Abstract
An early diagnosis of malignancies correlates directly with a better prognosis. Yet for many malignancies there are no readily available, non-invasive, cost-effective diagnostic tests with patients often presenting too late for effective treatment. This paper describes for the first time the use of fibre diffraction patterns of skin or fingernails, using X-ray sources, as a biometric diagnostic method for detecting neoplastic disorders including but not limited to melanoma, breast, colon, and prostate cancers. With suitable further development, an early low-cost, totally non-invasive yet reliable diagnostic test could be conducted on a regular basis in local radiology facilities, as a confirmatory test for other diagnostic procedures or as a mass screening test using suitable small angle X-ray beam-lines at synchrotrons.
Keywords: cancer diagnosis, fibre diffraction, skin, nails
Introduction
Diagnosis of malignancies currently requires direct targeting of affected organs with detection via mammogram, ultrasound, MRI, biopsy or other methods. Research over the last decade has demonstrated more “indirect” detection and diagnostic methods. Early research used hair samples but there also appears to be potential in the use of nail and skin samples. In ways not yet understood, malignancies elsewhere in the body appear to leave molecular level “signatures” in hair, skin and nail tissues as well as in the local tissues. These distinctive signatures can be distinguished using X-ray fibre diffraction techniques, as are now discussed.
Biological macromolecules such as DNA, muscle, collagen and keratin cannot be crystallized and so cannot be studied by routine protein crystallography. However, these tissues belong to a group of fibrous macromolecules where the long polymeric structures are parallel to each other either intrinsically or induced mechanically. The α–keratin plates in fingernails are composed of fibres which are intrinsically aligned whilst orientation of the collagen fibres in the dermal layer of the skin can be achieved by stretching to remove the natural crimp.
These pseudo crystalline rod-like structures can be studied by fibre diffraction. For this, the oriented fibres are placed in a collimated X-ray beam, so that the parallel fibres are at right angles to the beam and the pattern of the X-rays diffracted at very low angles is recorded and analysed1. Diffraction study of such fibres is now routinely achieved in minutes using laboratory-based rotating anodes combined with multi-layer optics or suitable small angle scattering synchrotron beams.
Fibre diffraction techniques have now been used extensively in the study of muscle, collagen and keratin 2,3,4. They have been used to examine changes from normal tissue in pathological tissue specific to a disease of that tissue 5,6,7,8,9. Additionally, changes in the molecular structures of hair10 and of the dermal layer of skin11 in breast cancer patients related changes in tissues remote from the affected area which could be associated with the malignancy. Specific changes in the structure of hair associated with colon cancer and Alzheimer's disease have also been published12,13. This “signature-at-a-distance” effect was surprising but has been consistently found in subsequent studies
What appears in diffraction analysis of the different cancers is a distinctive ring, different malignancies having specific ring patterning superimposed on the normal hard alpha keratin pattern14. This “normal” diffraction pattern for alpha keratin of hair and nails of all mammals, regardless of species or age, was first reported by Astbury and Street15 in 1931. Then followed rapid refinement of the experiment culminating in the highly detailed patterns of T.P.MacRae16,17
Since the keratin pattern itself remains unaltered for hair from cancer patients, the additional rings, radii specific to the cancer type, indicate that the randomly arranged extra material in the hair, which gives rise to these extra rings, is not associated in any way with the helical sections of the alpha-keratin. By contrast, the “normal” diffraction pattern of hair is changed with diseases such as insulin dependent diabetes mellitus where the intensity distribution of the meridional arcs is altered and the radius of the intermediate filaments is increased indicating that material is actually bound to the helical section of the alpha keratin18.
Interestingly, whilst breast and colon cancer caused specific changes in the diffraction pattern of hair, no change was found in the diffraction patterns of hair for patients with any lung cancer, liver cancer, basal cell skin carcinoma, melanoma, or prostate cancer14,19 even though more than 50 different samples from patients with each of these cancers were investigated in blinded trials. These samples included hair from patients with Grade 7 prostate cancer and Grade 7 adeno carcinoma of the lung. Since changes in the diffraction patterns of skin had been reported for breast cancer10, fetal tissue during embryogenesis20, and with insulin dependent diabetes and ageing21 and Mayo Clinic had reported changes in nails related to seven different diseases22, this investigation was carried out to determine whether any of these carcinomas might cause changes in the diffraction patterns of skin or nails. The results of trials on these alternative tissues are now discussed. The potential is for trouble-free and cost-effective diagnostic tests for a wider list of diseases.
Materials and Methods
The materials and methods for skin and nails are now described separately in terms of collection, preparation treatment and mounting of samples. A summary of common sections of the diffraction experiments and analysis is then provided.
(a) Skin Study
A total of 266 samples of skin (265 double blinded samples and 1 unblinded sample from a melanoma patient) have been studied from people aged between 18 and 90 using either rotating anode X-ray or synchrotron sources. After analysis these samples were shown to have included 197 controls, 52 samples from patients diagnosed with cancer,(12 prostate, 28 melanoma, 3 basal cell carcinomas), 9 from patients with a pathologically changed BRCA1 gene, 12 insulin dependent diabetic mellitus patients, and 5 radiation-hypersensitive patients. These samples were obtained from routine surgery, necropsies, or from punch biopsies. Unless the sample was removed during routine surgery for the carcinoma, the skin samples were isolated from the underarm of patients because of the relatively low level of ultra violet exposure/damage at this site. All controls had been cleared of the diseases being studied and other skin complaints in routine medical checks. No prior treatment is required before harvesting of the sample as the surface layers are removed and not used. Only the dermal layer is used in the diffraction study.
Immediately after excision, the samples were placed in physiological saline and stored at −20°C until required. Previous test have shown that no deterioration occurs in samples stored in this way for at least 12 months. Before mounting in the cells, which have been specifically designed to maintain 100% humidity throughout the experiment, the skin samples, approximately 1mmx5mm in size, were gently scraped to remove the epithelial and epidermal layers and so to expose the dermal layer. These cells also allowed for the skin samples to be stretched slightly removing the natural crimp, using the sutures surgically applied to the 1mm ends. The 2D collagen sample is thereby aligned preferentially in the direction of the applied stretch thus giving rise to the arcs in the meridional (vertical) pattern. The angular spread of the arcs is determined by the degree of alignment. If perfectly aligned, spots not arcs would be seen, as is the case in the diffraction patterns of tendon. The meridional lattice spacing for wet skin from controls is 65.2nm23,24. The equatorial (horizontal) pattern reflects the cylindrical packing arrangement of the collagen.
(b) Nails Study
Nails consist of rigid and durable dense keratinised plates. Some human and animal samples were examined on the BioCAT Facility, Advanced Photon Source, and were found to provide excellent diffraction pictures. Ninety-six blinded human nail clippings were examined in total using either small angle synchrotron X-ray beams or finely focussed X-ray beams from rotating anode sources enhanced by multi-mirror optics. After analysis, the samples were found to include 47 controls and 49 samples from cancer patients including 22 samples from breast cancer patients, 7 samples from persons with colon cancer, 3 samples each from persons with melanoma and lung cancer and 14 samples from persons with prostate cancer. The age range of the samples was from 3 months to 80 years. Although the selection of controls for these initial experiments was far from ideal, those from over age 40 had been cleared by two-recent successive mammograms and/or had been cleared in a recent colonoscopy. Those from under 40 were in good health and had no family history of breast or colon cancer.
For the diffraction experiment, small flat sections of the nails, approximately 1mm x 2mm, were cut from the nail clippings with the shorter section in the direction of the keratin fibres. These “crystallites” were fixed to the ends of quartz micro-tubules or pins which were then attached to a plate, specially grooved to accommodate the micro-tubules or pins. In the rotating anode experiments, the “crystallites” were attached to goniometers to further assist alignment. This optimum size of the nail sample eliminates the inherent curvature and as a consequence eliminates the disorder resulting from the curvature. This disorder, if present, would mask the breast cancer ring but not the colon cancer ring, as illustrated in Figure 1. The sample holders were positioned so that the nail to be irradiated protruded beyond the edge of the plate in the direction of growth of the keratin fibrils thus giving rise to the meridional (vertical) pattern for keratin. The arcs in the meridional pattern result from the repeat distances within the helical sections of the alpha-keratin fibrils giving rise to the 2 infinite lattices with repeat spacings of 46.7nm and 62.6nm and a number of finite lattices4,26. The equatorial (horizontal) pattern of spots arises from the transverse cylindrical packing of the intermediate filaments of the keratin4,25,26. The arcs in the equatorial pattern result from soaps between the filaments27.
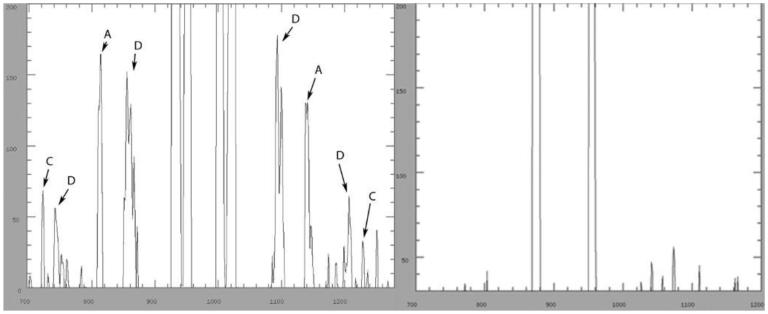
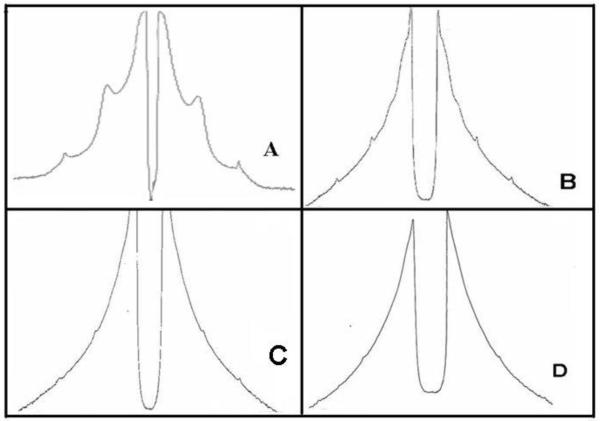
Figure 1.
The graphs are diagonal intensity cuts through 2 patterns from fingernails. The right hand graph is from the pattern of a control which has been correctly mounted so that there is no disorder. The left hand pattern shows the first and second order of diffuse disorder rings, D, resulting from a curved sample. Peaks indicated by A denote the disordered 67Å peaks which are elongated to form a ring. The presence of the colon cancer ring is evidenced by the peaks C.
It was not necessary to attach the samples at both ends as the nail sample is absolutely rigid. However, it was found that manicured nails gave the best results. They were flatter and smoother. So all nail clippings, both toe and finger, were buffed on both sides using in turn the coarse, the soft and the silky sides of a buffing block. The nails were then dissected into the small flat sections used for the study. No treatment of the nail was required prior to the clipping of the nail sample; any polish is easily removed prior to buffing. All layers of the nails exhibited the changes if any were present.
(c) Diffraction and Analysis Common to Both Skin and Nails
After successful initial tests using the GX21 Rotating Anode X-ray generator at the Rex Vowels Low Angle Diffraction Laboratory, UNSW, using sample to detector distances of 200mm and 1000mm, skin samples were studied at the BL15A Photon Factory, Tsukuba, Japan using sample to detector distances of 2000mm, 400mm and 250mm. Calibration was achieved by comparison with diffraction patterns from moist rat-tail tendon. Both skin and nails were also studied at the BioCAT Facility, the Advanced Photon Source (APS), Argonne, USA using sample to detector distances of 1048.5 mm, measured using silver behanate (first order spacing: 5.838 nm). The fingernail controls, animal and human, were also studied on the ChemMatCARS facility (APS). The synchrotron fibre diffraction intensity distributions were recorded on FUJI BASIII imaging plates at the Photon Factory and at BioCAT and the data was extracted by electronic scan. A MAR detector was used on-line on ChemMatCARS and also on BioCAT to aid in the alignment of the sample and to record the diffraction patterns. Further studies were conducted using rotating anode generators in Europe and Japan.
Initial analyses were carried out using a combination of MATLAB-R-2007 and ProcessFITS or SAX15ID. This was followed by a high precision analysis of the two dimensional data using the Smithsonian astronomy packages IRAF (1986) and SAO Image (1991). These packages were designed for viewing stars over a wide range of intensities from the very weak to the very strong by limiting the intensity range under observation. They are therefore perfectly designed for handling the wide dynamic range of intensities obtained from both imaging plates and detectors. This is especially important as changes associated with Grade 1 tumours can be extremely weak and not always apparent using other computer programs.
(d) Ethics
All experiments and procedures have been approved by the appropriate Ethics Review committees of the Institutions that supplied the samples as well as by the ethics committees of the respective synchrotrons and by the host institutions of the individual beam-lines at the synchrotrons.
Results
Both skin and nail samples produced excellent diffraction patterns. Detailed descriptions of the normal patterns for collagen and keratin have been given elsewhere12, 21.
Changes with ageing
A study of post-partum samples of keratin for ages ranging from 0 to 80 years, yielded patterns which showed no changes in the meridional pattern and a variation of only 4.0±0.5 nm to 3.87±0.5 nm in the radial dimensions of the intermediate filaments18. Changes in the diffraction pattern of skin with ageing have also been reported11. These changes indicate a parabolic distribution of the fibrillar radial dimension for normal skin with age, the maximum occurring in the 40-50 age range. No change in the meridional diffraction pattern for skin was observed over the entire age range (0 to 80 years). For diagnostic purposes, only the differences between normal age-matched patterns and the patterns for pathological samples have to be evaluated. All such changes and additions for the human samples investigated so far are listed in Table 1 for nails and in Table 2 for skin. To date, apart from the changes noted for Alzheimer's disease and insulin dependent diabetes, only BRCA gene and non-BRCA gene related breast cancer and colon cancer show specific changes in the diffraction patterns of nails. Unlike the additional near-equatorial spots observed in Alzheimer's disease and the change in the meridional intensity distribution observed in insulin dependent diabetes mellitus, the changes in fingernails for the neoplastic diseases are additional rings. These rings are superimposed on the normal pattern without causing any changes in the normal keratin pattern itself. Based on the accepted α-keratin D-spacing of 46.8±0.3nm, obtained from the 91st order of this lattice, the relative spacing in real space using the first order only of the colon cancer ring is 4.53 ± 0.05 nm and that of the breast cancer ring is 4.71 ± 0.05 nm. An example of the change in the diffraction pattern of finger-nails with non-BRCA gene related breast cancer is given in Figures 2.
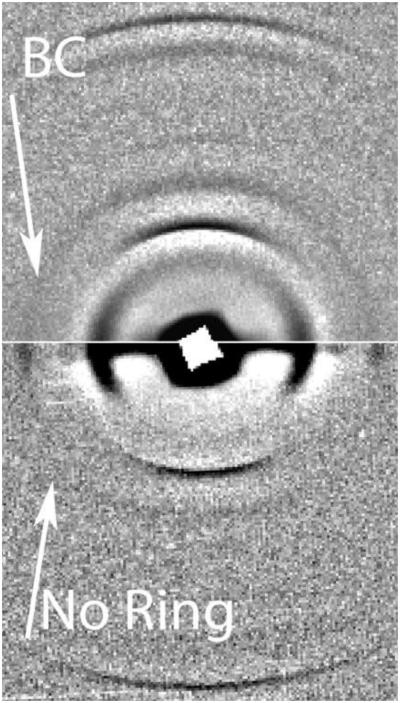
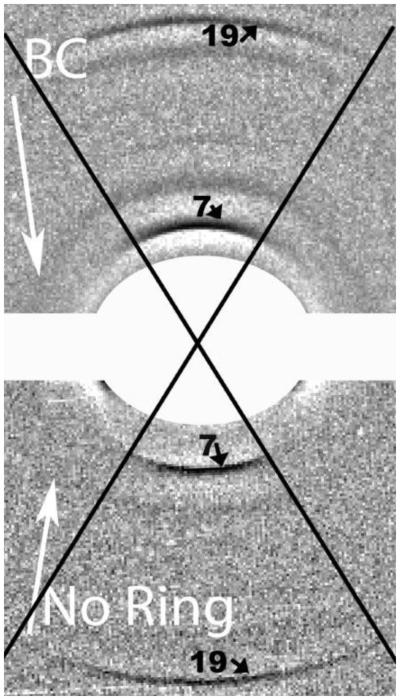
Figure 2.
This composite diffraction pattern is from fingernails. The lower pattern is from a control. The BC arrow on the right hand side of the upper pattern indicates the presence of the breast cancer diffraction ring obtained from a nail sample from a breast cancer patient. Figure 2 INSERT shows the central portion of the composite pattern. The strong 7th and 19th meridional orders of the 46.7nm lattice are indicated. The angular spread of the meridional orders is defined by the black cross. Only the very strong arcs of the α-keratin ever extend outside these lines but the angular spread of these arcs is defined by the alignment of the fibrils within the sample. The equatorial sections and the beam-stop section have been removed to eliminate the illusion of complete circles for all reflections. In the resulting picture the breast cancer ring is clearly visible in the top half (as indicated) and is absent in the lower half. When analysing, an inspection of the empty space away from the axes is carried out first. Intensity plots are first made in directions 40- 45° to the equatorial axis. As the breast cancer ring passes through one of the equatorial spots and lies very close to reflections on the meridional axis, it is best to steer clear of these congested areas.
Changes from normal were not found in the diffraction patterns for nail samples from patients with any form of lung cancer, prostate cancer, liver cancer or skin cancer even though samples from persons with Grade 7 tumours were used for the tests for adeno carcinoma of the lung and prostate cancer. However, clear and consistent changes were found in the diffraction patterns of skin samples taken from both Caucasian and African persons with prostate cancer and also for Caucasian persons with melanoma. As changes were first noted in African samples, it was necessary to eliminate the possibility that the keloid scarring of the epidermis that develops in the healing of African skin was responsible for the observed changes in the diffraction patterns. No such connection was found.
The changes noted in the diffraction patterns for both cancers were additional rings with spacings of 4.75 ± 0.05 nm for prostate cancer and 4.03±0.05nm for melanoma samples. These rings were again superimposed on the “normal-skin” collagen pattern obtained for the controls. The changes associated with melanoma and prostate cancer, together with those of other pathologies that have produced changes in the diffraction pattern of skin, are listed in Table 2 showing that these changes are unique to each of these neoplastic conditions. Figure 3a illustrates the pattern obtained for a normal skin sample, Figure 3b illustrates the pattern obtained that for the skin of melanoma patients using MATLAB and ProcessFITS and Figure 3c illustrates the pattern obtained for the skin of prostate cancer patients using the IRAF and SAO image packages. The additional rings superimposed on the normal collagen pattern for skin in melanoma patients and prostate cancer patients are indicated by the arrows marked M and P in Figures 4b and 4c respectively.
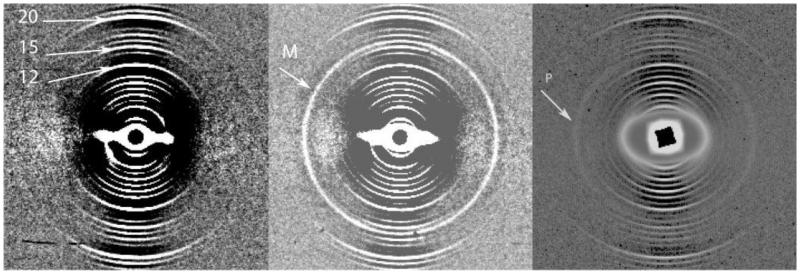
Figure 3.
The patterns in Figures 3a and 3b were obtained, using a combination of MATLAB and ProcessFITS, from the skin samples of a control and a female melanoma patient, respectively. The arrow (M) indicates the additional ring in the pattern from the melanoma patient which is centred slightly below the centre of the 16th order of the meridional pattern of the collagen. The diffraction pattern in Figure 3c is a typical pattern obtained for samples from prostate cancer patients. The numbers on the Figure 3a indicate the very sharp 12th, 15th and 20th orders respectively of the skin collagen meridional pattern. The extra diffuse ring, P, on the pattern in 3C is superimposed on the collagen pattern and falls between the 13th and 14th orders of the collagen pattern. This ring is specific to the presence of prostate cancer. Both these rings are different in radius from that reported for breast cancer.
Figure 4.
These intensity distribution graphs were obtained from equatorial cuts (1mm on either side of the x-axis) of diffraction patterns from skin samples. A is the pattern from a normal female. The others are from patients with basal skin carcinoma and were sections removed in surgery. B (91year-old female) is from skin adjacent to a basal cell carcinoma of the lip, frozen section pathology indicated 40% increased hyaline late stage elastin, 60% collagen. C (30 year-old female) was from skin of central forehead adjacent to a benign dermal naevus with only minor actinic damage. The decrease in the intensity of the equatorial peaks compares with the estimate from frozen section of 30% elastosis of the papillary layer only of the dermis. D (76 year old female) was from the skin of the left cheek adjacent to a basal cell carcinoma showing long standing damage of the dermal layer, estimate of 70% elastosis agreed with that from frozen section pathology. Although interesting, these changes are not sensitive enough to be used for a diagnostic test.
Figure 5 is a graphical illustration of changes found in the skin of patients with basal skin carcinoma which agree with the pathological findings in so far as the intensity of equatorial peaks which relate to collagen in the dermal layer decreases with the severity of the cancer. However, these changes cannot be measured reliably.
Discussion
Results reported in this paper indicate that diffraction analysis of nails and skin can be used in the early diagnosis of several malignancies. Combined nail and skin diffraction studies may provide an alternative diagnostic test for melanoma, breast, colon and prostate cancers.
Such molecular “signatures” of malignancies, evident in skin and nails as well as in hair, offer not just powerful early diagnostic methods but also further fascinating research. Currently there is no biological mechanism demonstrated. Whatever the mechanism, diffraction patterns are consistent and specific to the type of malignancy. To date there have been no false negatives in the skin and nail studies of blinded samples.
Diagnostic tests using skin and nail can be routinely studied at a reasonable time-rate and personnel cost in local laboratories using rotating anode X-ray generators and multi-mirror optics. At this stage insufficient samples have been studied to obtain statistically reliable specificities and sensitivities. Clinical trials are planned to commence in 2009.
Subject to further successful research outcomes, there does appear to be good potential for early low-cost, totally non-invasive yet reliable tests using easily accessible biological tissues which can be harvested at remote locations. Samples do not deteriorate with correct storage. Use of local radiology units for routine testing, using similar equipment to that used for the last 50 years, would remove the need for transportation to remote synchrotron facilities and the use of relatively high cost equipment. Suitable synchrotron beam-lines would be needed for high volumes of samples. Perhaps in the not-too-distant future, reliable, malignancy-sensitive and cost-effective diagnosis using fibre diffraction analysis of nails and skin may be a routine reality.
Acknowledgements
The author would like to acknowledge the financial support of the Whittington Hospital Postgraduate Research Fund, The Courthouse Collection, Western Australia, and of the Access to Major Research Facilities Program and the Australian Synchrotron Research Program, which are funded by the Commonwealth of Australia. The author also acknowledges the generous support of the Photon Factory for use of Beamline BL15A, of the BioCAT and ChemMatCARS facilities (Advanced Photon Source), Argonne National Laboratory and for the assistance provided by the beam-line staff of these facilities. The Advanced Photon Source is supported by the US Department of Energy, Basic Energy Sciences, Office of Science under Contract No.W-31-109-ENG-38 W-31-109-Eng-38. BioCAT is a National Institute of Health-supported Research Centre RR-08630. The author is deeply indebted to Rigaku Inc. for the generous support in the use of their rotating anodes and for the assistance provided by diffraction experts. The author is also indebted to Professors Albert Singer (Whittington Hospital, London), Mark McGovern, John Papadimitriou, Drs Terry Robertson and Graeme Langsford for their valuable assistance with this manuscript and/or their advice regarding this project. The author would also like to thank Associate Professor David Miller and Dr Marlene Read (UNSW, Australia) for their generous assistance at the synchrotrons and Dr Murugesan (Whittington Hospital) for assistance in the loading of samples. The author is deeply indebted to Daniel Greenfield (Cambridge University) for the computer program, ProcessFITS (2008), specially designed and developed for my use to expedite the inspection of raw data.
Reference List
- 1.Chandrasekaran R, Stubbs G. Crystallography of biological macromolecules. In: Rossmann MG, Arnold E, editors. International Tables for Crystallography, Vol F. Vol. 19.5. Kluwer Academic; Dordrecht: 2006. pp. 444–450. [Google Scholar]
- 2.Squire JM. Fibre and Muscle Diffraction. In: Fanchon E, Geissler E, Hodeau L, Regnard J, Timmins P, editors. Structure and Dynamics of Biomolecules. Oxford University Press; 2000. pp. 272–301. [Google Scholar]
- 3.Orgel JP, Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ. The in-situ supermolecular structure of type I collagen. Structure (Camb) 2001;9(11):1061–1069. doi: 10.1016/s0969-2126(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 4.Feughelman M, Willis B, James V. The Tetrameric Structure of the Intermediate Filaments of Alpha-keratin Fibres; Proc 11th Int Wool Research Conf; Leeds, UK. September, 2005; 65FI, electronic publication. [Google Scholar]
- 5.Hayes S, Boote C, Huang Y, Meek KM. The Relationship between Surface Corneal Topography and Stromal Collagen Organisation in Normal and Keratoconus Corneas. Fibre Diffraction Review. 2006;14:15–21. [Google Scholar]
- 6.Bradshaw JP, Miller A. Osteogenesis imperfecta: an X-ray fibre diffraction study. Annals of the Rheumatic Diseases. 1986;45:750–756. doi: 10.1136/ard.45.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connon CJ, Nakamura T, Hopkinson A, Quantock A, Yagi N, Doutch J, Meek KM. The Biomechanics of Amnion Rupture: An X-ray Diffraction Study. PLoS ONE. 2007;2(11):e1147. doi: 10.1371/journal.pone.0001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James V. Synchrotron fibre diffraction identifies and locates foetal collagenous breast tissue associated with breast carcinoma. J. Synchrotron Radiation. 2002;9(2):71–76. doi: 10.1107/s0909049502001504. [DOI] [PubMed] [Google Scholar]
- 9.James VJ, McConnell JF, Capel M. The d-spacing of collagen of mitral heart valves changes with ageing but not with collagen Type III content. Biochim. Biophys Acta. 1991;1078:19–22. doi: 10.1016/0167-4838(91)90086-f. [DOI] [PubMed] [Google Scholar]
- 10.James V, Kearsley J, Irving T, Amemiya Y, Cookson D. Using hair to screen for breast cancer. Nature. 1999;398:33–34. doi: 10.1038/17949. [DOI] [PubMed] [Google Scholar]
- 11.James VJ, Willis BE. Molecular changes in skin predict predisposition to breast cancer. JMedGenet. 2002;39(2):1e. doi: 10.1136/jmg.39.2.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James VJ. Fibre diffraction from a single hair can provide an early non-invasive test for colon cancer. Medical Science Monitor. 2003;9(8):MT79–84. [PubMed] [Google Scholar]
- 13.James VJ, Richardson JC, Robertson TA, Papadimitriou JM, Dutton NS, Maley MAL, Berstein LM, Lantseva OE, Martins RN. Fibre diffraction of hair can provide a screening test for Alzheimer's Disease: a human and animal model study. Medical Science Monitor. 2005;11(2):CR53–57. [PubMed] [Google Scholar]
- 14.James VJ. A place for fibre diffraction in the detection of breast cancer? Review Article. Cancer Detection and Prevention. 2006;30:233–238. doi: 10.1016/j.cdp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Astbury WT, Street A. X-ray studies of the structures of hair, wool and related fibres. I General Trans R. Soc. Lond. 1931;230:75–101. [Google Scholar]
- 16.Fraser RDB, MacRae TP, Miller A. X-ray diffraction patterns of α-fibrous proteins. J Mol Biol. 1965;14:432–442. doi: 10.1016/s0022-2836(65)80193-0. [DOI] [PubMed] [Google Scholar]
- 17.Parry DAD, Fraser RDB, Squire JM. Fifty years of coiled-coils and α-helical bundles: A close relationship between sequence and structure. J Struct Biol. 2008;163:258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 18.James VJ, Yue DK, McLennan SV. Changes in the molecular Structure of Hair in Insulin Dependent Diabetes. Biochim Biophys Res Com. 1997;233:76–80. doi: 10.1006/bbrc.1997.6405. [DOI] [PubMed] [Google Scholar]
- 19.James VJ. CAMBIOS DEL PATRON DE DIFRACCION DEL PELO EN CANCER DE MAMA. SIIC. 2007 Dec. 6 Section Expertos Invitados of www.siicsalud.com.
- 20.James VJ, McConnell JF, Amemiya Y. Molecular structural changes in human fetal tissue during the early stages of embryogenesis. Biochim Biophys Acta General Subjects. 1998;1379(2):282–288. doi: 10.1016/s0304-4165(97)00108-6. [DOI] [PubMed] [Google Scholar]
- 21.James J, Delbridge L, McLennan SV, Yue DK. Use of X-ray diffraction in the study of human diabetic and ageing collagen. Diabetes. 1991;40:391–394. doi: 10.2337/diab.40.3.391. [DOI] [PubMed] [Google Scholar]
- 22. www.mayoclinic.com/health/nails/WO00055.
- 23.Brodsky B, Eikenberry EF, Cassidy K. An unusual collagen periodicity in skin. Biochim Biophys Acta Proteins. 1980;621(1):162–166. doi: 10.1016/0005-2795(80)90072-0. [DOI] [PubMed] [Google Scholar]
- 24.Stinson RH, Sweeney PR. Skin collagen has an unusual d-spacing. Biochim Biophys Acta Proteins. 1980;621(1):158–161. doi: 10.1016/0005-2795(80)90071-9. [DOI] [PubMed] [Google Scholar]
- 25.James VJ, Amemiya Y. Intermediate Filament Packing in a -Keratin of Echidna Quill. Textile Res J. 1998;68(3):167–170. [Google Scholar]
- 26.Feughelman M, James VJ. Hexagonal Packing of Intermediate Filaments in α-keratin fibres. Textile Res J. 1998;68(2):110–114. [Google Scholar]
- 27.Briki F, Mérigoux C, Sarrot-Reynauld F, Salomé M, Fayard B, Susini J, Doucet J. Evidence of calcium soaps in human hair shafts revealed by sub-micrometer X-ray fluorescence. J. Phys. IV France. 104:337–340. doi: 10.1016/s0304-4165(02)00441-5. [DOI] [PubMed] [Google Scholar]