Abstract
Catalysis for reaction of electophilic fluorine addition to a fluorinated double bond is described. The presence of small amounts of iodine, bromine, or boron trifluoride increases an overall product yield and degree of fluorine incorporation, making the reaction a more efficient method for preparation of 18F positron emission tomography agents. A possible mechanism of catalytic action of iodine is discussed.
Keywords: electrophylic fluorination, 18F-labeling, catalysis, EF5
Introduction
Direct electrophilic addition of elemental fluorine to fluorinated double bonds can be a practical method for preparation of 18F-labeled PET agents, as witnessed by our earlier report of its application to the synthesis of the hypoxia marker 18F-EF5 [Dolbier et al, 2001], which has proven useful for non-invasive imaging of tumor hypoxia [Ziemer et al, 2003; Evans et al, 2006; Komar et al, 2008]. Although we found the yield of 18F-EF5 synthesis to be sufficiently high for the purpose, an improvement of its efficiency was nevertheless desirable for practical application of the process. This is especially important because of the inherently low specific activity of 18F-F2 gas, which requires the addition of non-radioactive carrier gas as part of its preparation. In the current paper we report a catalytic enhancement of the reaction of fluorination of the trifluorovinyl group of EF5-precursor 1 by addition of minute quantities of I2, as well as derivatives of bromine and boron trifluoride, such that the overall yield of EF5 and the efficiency of consumption of F2 are increased.
Materials and methods
Most of reagents and solvents (except as otherwise noted) were purchased from Sigma-Aldrich. Reaction precursor 2-(2-nitro-1[H]-imidazol-1-yl)-N-(2,3,3-trifluoroallyl)-acetamide (EF12A) was prepared as described before [Dolbier et al, 2001].
Fluorination of the precursor was carried out in trifluoroacetic acid via two similar procedures. In one case we were using conditions similar to synthesis of [18F]-EF5 for PET experiments. 0.1% F2 gas was obtained by premixing of 3% F2/Ar (BOC gases) with argon in a 200 mL monel cylinder and used in quantities comparable with amount of precursor. Total amount of fluorine gas in the resulted mixture was calibrated in separate experiments by trapping the gas with KI, followed by titration of obtained I2 with Na2S2O3 in presence of starch indicator. To prepare the iodine catalyst, an excess (several crystals) of iodine was partially dissolved in ~ 1mL TFA, to form a saturated solution (of concentration 0.55 mM, as determined spectroscopically by comparison of absorption at 510 nm with I2 solution in CHCl3, for which was found ε = 930). The 0.1% F2/Ar mixture was added slowly (over about a 10 minute period) by bubbling the gas through a solution of 6–8 mg 1 in 10 mL trifluoroacetic acid (TFA) at −10 °C to which had been added various quantities of the I2 solution. Other catalysts were diluted in TFA prior to using; bromine and boron trifluoride were used in forms of 33% HBr solution in CH3COOH and BF3·2CH3COOH complex. A fraction of the resulting reaction mixture was diluted in HPLC buffer and analyzed by Jasco binary pump system with Borwin software, 4.6×250 mm C-18 Alltech column, 1 mL/min 0.1 M CH3COOH-CH3COONH4 buffer pH 4.7 with CH3CN gradient 0–40% for 25 min; 325nm UV detection. The yield of product (EF5) was determined by comparison of AUC with the signal from a standardized solution of authentic EF5 [2-(2-nitro-1[H]-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide] that had been prepared by National Cancer Institute.
To confirm the relevance of our PET experiment conditions to maximal possible yield of the reaction, we also performed the reaction with an excess of fluorine gas. Iodine (2.5mg, 0.01mmol) was dissolved in trifluoroacetic acid (300 mL) at room temperature and used as a reaction catalyst. A 100 mL three-necked flask was equipped with a magnetic stirrer, a gas inlet tube (ID 0.5mm) for introduction of the 1% F2/Ar mixture, and an outlet to a bottle trap containing saturated potassium iodide solution. 30 ML of the I2-trifluoroacetic acid solution (containing 0.001 mmol of I2) was added to this flask and cooled to −10 °C. Precursor 1 was added in one portion with stirring to create a solution with the required ratio of I2 to precursor: for example, 26.4 mg (0.1 mmol) of precursor for ratio 0.01 (1%). Then F2 (1% in Ar, and obtained by dilution of 10% F2/Ar with argon) was introduced slowly (flow rate = 10.5 mL/min.) for about 20 minutes, with the excess of F2 being trapped by the potassium iodide solution. After removal of solvent, the residue was dissolved in d6-acetone (1 mL), and trifluoromethylbenzene was added to the solution as an internal standard in order to determine the yields using 19F NMR.
Results
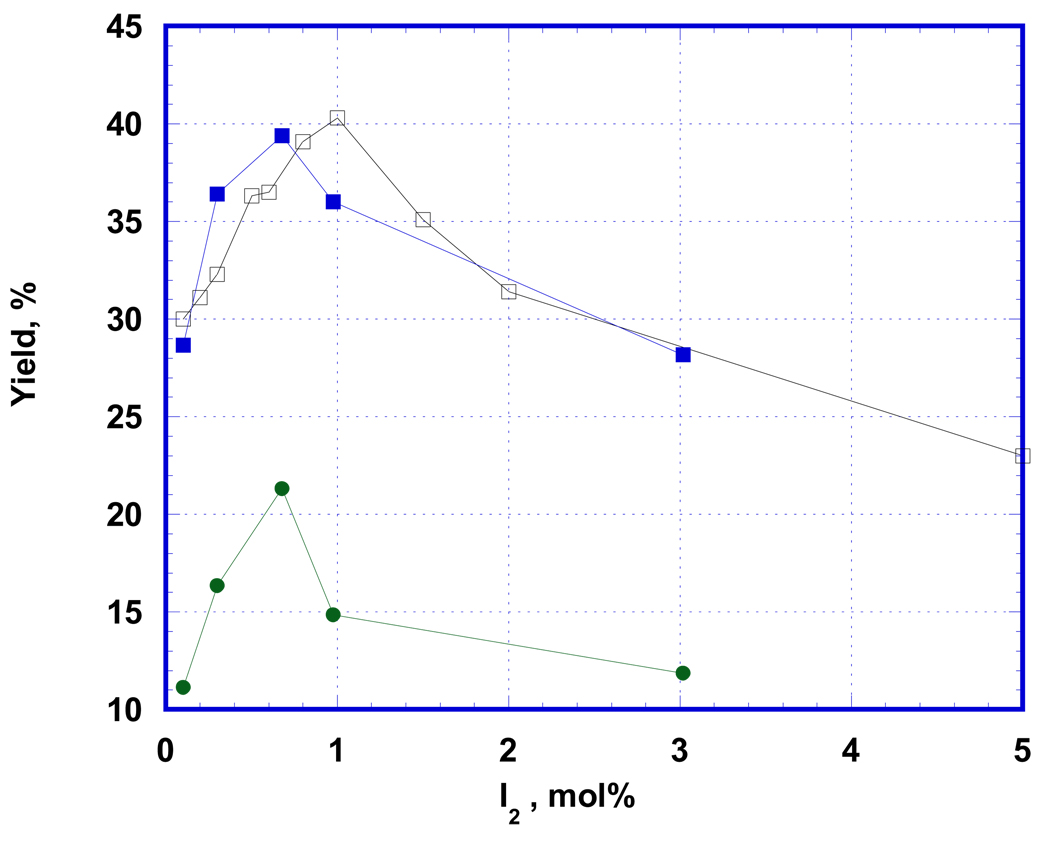
The beneficial effect of iodine on the conversion of precursor 1 to EF5 (Fig.1) is observed only at very low catalytic concentrations of I2. Based upon results obtained at different conditions, maximal enhancement of product yield occurs between 0.5 and 1 mol. % of I2 vs. total amount of precursor used. Optimal catalyst concentration causes an increase in EF5 production of about fifty percent (relative to the amount of precursor 1) (Fig. 1, curve 1), and almost doubles the level of fluorine uptake into product (represented as yield vs. F2 in Fig. 1, curve 3). The difference between the two methods of yield calculation derives from both the non-stoichiometric ratio of the reagents and the incomplete consumption of precursor 1 during the reaction. Although the overall reaction has formal equimolar stoichiometry, a significant amount of precursor remains in solution even under conditions of two-fold excess of fluorine being used to obtain the data in Fig. 1 (60–70 µmols of F2 bubbled through a solution of 25–30 µmols of 1 in 8 mL TFA). This probably occurs because highly reactive fluorine gas may participate in various unquantified side reactions.
Figure 1.
Catalytic effect of iodine (mol. % vs. precursor) in conditions of [18F]-EF5 synthesis (25–30 µmols EF12A in 8 mL TFA with 60–70 µmols of 0.1% F2) on the EF5 yield (upper curve, closed squares) and degree of fluorine incorporation. For comparison, upper line with open squares shows the product yield at F2 excess, when conversion of precursor into product is maximal. Optimal amount of catalyst (between 0.5 and 1 % of iodine) almost doubles the degree of fluorine incorporation into product, while high concentrations of product lose the catalytic effect.
Similar results were obtained when using excess 1% F2/Ar gas, with optimal results being observed at about 1% (Fig. 1, curve 2). In this study, it was found that the conversion of 1 to EF5 was actually decreased at concentrations of I2 as high as 5%.
With increasing iodine concentrations above the optimal, the product yield gradually decreases, approaching the level of the non-catalyzed reaction at about 3%. Not only does the presence of high concentrations of iodine in the reaction mixture not cause an enhancement of product yield, but it results in observance of additional radioactive signals during separation of crude EF5 product by semi-preparative HPLC in comparison with pure F2 gas (data not shown). This suggests that iodine may be involved in other, non-EF5-productive processes that somehow negate its catalytic effect. Iodine itself does not react with 1. When I2 is added to precursor 1 in the absence of F2, its light purple color persists indefinitely in solution. Once fluorine addition commences, the solution becomes colorless, with the purple color being restored shortly after F2 addition is completed. These observations suggest that the catalytic action of iodine is mediated by some iodine-fluorine intermediate compound of low stability.
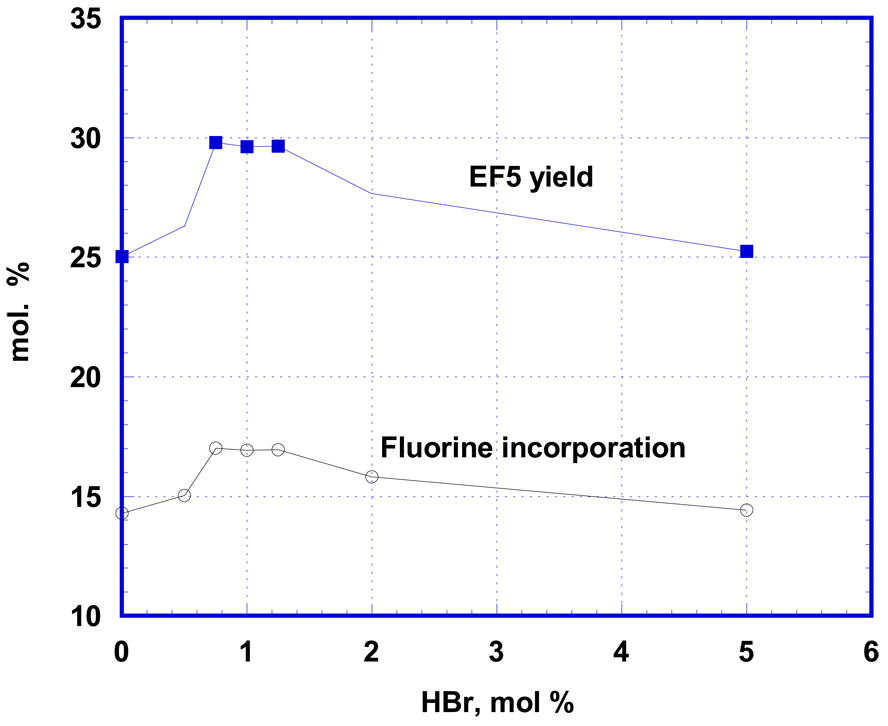
The observed catalytic action of iodine led us to examine the possibility of similar influence by bromine. Use of pure Br2 as a catalyst posed experimental problems deriving from the handling of small quantities of this volatile liquid, and there was also the possibility of side reactions of this more reactive halogen with precursor 1. Because of these potential problems, a commercial solution of HBr in CH3COOH was used, with the anticipation that its contact with F2 would serve to generate Br2 or BrF. In the event, the effect of small concentrations of HBr on conversion of 1 to EF5 (Fig. 2) turned out to be similar to, but less profound than the effect of I2. It gave rise to about 20% enhancement of the reaction yield with maximum effect at concentrations between 0.7 and 1.5 mol. % of HBr. The fact that the maximum efficacy of HBr as a catalyst occurred at a concentration about twice that of I2 catalysis suggests that one bromine atom from HBr (versus two iodine atoms from I2) is utilized in providing the catalytic activity and that both have a similar mechanism of action.
Figure 2.
Catalytic effect of HBr (20 µmols EF12A in 6 mL TFA with 35 µmols of F2) is similar, but less profound. Maximal catalytic effect is shifted to higher concentration of HBr, suggesting involvement into catalytic action one bromine atom from HBr molecule vs. two iodine atoms in previous case.
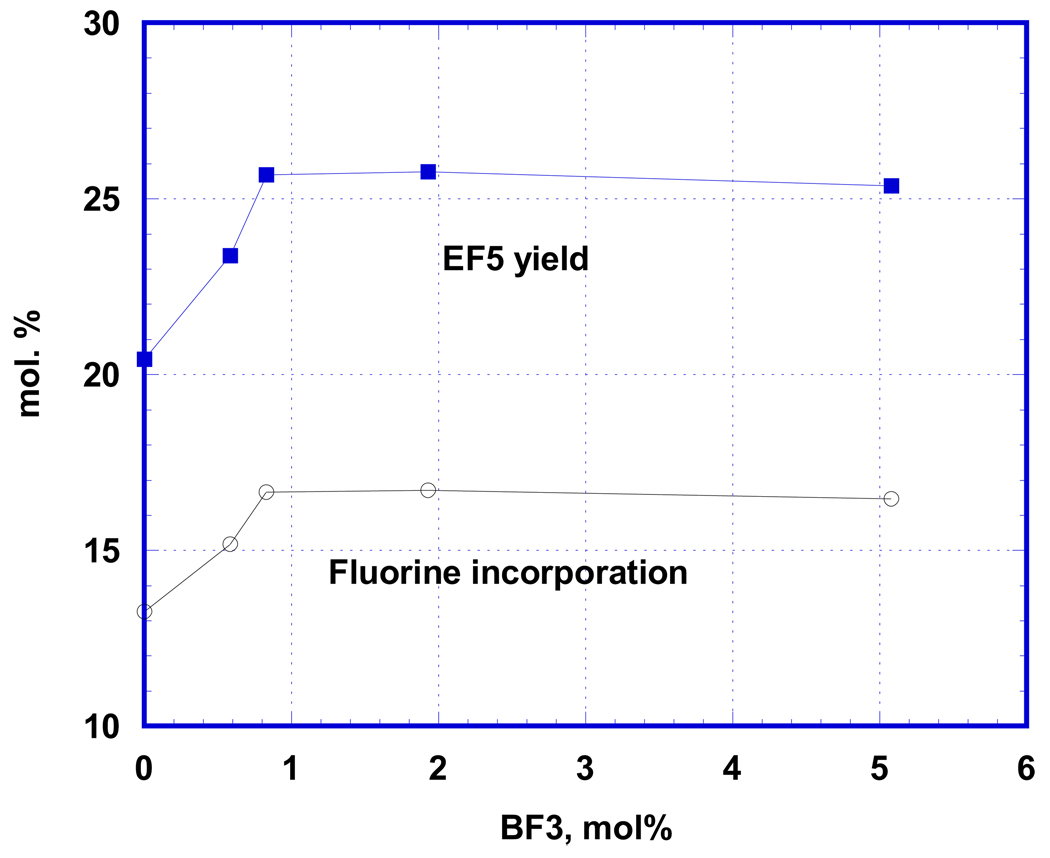
We hypothesized, that the above catalysts of the reaction could act as Lewis acids and tested several other compounds. Strong oxoacids (HClO4 and H2SO4) had no effect on the reaction (most likely due to lack of dissociation in strong acidic media of TFA), nor did triethylborane or complexes of BF3 with water or diethyl ether. However, the boron trifluoride complex with acetic acid (BF3·2CH3COOH) caused about a 30% enhancement of product yield (Fig. 3). The effect again reached its maximum at a relatively low concentration (0.8 mol. % of BF3), this time retaining its effect at higher concentrations.
Figure 3.
Effect of BF3 (23 µmols EF12A in 6 mL TFA with 35 µmols of F2) does not demonstrate loss of catalytic activity at high concentrations. This suggests that BF3 (unlike bromine and iodine in previous cases) is not involved in side reactions.
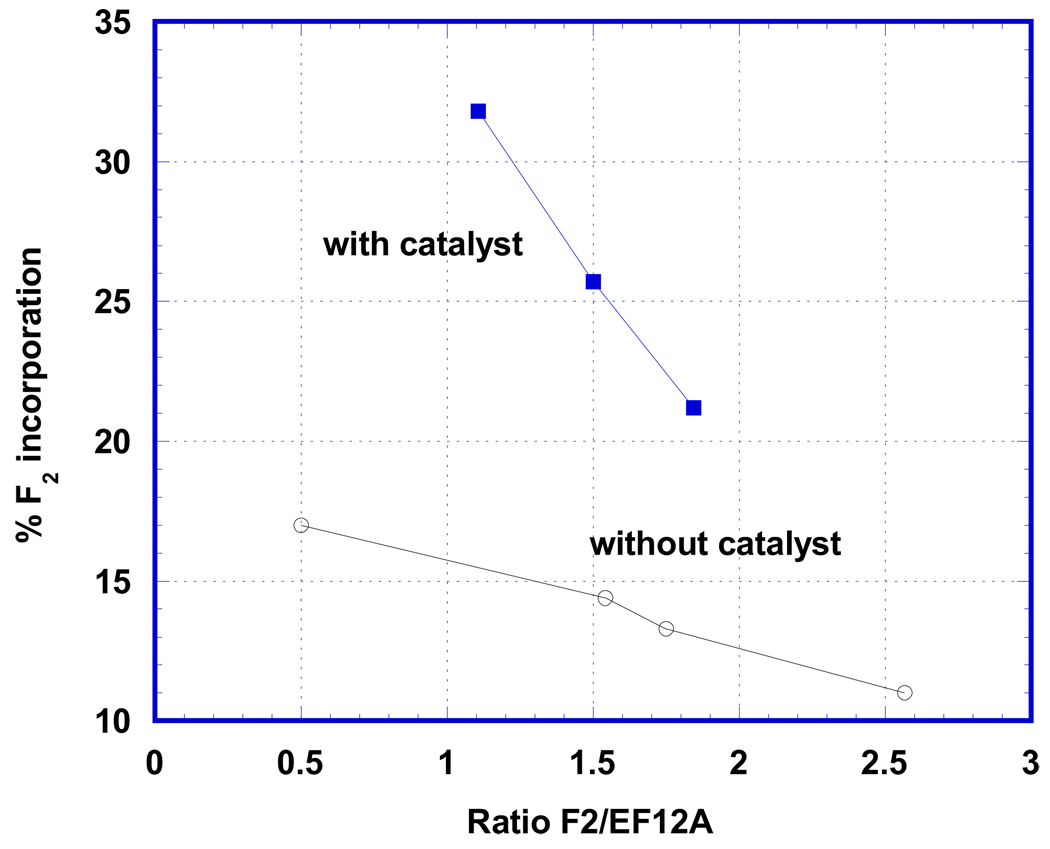
Due to importance of the reaction in the synthesis of 18F PET agents, our ultimate goal in this study was to increase the level of fluorine incorporation into the final product. One possibility for accomplishing this involved variation of the ratio of fluorine to precursor (Fig. 4, lower curve). Indeed, using an excess of precursor led to some increase of yield vs. fluorine, but such approach was rather impractical for the preparation of PET markers because of the aforementioned problems of product separation. At the optimal level of iodine catalyst (0.7 mol. %) a significant increase of fluorine incorporation was also observed. The upper curve of Fig. 4 shows the variation of % fluorine uptake as a function of total fluorine/precursor 1 ratio under optimal conditions of I2-catalyzed [18F]-EF5 synthesis. The degree of fluorine incorporation in presence of catalyst (32%) is comparable with the best yield of the reaction that had been obtained earlier using a large excess of fluorine [Dolbier et al, 2001]. Overall, the results described above clearly show that use of the iodine catalyst allows optimization of EF5 preparation, and they offer the possibility of similar catalysis of preparations of other 18F PET agents that are prepared by use of [18F]F2.
Figure 4.
Fluorine incorporation into product at different ratios of F2/precursor. Lower line represents data without catalyst (one more point 5% at ratio 6 [Dolbier et al, 2001] is not shown) and upper line was obtained in the presence of optimal (0.7 mol. %) iodine concentration.
Discussion
In our initial report of the reaction of [18F]-EF5 synthesis [Dolbier et al, 2001] we found that the yield of EF5 measured vs. amount of precursor, could be as high as 32% using a 6-fold excess of fluorine gas. However, the degree of F2 uptake (yield vs. F2) at these conditions was only 5%, which was far from optimal for preparation of 18F PET agents, where incorporation of radioactive fluorine should be as high as possible. In contrast, use of a two fold excess of precursor led to an increase of F2 incorporation up to 17%. Still, using an excess of precursor can be problematic in practice because of resultant problems with purification of the final product. Although EF5 is the major product of the reaction, numerous side-products are evident as there are dozens of small UV325/radioactive peaks observed on the chromatogram of crude product. Presence of these impurities requires a separation of reaction mixture with semi-preparative HPLC rather than simple SepPack cartridges. However, since the amount of fluorine gas in the cyclotron target can not be lower than certain limits (determined by target volume, minimal gas pressure for efficient stopping of deuteron beam, and at least 0.1% fluorine concentration in gas mixture), more efficient trapping of activity would also require high amount of precursor. This creates problems with crude product solubility and HPLC column overload, leading to deterioration in separation of compounds with a resultant decrease in purity of final product.
The addition of a catalyst to the reaction mixture leads to both an increase in product yield and enhanced fluorine uptake, making the overall method more efficient. The catalytic effect of iodine in the reaction was discovered rather accidentally as a result of contamination of the reaction mixture by traces of iodine from the solution of KI, which was used as a trap for excess fluorine in the reaction. The amount of contaminant was sufficiently low (estimated as dozens of micrograms) to be not clearly visible, yet sufficient to give rise to a noticeable catalytic effect. Interestingly, greater amounts of iodine contamination would have led to no enhancement, and would have been ignored.
In considering a possible mechanism of action for the observed I2 catalysis, one first must consider that IF is formed quantitatively from the reaction of I2 with F2 [Rosen and Zamir, 1991], and secondly that IF undergoes efficient electrophilic addition to fluorinated carbon-carbon double bonds [Sartori and Lehnen, 1971]. Moreover, it has been found that perfluoroalkyl iodides, in the presence of F2, will be converted to the respective hypervalent difluorides, which decompose to eliminate IF and form a C-F bond [Rondestvedt, 1969; Alam and Janzen, 1987; Zupan and Pollak, 1976]. Also, iodine monofluoride is less reactive in comparison with fluorine gas, making an overall reaction less vigorous and decreasing level of unwanted side reactions. Thus, it is certainly feasible that the presence of catalytic amounts of I2 during the fluorination of fluorinated alkene precursor 1 could lead to the observed enhancement of yield and efficiency of the reaction.
Possible mechanism of iodine action includes the following reactions:
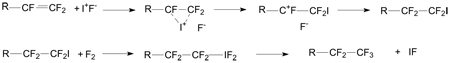 |
An intermediate compound with addition of iodine to another carbon atom with the same result can also be proposed, although consideration of steric factors makes the mechanism above more preferable.
The same arguments can be applied to a catalytic action of bromine. Hydrogen bromide reacts with fluorine producing Br2, which can further form bromine monofluoride, described long ago [Ruf and Braida, 1933]. It has also been reported that Br-F behaves similarly to I-F in its reactions with olefins [Rosen and Brand, 1985], and this analogy can explain similarities in catalytic action of iodine and bromine. A reduced effect of bromine in comparison with iodine may correspond to its higher electronegativity, which decreases partial positive charge on the bromine atom and subsequently makes it a weaker Lewis acid less efficiently attacking double bond. Also bromine monofluoride should be more reactive in comparison with IF causing higher level of unwanted side reactions. The catalytic effect of boron trifluoride can be attributed to its behavior as Lewis acid and formation of π-complex with double bond, which makes it more susceptible to electrophilic attack. Still these catalysts are less efficient and convenient for practical using in comparison with iodine.
Conclusions
Presence of small (0.5–1 mol. %) amounts of iodine, bromine, or boron trifluoride increases overall reaction yield in the reaction of electophilic fluorine addition to a fluorinated double bond. This catalytic action of heavy halogens can be explained by generation of their monofluorinated derivatives acting as intermediate compounds in the addition reaction. Presence of catalysts causes a significant (up to 50%) increase of degree of fluorine incorporation, which makes the reaction more efficient for preparation of 18F PET agents.
Acknowledgements
The work was supported by NIH grant CA87645.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Alam K, Janzen AF. Reactions of xenon difluoride. Part 6. Some reactions of phosphorus, arsenic and iodine compounds. Journal of Fluorine Chemistry. 1987;36:179–184. [Google Scholar]
- 2.Dolbier WR, Jr, Li AR, Koch CJ, Shiue CY, Kachur AV. [18F]-EF5, a marker for PET detection of hypoxia: synthesis of precursor and a new fluorination procedure. Applied Radiation & Isotopes. 2001;54:73–80. doi: 10.1016/s0969-8043(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 3.Evans SM, Hahn SM, Judy KD, Lustig R, Saffer JR, Karp JS, Freifelder RH, Kachur A, Alavi A, Koch CJ. 18F EF5 PET imaging with immunohistochemical validation in patients with brain lesions. International Journal of Radiation Oncology, Biology and Physics. 2006;66:S248. [Google Scholar]
- 4.Komar G, Seppanen M, Eskola O, Lindholm P, Gronroos TJ, Forsback S, Sipila H, Evans S, Solin O, Minn H. F-18-EF5: A new PET tracer for imaging hypoxia in head and neck cancer. Journal of Nuclear Medicine. 2008;49:1944–1951. doi: 10.2967/jnumed.108.053785. [DOI] [PubMed] [Google Scholar]
- 5.Rondestvedt CS., Jr Organic polyvalent iodine. Perfluoroalkyl iodide polyfluorides. I. Preparation and properties. Journal of the American Chemical Society. 1969;91:3054–3061. [Google Scholar]
- 6.Rozen S, Brand M. A new method for introducing iodo and bromo fluorides into organic molecules using elemental fluorine. Journal of Organic Chemistry. 1985;50:3342–3348. [Google Scholar]
- 7.Rozen S, Zamir D. Conversion of the carbonyl group the CF2 using IF. Journal of Organic Chemistry. 1991;56:4695–4700. [Google Scholar]
- 8.Ruf O, Braida A. Bromine monofluoride. Z. Anorg. Allgem. Chem. 1933;214:81–90. [Google Scholar]
- 9.Sartori P, Lehnen AJ. Addition of iodine monofluoride to halogenated olefins. Chemische Berichte. 1971;104:2813–2820. [Google Scholar]
- 10.Ziemer LS, Evans SM, Kachur AV, Shuman AL, Cardi CA, Jenkins WT, Karp JS, Alavi A, Dolbier WR, Jr, Koch CJ. Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-EF5. European Journal of Nuclear Medicine & Molecular Imaging. 2003;30:259–266. doi: 10.1007/s00259-002-1037-5. [DOI] [PubMed] [Google Scholar]
- 11.Zupan M, Pollak A. Stereochemistry of iodofluorination of phenyl-substituted olefins with (difluoroiodo)methane. Journal of the Chemical Society. Perkin Transactions. 1976;1:745–748. [Google Scholar]