding (Beckman Coulter Immunomics). APC or PE-conjugated Class I tetramers with the EBV BMLF1 peptide (GLCTLVAML), Influenza-M1 (GILGFVFTL), and Her-2/neu (KIFGSLAFL) were also used. The Her-2/neu peptide containing HLA-A2 tetramer was used to assess non-specific staining.
Table 1.
Peptides used to prepare Class I MHC tetramers
The following antibodies were used for staining: R-phycoerythrin (PE)-labeled anti CD8–(clone3B5) (Caltag Laboratories, Invitrogen Corp.) PE anti–CD45RA (clone HI100) (BD Biosciences), phycoerythrin-cyanine5 (PE-Cy5)-labeled anti-CXCR3 (clone 1C6/CXCR3) and anti-CD137 (clone 4B4-1) (BD Biosciences), phycoerythrin-cyanine7 (PE-Cy7)-labeled anti-CCR7 (clone 3D12) (BD Biosciences) and anti-CD39 (clone eBioA1) (eBioscience), fluroscein isothiocyanate (FITC)-conjugated anti-CD44 (cloneG44-26) and anti-CD28 (clone CD28.2), allophycocyanin-Alexa Fluor 750 (APC-Al.Fl.750)-labeled anti-CD62L (clone Dreg-56) (Caltag Laboratoires, Invitrogen Corp.). pacific blue-conjugated anti-CD8 (clone RPA-T8)(BD Biosciences). Relevant isotype- and fluorochrome-matched control antibodies, were also used for these studies.
The washed PBMC (1×106 cells/tube) were stained with APC-tetramers (1:500 dilution) and anti-CD8-PE (Caltag Laboratoires) (1: 20 dilution) and other mAbs in 200 μl final volume of FACS buffer (PBS with 1% BSA and 0.1% sodium azide). Cells were incubated for 30 min in the dark at 4 °C, then washed twice in FACS buffer, fixed with 2% paraformaldehyde, resuspended in FACS buffer and analyzed on FACS Calibur or LSRII (Becton Dickinson). A minimum of 150,000 lymphocytes were analyzed.
Flow cytometry analysis
In preliminary studies (see supplemental Figure 1) we established the specificity of staining with the tetramers. Three approaches were used. First, we compared staining with diabetes specific and a negative tetramer with staining with diabetes tetramers with tetramers with a tetramer with an irrelevant peptide (Hep C)(Supplemental Figure 1A). Second, we co-stained with PE conjugated tetramers with viral (i.e. EBV) peptides and APC-conjugated tetramers with diabetes peptides and determined whether any of the tetramer+ subpopulations overlapped (indicating non-specific binding) or were separate. Co-staining with tetramers in different fluorochromes identified separate subpopulations of CD8+ T cells (Supplemental Figure 1B), suggesting that the diabetes and viral tetramers recognized different subpopulations of CD8+ T cells and that non-specifically stained cells, identified with both tetramers, were not detected. Third, the thresholds used to designate “positive staining” with the tetramers were derived from the results from staining CD8+ T cells from HLA-A2− normal healthy blood donors (n=12 for frozen and n=10 for fresh samples) (Supplemental Table 1). The average percent staining of CD8+ T cells (minus staining with a negative tetramer) + 3SD was set as the threshold for positive staining in HLA-A2+ subjects. These mean, SD, and thresholds are shown in Supplemental Table 1.
Statistical analysis
A Chi-squared or Fisher’s exact test was used for categorical analyses and t-test and Mann-Whitney tests were used for comparison of continuous variables in the case of normally and non-normally distributed data respectively. The phenotype of tetramer+ cells was analyzed by comparing the frequency of the phenotype of cells from subjects who were either untreated or before treatment with anti-CD3 mAb to cells from subjects after treatment with anti-CD3 mAb by ANOVA. The analyses were two-tailed and the statistical significance was declared at p<0.05.
Results
Identification of diabetes-peptide specific CD8+ T cells with Class I MHC tetramers
We used a panel of 6 Class I MHC tetramers to enumerate antigen specific T cells in patients with T1DM up to 1 year after diagnosis, and in blood donors. The threshold for positive staining, obtained from tetramer staining of HLA-A2− samples, was applied when samples from HLA-A2+ patients and blood donors were studied with the tetramers.
There was a significantly greater proportion of individuals with T1DM with positive staining with tetramers in comparison with healthy donors when either fresh or frozen cells were used for analysis (Figure 1, Table 2). With frozen cells, 45% of patients with T1DM were positive for 1 or more tetramers compared to 2/18 healthy control subjects (p<0.001). With freshly isolated lymphocytes, 25/31 subjects who were studied within 1 year of diagnosis were positive for at least 1 tetramer compared to 5/13 healthy control subjects (p=0.01). In patients with T1DM, the highest proportion of subjects (61%) was positive for IGRP tetramer and the lowest (6%) was positive for preproinsulin tetramer. The proportion positive for GAD65, InsA1, InsA2, and Ins B tetramers were 22%, 14%, 21% and 8%. Of the positive tetramers in healthy control subjects, 3 were with the GAD65 and 3 with the IGRP tetramer.
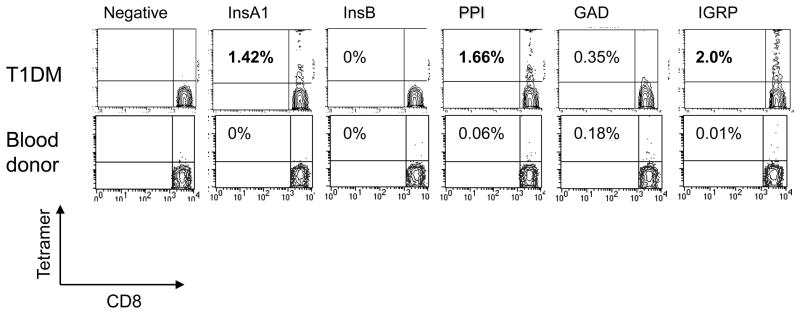
Figure 1. Identification of antigen specific CD8+ T cells with Class I MHC tetramers.
The representative staining of fresh cells from a representative patient with T1DM and a blood donor (both HLA-A2+) are shown. The X axis shows staining with anti-CD8 mAb and the Y axis represents staining with tetramer. Electronic gates were placed around lymphocytes on the basis of forward and side scatter and around CD8+ cells. The numbers in each contour plot represents the percentage of CD8+ T cells that are tetramer+ minus the background staining with the negative tetramer (% negative tetramer: 0.06% [T1DM] and 0.03% [Blood donor], respectively). Bolded numbers represent positive staining (above threshold for positivity from staining of HLA-A2− control subjects (see Supplemental Table 1)).
Table 2.
Number of subjects with tetramer positive CD8 cells
| A. Frozen cells | ||||||
|---|---|---|---|---|---|---|
| No. of tetramers | 0 | 1 | 2 | 3 | 4 | 5 |
| T1DM (n=20)+ | 4 | 7 | 0 | 3 | 2 | 4 |
| Blood donors (n=18) | 16 | 2 | 0 | 0 | 0 | 0 |
| B. Freshly isolated cells | ||||||
|---|---|---|---|---|---|---|
| No of tetramers | 0 | 1 | 2 | 3 | 4 | |
| T1DM (n=31)+ | 6 | 13 | 6 | 4 | 2 | |
| Blood donors (n=13) | 8 | 4 | 1 | 0 | 0 | |
p≤ 0.001 vs blood donors
p=0.01 vs blood donors
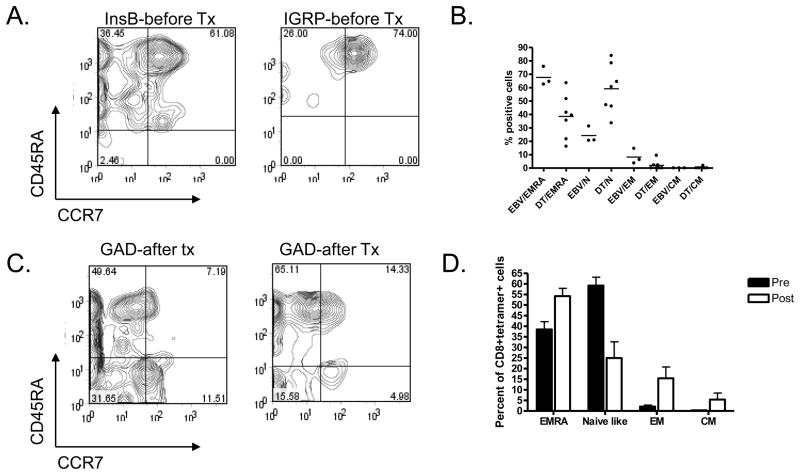
We compared the phenotype of the diabetes antigen specific CD8+ T cells to the phenotype of CD8+ cells reactive to EBV antigens in patients with T1DM. We differentiated the CD8+ cells into functional phenotypes based on the basis of the expression of CD45RA and CCR7 into naïve (N)(CD45RA+CCR7+), central memory (CM) (CD45RA−CCR7+), effector memory (EM)(CD45RA−CCR7−) or terminally differentiated effector memory cells (EMRA) (CD45RA+CCR7−)[24, 25]. Frozen cells were used for these analyses in order to minimize the number of cells required for the studies. 59.2±3.9% and 38.4±3.68% of the diabetes tetramer+ cells were of naïve and EMRA phenotypes respectively whereas 2.01±0.73% and 0.32±0.16% of cells were effector and central memory cells respectively. In patients with T1DM, the EBV reactive cells were more frequently terminally differentiated EMRA cells: 67.6% were CD45RA+CCR7− (p=0.02) and only 24.2% were CD45RA+CCR7+ (Figure 2B). The EBV reactive cells had a similar phenotype in patients with T1DM and normal control subjects [26]. Even in the same individual, in which the EBV specific T cells were predominantly of an EMRA phenotype, the diabetes-antigen specific T cells were CCR7+ and CD45RA+ suggesting a naïve phenotype (Figure 2A and B)(p<0.001).
Figure 2. Phenotype of tetramer+ cells.
A: PBMC were stained with mAbs to CD8, CCR7, CD45RA, and the indicated tetramers. Electronic gates were placed around the CD8+tetramer+ cells and the expression of CCR7 (X axis) and CD45RA (Y axis) was analyzed. B: The functional phenotypes, on the basis of expression of CCR7 and CD45RA are shown for diabetes antigen specific CD8+ T cells (DT) and EBV specific T cells (EBV) from patients with T1DM. There was a significant difference in the distribution of phenotypes among the DT+ vs EBV+ T cells (p<0.001). EMRA: terminally differentiated effector memory RA+; N: naïve-like; EM: effector memory; CM: central memory; C: The expression of CCR7 and CD45RA among diabetes antigen specific T cells after treatment with anti-CD3 mAb is shown. D: The frequency of phenotypes of CD8+diabetes tetramer+ T cells before and after treatment with anti-CD3 mAb are shown. There was a significant change in the distribution of phenotypes after treatment (p<0.001).
Diabetes antigen specific CD8+ T cells increase in frequency after treatment with anti-CD3 mAb
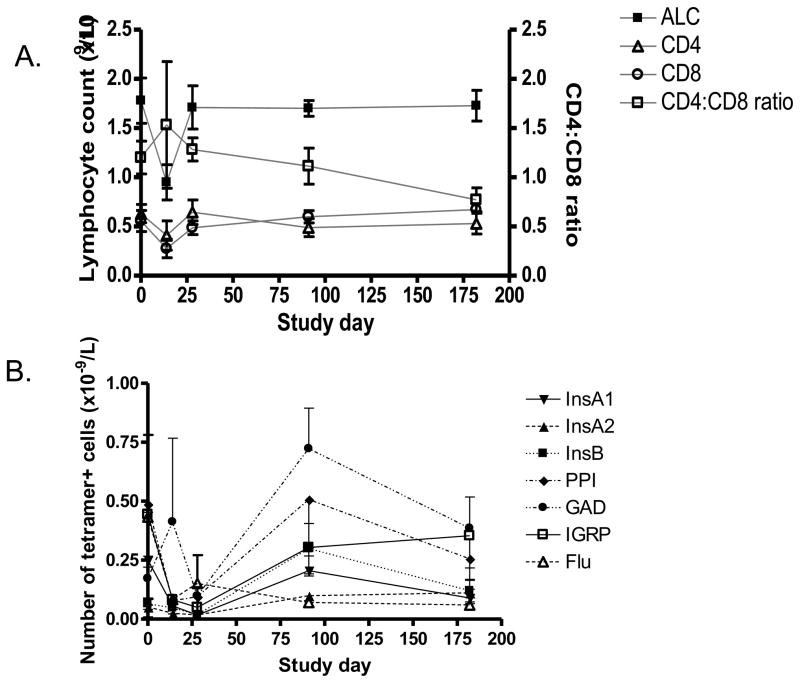
Six of the study subjects were treated with anti-CD3 mAb, Teplizumab, for 14 days (5 subjects were participants in NCT00385697). The lymphocyte counts, CD4+ and CD8+ T cells and tetramer+ cells were studied before and after treatment. During the 14 d course of mAb administration there was a decline and a recovery of circulating lymphocytes (Figure 3A). The absolute lymphocyte count reached a nadir of 24.9±5.6% of the baseline value (day 6) but then increased: by month 2–3 (6–10 weeks after the last dose of drug) the absolute lymphocyte count was 102±10.8% of the baseline value. There was a trend for a decrease ratio of CD4:CD8+ T cells during the time of T cell recovery which reflected a slight increase in the number of CD8+ T cells (from 0.56±0.11 × 109/L to 0.6±0.06 × 109/L at month 2–3) and a decrease in the number of CD4+ cells (from 0.63±0.09 to 0.49±0.09 × 109/L) but the cell counts at these two time points were not statistically different (p>0.5).
Figure 3. Changes in the numbers of lymphocytes during and following treatment with Teplizumab.
A: The absolute lymphocyte (ALC) (n=6), CD4, CD8 (L axis), cell counts, and CD4:CD8 ratios (R axis)(n=6) were calculated for subjects receiving Teplizumab for 14 days. B. The absolute numbers of tetramer+ cells are shown.
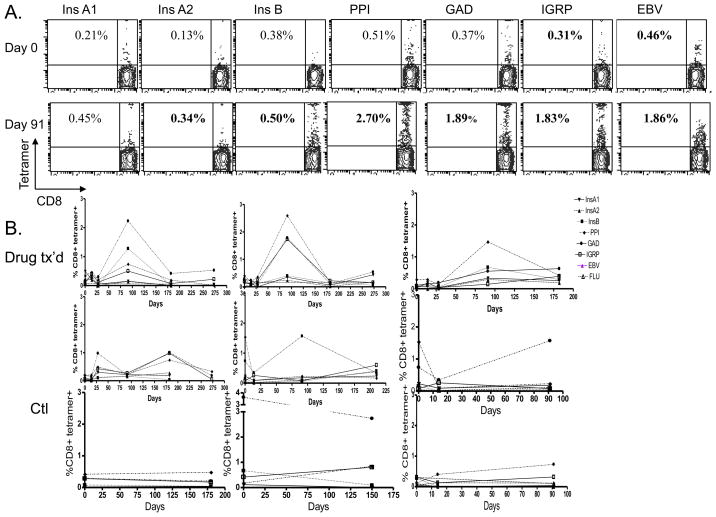
There was an increase in the frequency of GAD-reactive T cells as a percentage of the CD8+ T cells 2–3 months after treatment with the anti-CD3 mAb from 0.30±0.1% to 1.25±0.31% (Figure 4A,B and 3B, p=0.03), and an increase in the proportion of InsB specific CD8+ T cells from 0.11±0.04% to 0.48±0.18% (p<0.05). Small changes (not statistically significant) were seen in InsA2 (from 0.058±0.03% to 0.19.±03%, PPI (0.74±0.27% to 0.90±0.40%), and IGRP (0.53±0.42 to 0.52±0.25%) specific CD8+ T cells as well. The increase in the proportion of InsB peptide-reactive cells did not appear to be due to the use of insulin by the patients since the proportion of InsA1 reactive cells declined (by 0.082%) and the increase in the InsB reactive T cells (0.3%) was significantly greater in the drug treated subjects than in untreated control subjects (a decline of 0.4%) studied over the same time period (p=0.04). Before treatment, ½ of the patients showed positive staining for at least 1 tetramer whereas after treatment, all of the drug treated subjects had positive staining for at least 1 tetramer (p=0.11). In 2 subjects, initially negative for all 6 tetramers, there was positive staining for 4 tetramers after treatment. There was no overall change in the proportion of subjects or number of tetramers with positive staining in the untreated group studied at similar time points. The increased frequency of the diabetes antigen specific T cells that had been seen at day 90 in the drug treated group declined by day 182.
Figure 4. Changes in diabetes antigen specific CD8+ T cells with treatment with Teplizumab.
A Representative staining for CD8 and tetramers from a single participant is shown before (day 0) and 3 months after (day 90) treatment with Teplizumab. The numbers refer to the percentage tetramer+ of the CD8+ T cells. Bolded numbers are considered positive staining based on threshold values shown in Supplemental Figure 1. B: Changes in the frequency of diabetes antigen specific T cells in individual patients treated with Teplizumab and untreated patients with T1DM. (tetramers: InsA1 ▼;, InsA2 ▲, Ins B ■ PPI ◆ GAD ● IGRP □, Flu ○).
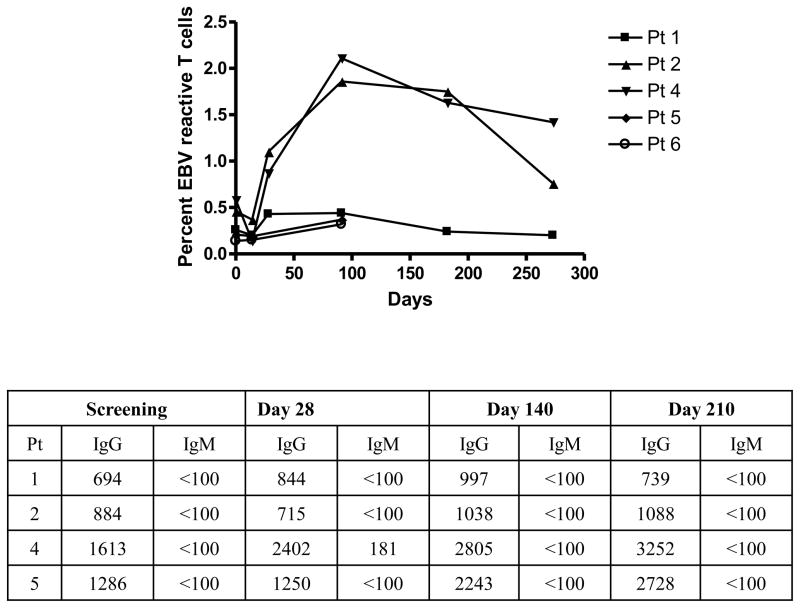
We also enumerated the number of EBV and Flu specific T cells in the drug treated patients (n=4 and 2 respectively) (Figure 5). EBV-reactive T cells were detectable in 4 subjects. The frequency of these cells increased in 2/4 drug treated subjects although the change in the frequency of the EBV reactive cells was not statistically significant (average increase at peak = 1.04%, p=0.14). After day 91, the frequency of the EBV-specific CD8+ T cells decreased in both patients (at day 182 and 210) (Figure 5). At the time points for sampling (screening, day 28, day 140, and day 210) the EBV viral loads did not increase and subjects did not report a mono-like illness. However, in the two subjects in whom the frequency of the EBV specific T cells increased, the titer of anti-EBV IgG increased as well (Figure 5). The frequency of EBV reactive T cells in the untreated subjects did not change significantly over the same time period (data not shown). The frequency of Flu specific T cells was similar at baseline and at day 91 in the 2 drug treated subjects in whom these cells could be detected (Figure 4B).
Figure 5. Changes in EBV reactive T cells and anti-EBV immunoglobulins after treatment with Teplizumab.
The changes in EBV reactive CD8+ T cells were monitored in parallel with the diabetes-antigen specific T cells. The EBV IgG and IgM titers were measured at the indicated study days (the mAb was given from days 0–14).
Changes in the phenotype of diabetes antigen specific T cells with drug treatment
We compared the phenotype of the tetramer+ T cells from patients before and after treatment with anti-CD3 mAb (Figure 2A, C and D). Three months after treatment with anti-CD3 mAb, the frequency of diabetes-antigen specific EMRA, EM, and CM CD8+ T cells increased whereas the frequency of naïve cells (CD45RA+CCR7+) decreased (Figure 2C and D, p<0.001). The percentage of diabetes-specific tetramer+ T cells expressing CCR7 decreased from 66.1±20% (pretreatment) to 39.1±10% (post-treatment)(p<0.01), while those that expressed CD45RA decreased from 97.6±4% (pretreatment) to 67.2±15% (post-treatment)(p<0.01). The proportion of tetramer+ cells that expressed CD62L and CD44 decreased after treatment (39.13±10% vs. 66.1±20%, p<0.05 and 67.3±5% vs. 94.7±7%, p<0.01, respectively) compared to before treatment. The other surface markers (such as CXCR3) did not change significantly after treatment (not shown).
Discussion
We have studied antigen specific CD8+ T cells with Class I MHC tetramers in patients with T1DM, and followed the changes in these cells after treatment with anti-CD3 mAb. We were able to identify CD8+ T cells specific for 6 diabetes antigens in freshly isolated or frozen PBMC from patients without the need for expansion in vitro. The frequency of diabetes-tetramer+ T cells is greater in patients than in blood donors.
We found staining for at least one diabetes-tetramer in 76% and 80% of freshly isolated and frozen samples from patients with T1DM and 36% and 30% of fresh or frozen samples had two or more tetramer positive results. In a similar manner, only 1/13 freshly isolated samples and 0/18 frozen samples from blood donors showed positive staining with more than 1 tetramer. In other bioassays in T1DM, criteria used to designate “positive” responses have relied on the number of positive responses to different autoantigens rather than the magnitude of the response to any particular antigen [12, 27]. The 6 tetramers used in our analysis did not identify all patients with diabetes and this might indicate that other β-cell antigens are important for an all-inclusive T1DM profile, suggested by the recent identification of a new β-cell antigen (ZnT8) [28].
When patients were treated with anti-CD3 mAbs, the proportion of the GAD and InsB specific CD8+ cells increased significantly even though there was not a significant change in the overall number of CD8+ T cells. There was a trend for an increase in the number of positive tetramers found in subjects that did not reach statistical significance. The data therefore is consistent with expansion of preexisting antigen specific cells, although studies with additional patients treated with anti-CD3 mAb may identify positive staining with new specificities. It is unlikely that the changes we observed with anti-CD3 mAb treatment reflect changes of the antigen specific T cells that occur during the natural history of the disease since, similar to other studies of antigen specific CD8+ T cells in patients with T1DM, we did not find an increase in tetramer+ cells with time after the diagnosis of diabetes (data not shown). The increase in the diabetes antigen specific T cells also did not appear to reflect a non-specific stimulation of CD8+ cells: The changes we found overall in CD8+ T cells were modest and the proportion of Flu reactive CD8+ T cells did not change over the same time period, but we cannot exclude homeostatic proliferation of autoreactive cells possibly occurring after margination of T cells or even elimination of a small proportion of these cells. We did find an increase in EBV reactive T cells in 2/4 subjects in whom these cells were detected before treatment but the changes in frequency of these cells were not statistically significant. EBV is a latent virus that may show reactivation with immune suppression or disorders associated with immune dysregulation [29, 30]. The change in the frequency of these cells following treatment with anti-CD3 mAb therefore likely reflects a cellular response to a subclinical reactivation of EBV since the titers of anti-EBV reactive antibodies also increased during this time in the 2 subjects in whom the tetramer+ cells increased. However, the viral loads did not increase and mono-like symptoms were not seen. These findings suggest that any effects of the anti-CD3 mAb to induce immune suppression are transient since a brisk anti-viral response was mounted. However, these analyses entail a very small number of subjects: further studies on a larger group of subjects will be needed to clarify the changes in anti-viral responses following anti-CD3 mAb treatment. Nonetheless, the mechanisms underlying these changes in the viral antigen specific cells may be different from those related to the changes in diabetes antigen specific T cells since the latter antigens are not changing.
A surprisingly high proportion of the diabetes antigen specific T cells had a naïve-like phenotype (CCR7+CD45RAhiCD62L+) before treatment. One possible explanation for this finding is a selective sequestration of effector cells in the pancreas. Similarly, a recent study reported a high expression of CCR7 and CD45RA expressing cells overall in the CD8+ T cell population in patients with T1DM [31]. In another recent report by Monti et al, pentamer+ cells were detected in patients and control subjects with equal frequency among the CD45RO− population but in higher proportion among patients when the analysis was restricted to the CD45RO+ subpopulation suggesting that in patients [32]. We did not specifically analyze the tetramer+ cells on the basis of CD45RO expression, but the phenotype of the diabetes antigen-specific T cells differed from EBV reactive T cells from the same individual, which showed a EMRA phenotype. Analyses of the antigen specific CD8+ T cell response to viral antigens (vaccinia virus) have shown the loss of CCR7 expression during the transition from naïve to effector to memory cells, but CCR7 expression was bimodal among the memory CD8+ T cell pool[33]. Likewise, CD45RA may be reexpressed on memory T cells. Therefore, we cannot be certain whether our findings of the phenotype of antigen specific T cells represent a truly naïve subpopulation or a long lived subpopulation of CD8+ T cells. Further studies in which the phenotype of cells in the tetramer+ healthy control subjects and patients with T1DM will be needed to determine whether these antigen specific subpopulations differ among subjects and to compare our results with those previously published. Nonetheless, the difference in the phenotype among viral and diabetes-antigen specific T cells suggests that activation of these separate populations of antigen specific cells differs, possibly resulting from exposure to less available self antigen and/or lower avidity for those antigens compared to viral epitopes during infection. This hypothesis is supported by the work of Betts el al., which showed heterogeneity of viral antigen specific CD8+ T cell clonotypes depending on the antigen concentration and the TCR avidity for the MHC/antigen complex (low antigen levels determining CD8 T cells only to elaborate cytolytic function)[34]. After treatment with anti-CD3 mAb, a greater proportion of the cells reactive with the diabetes antigens displayed markers of EMRA+ and effector cells, similar to the viral antigen reactive CD8+ T cells. This change in phenotype is consistent with the effects of the mAb on activation of CD8+ T cells that we have previously reported [35, 36].
The functional consequences of the activated and expanded CD8+ T cells remain unknown. In previous studies, we found an increased proportion of CD8+ T cells after anti-CD3 mAb treatment in subjects who showed a clinical response to the drug in terms of a reduced rate of decline of C-peptide over time [16, 17]. We have previously reported that when activated by anti-CD3 mAb, some CD8+ T cells acquire regulatory function [36]. Further functional studies of the antigen specific T cells before and after treatment will be needed to address this question.
In summary, we have found that diabetes-antigen specific CD8+ T cells can be found ex vivo in the peripheral blood of patients with T1DM. Our data indicates that the mechanism of action the anti-CD3 mAb does not simply reflect elimination of T cells in a manner similar to agents such as anti-CD52 mAb since the proportion of certain antigen specific CD8+ T cells increases after treatment with anti-CD3 mAb [37]. The functional phenotype of these cells is modulated after anti-CD3 mAb treatment but functional analyses of the antigen specific cells will require further studies with expanded cells.
Supplementary Material
Supplemental Figure 1: Frequency of diabetes specific CD8+ cells simultaneously stained with a negative tetramer or Her-2/neu (irrelevant) tetramer (gated on CD8 cells). A: Frequency of diabetes specific CD8+ cells simultaneously stained with a negative tetramer or Her-2/neu (irrelevant) tetramer (gated on CD8 cells). PBMC from a patient with T1DM were stained with tetramer with IGRP peptide (top) or GAD peptide (bottom) and either a negative tetramer or a tetramer with an irrelevant peptide (Hep C) using different flurochromes. The staining with the IGRP or GAD was compared under the two staining conditions. There was not a detectable difference in the frequency of tetramer+ cells when cells were stained with a negative tetramer (with no known binding ligands) or with an irrelevant tetramer. B: Staining with tetramers containing diabetes and EBV peptides identifies distinct subpopulations: Cells from 2 patients with T1DM were stained with tetramers containing a diabetes peptides that had been found to have detectable staining (L: IGRP, InsB, and GAD65, and R: InsA2, PPI, GAD65, and IGRP Y axis) conjugated to APC or with EBV peptide conjugated to PE (X axis). Staining with the tetramers identified distinct subpopulations of cells indicating that staining with the tetramers was not non-specific.
Acknowledgments
Supported by grants: DK057846 and RR024139 from the NIH and 2006-351, 2006-502, and 2005-1168 from the Juvenile Diabetes Research foundation, and a gift from the Brehm Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkinson MA. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can’t we prevent or reverse this disease? Diabetes. 2005;54:1253–63. doi: 10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- 2.Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–8. [PubMed] [Google Scholar]
- 3.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD. NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 5.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–42. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 6.Qin H, Trudeau JD, Reid GS, Lee IF, Dutz JP, Santamaria P, Verchere CB, Tan R. Progression of spontaneous autoimmune diabetes is associated with a switch in the killing mechanism used by autoreactive CTL. Int Immunol. 2004;16:1657–62. doi: 10.1093/intimm/dxh167. [DOI] [PubMed] [Google Scholar]
- 7.Santamaria P. Kinetic evolution of a diabetogenic CD8+ T cell response. Ann N Y Acad Sci. 2003;1005:88–97. doi: 10.1196/annals.1288.010. [DOI] [PubMed] [Google Scholar]
- 8.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–22. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–20. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reijonen H, Kwok WW. Use of HLA class II tetramers in tracking antigen-specific T cells and mapping T-cell epitopes. Methods. 2003;29:282–8. doi: 10.1016/s1046-2023(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 11.Nepom GT, Buckner JH, Novak EJ, Reichstetter S, Reijonen H, Gebe J, Wang R, Swanson E, Kwok WW. HLA class II tetramers: tools for direct analysis of antigen-specific CD4+ T cells. Arthritis Rheum. 2002;46:5–12. doi: 10.1002/1529-0131(200201)46:1<5::AID-ART10063>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Seyfert-Margolis V, Gisler TD, Asare AL, Wang RS, Dosch HM, Brooks-Worrell B, Eisenbarth GS, Palmer JP, Greenbaum CJ, Gitelman SE, Nepom GT, Bluestone JA, Herold KC. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes. 2006;55:2588–94. doi: 10.2337/db05-1378. [DOI] [PubMed] [Google Scholar]
- 13.Martinuzzi E, Lemonnier FA, Boitard C, Mallone R. Measurement of CD8 T cell responses in human type 1 diabetes. Ann N Y Acad Sci. 2008;1150:61–7. doi: 10.1196/annals.1447.015. [DOI] [PubMed] [Google Scholar]
- 14.Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, Bruno G, Chatenoud L, van Endert P, Mallone R. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes. 2008;57:1312–20. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- 15.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102:18425–30. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 18.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 19.Herold KC, Bluestone JA, Montag AG, Parihar A, Wiegner A, Gress RE, Hirsch R. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–91. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch R, Bluestone JA, DeNenno L, Gress RE. Anti-CD3 F(ab′)2 fragments are immunosuppressive in vivo without evoking either the strong humoral response or morbidity associated with whole mAb. Transplantation. 1990;49:1117–23. doi: 10.1097/00007890-199006000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch R, Eckhaus M, Auchincloss H, Jr, Sachs DH, Bluestone JA. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988;140:3766–72. [PubMed] [Google Scholar]
- 22.Woodle ES, Bluestone JA, Zivin RA, Jolliffe LK, Auger J, Xu D, Thistlethwaite JR. Humanized, nonmitogenic OKT3 antibody, huOKT3 gamma(Ala-Ala): initial clinical experience. Transplant Proc. 1998;30:1369–70. doi: 10.1016/s0041-1345(98)00278-4. [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 24.Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–8. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 26.Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–8. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- 27.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–33. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 28.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter PA, Appelbaum FR, Corey L, Deeg HJ, Doney K, Gooley T, Krueger J, Martin P, Pavlovic S, Sanders J, Slattery J, Levitt D, Storb R, Woolfrey A, Anasetti C. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood. 2002;99:2712–9. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 30.Kang I, Quan T, Nolasco H, Park SH, Hong MS, Crouch J, Pamer EG, Howe JG, Craft J. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004;172:1287–94. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- 31.Hedman M, Faresjo M, Axelsson S, Ludvigsson J, Casas R. Impaired CD4 and CD8 T cell phenotype and reduced chemokine secretion in recent-onset type 1 diabetic children. Clin Exp Immunol. 2008;153:360–8. doi: 10.1111/j.1365-2249.2008.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, Chiumello G, Ziegler AG, Bonifacio E. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;179:5785–92. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 33.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–17. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 35.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–18. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166–73. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi K, Honeyman MC, Harrison LC. Cytotoxic T cells to an epitope in the islet autoantigen IA-2 are not disease-specific. Clin Immunol. 2001;99:360–4. doi: 10.1006/clim.2001.5031. [DOI] [PubMed] [Google Scholar]
- 39.Hassainya Y, Garcia-Pons F, Kratzer R, Lindo V, Greer F, Lemonnier FA, Niedermann G, van Endert PM. Identification of naturally processed HLA-A2−-restricted proinsulin epitopes by reverse immunology. Diabetes. 2005;54:2053–9. doi: 10.2337/diabetes.54.7.2053. [DOI] [PubMed] [Google Scholar]
- 40.van Endert P, Hassainya Y, Lindo V, Bach JM, Blancou P, Lemonnier F, Mallone R. HLA class I epitope discovery in type 1 diabetes. Ann N Y Acad Sci. 2006;1079:190–7. doi: 10.1196/annals.1375.030. [DOI] [PubMed] [Google Scholar]
- 41.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach JM, van Endert P. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–21. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 42.Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995;181:1923–7. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–65. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Frequency of diabetes specific CD8+ cells simultaneously stained with a negative tetramer or Her-2/neu (irrelevant) tetramer (gated on CD8 cells). A: Frequency of diabetes specific CD8+ cells simultaneously stained with a negative tetramer or Her-2/neu (irrelevant) tetramer (gated on CD8 cells). PBMC from a patient with T1DM were stained with tetramer with IGRP peptide (top) or GAD peptide (bottom) and either a negative tetramer or a tetramer with an irrelevant peptide (Hep C) using different flurochromes. The staining with the IGRP or GAD was compared under the two staining conditions. There was not a detectable difference in the frequency of tetramer+ cells when cells were stained with a negative tetramer (with no known binding ligands) or with an irrelevant tetramer. B: Staining with tetramers containing diabetes and EBV peptides identifies distinct subpopulations: Cells from 2 patients with T1DM were stained with tetramers containing a diabetes peptides that had been found to have detectable staining (L: IGRP, InsB, and GAD65, and R: InsA2, PPI, GAD65, and IGRP Y axis) conjugated to APC or with EBV peptide conjugated to PE (X axis). Staining with the tetramers identified distinct subpopulations of cells indicating that staining with the tetramers was not non-specific.