Abstract
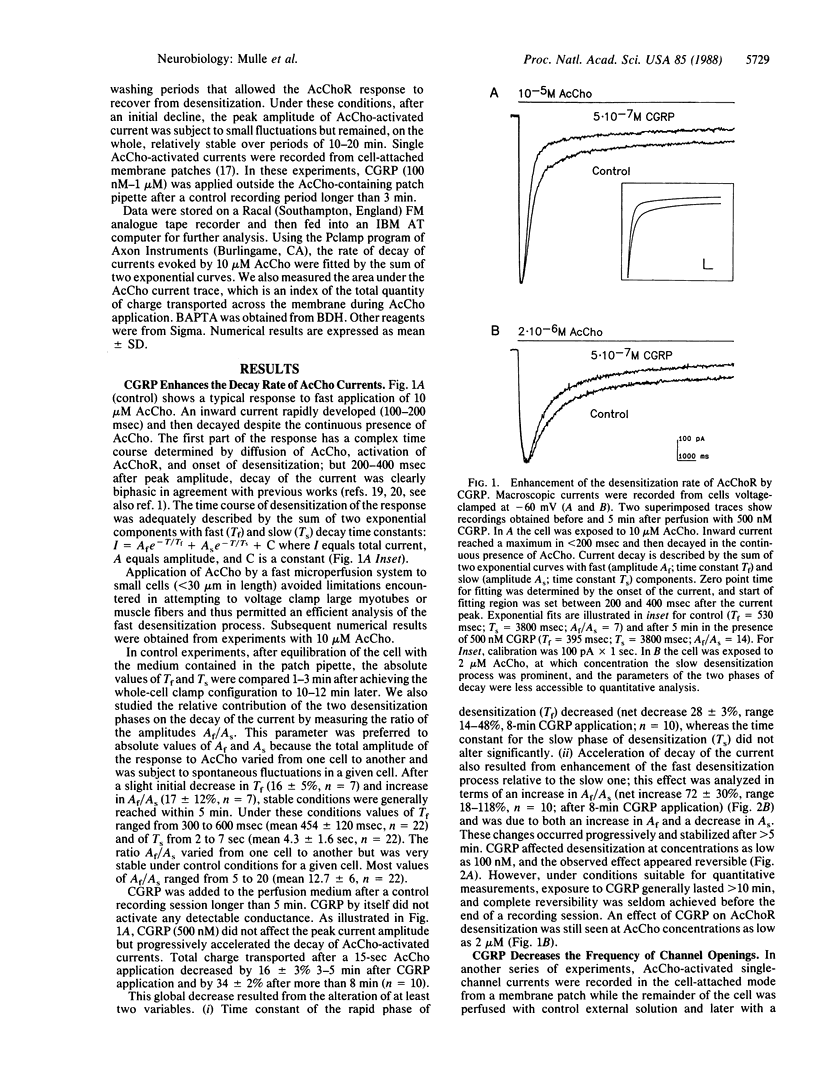
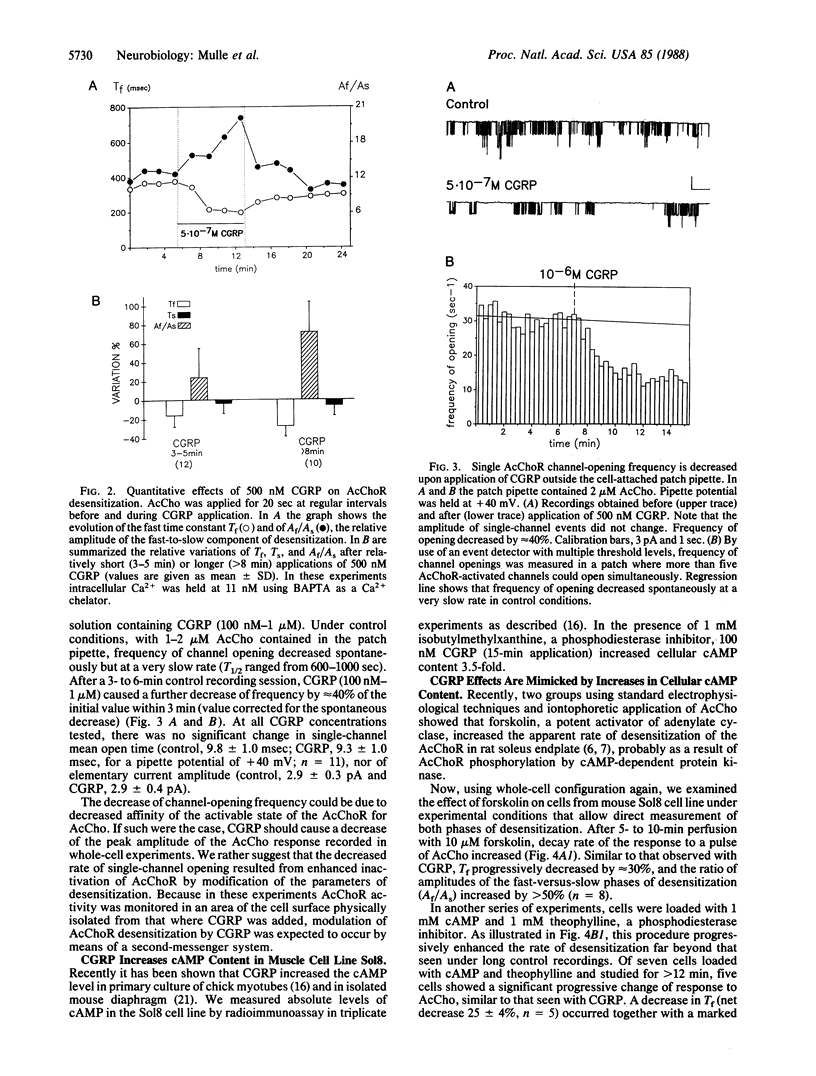
Calcitonin gene-related peptide (CGRP) is a neuropeptide that coexists with acetylcholine in spinal cord motoneurons. The effects of CGRP on the functional properties of the nicotinic acetylcholine receptor (AcChoR) were examined by electrophysiological methods. Using the whole-cell patch-clamp technique and a mouse cell line derived from soleus muscle, we found that CGRP produces a progressive and reversible enhancement of the rapid-decay phase of AcChoR desensitization. Single-channel data further show that CGRP decreases acetylcholine-activated channel opening frequency. This decrease occurs when CGRP and acetylcholine are applied on different cell-surface areas and thus is likely mediated by a second-messenger system. CGRP is also shown to increase cAMP accumulation in this cell line. The effects of CGRP on macroscopic acetylcholine-activated currents are mimicked by external application of forskolin (10 microM) or by internal perfusion of the cell with cAMP (1 microM). In both these cases, further application of CGRP produces no additional enhancement of AcChoR desensitization. These results suggest that, on mouse muscle cells, CGRP regulates AcChoR desensitization by a mechanism that involves, at least in part, cAMP-dependent phosphorylation of the AcChoR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., Zani B. M., Nervi C., Senni M. I., Molinaro M., Eusebi F. Acetylcholine stimulates phosphatidylinositol turnover at nicotinic receptors of cultured myotubes. FEBS Lett. 1985 Oct 7;190(1):161–164. doi: 10.1016/0014-5793(85)80449-x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Blosser J. C., Appel S. H. Properties and distribution of mammalian skeletal muscle guanylate cyclase. Alterations in denervated and dystrophic muscle. J Biol Chem. 1978 May 10;253(9):3088–3093. [PubMed] [Google Scholar]
- Changeux J. P. Coexistence of neuronal messengers and molecular selection. Prog Brain Res. 1986;68:373–403. doi: 10.1016/s0079-6123(08)60252-6. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. E., Role L. W. Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7739–7743. doi: 10.1073/pnas.84.21.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem. 1987 Nov;49(5):1634–1643. doi: 10.1111/j.1471-4159.1987.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci Lett. 1986 Oct 30;71(1):59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Un modèle moléculaire de régulation d'efficacité au niveau postsynaptique d'une synapse chimique. C R Seances Acad Sci III. 1982 Dec 6;295(12):665–670. [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Holets V. R., Staines W., Meister B., Melander T., Schalling M., Schultzberg M., Freedman J., Björklund H., Olson L. Coexistence of neuronal messengers--an overview. Prog Brain Res. 1986;68:33–70. doi: 10.1016/s0079-6123(08)60230-7. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle: possible neurotrophic role for a coexisting neuronal messenger. EMBO J. 1987 Apr;6(4):901–906. doi: 10.1002/j.1460-2075.1987.tb04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K., Dionne V. E. Cyclic AMP regulates the proportion of functional acetylcholine receptors on chicken ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8155–8159. doi: 10.1073/pnas.84.22.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K. Enkephalin and substance P modulate synaptic properties of chick ciliary ganglion neurons in cell culture. Neuroscience. 1986 May;18(1):175–182. doi: 10.1016/0306-4522(86)90186-7. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K., Anthony D. T., Rubin L. L., Greengard P., Huganir R. L. Regulation of nicotinic acetylcholine receptor phosphorylation in rat myotubes by forskolin and cAMP. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6591–6595. doi: 10.1073/pnas.84.18.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New H. V., Mudge A. W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. 1986 Oct 30-Nov 5Nature. 323(6091):809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Revah F., Mulle C., Pinset C., Audhya T., Goldstein G., Changeux J. P. Calcium-dependent effect of the thymic polypeptide thymopoietin on the desensitization of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1987 May;84(10):3477–3481. doi: 10.1073/pnas.84.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Role L. W. Substance P modulation of acetylcholine-induced currents in embryonic chicken sympathetic and ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1984 May;81(9):2924–2928. doi: 10.1073/pnas.81.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Merlie J. P., Lawrence J. C., Jr Regulation of phosphorylation of nicotinic acetylcholine receptors in mouse BC3H1 myocytes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6601–6605. doi: 10.1073/pnas.84.18.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami K., Hashimoto K., Uchida S., Tohyama M., Yoshida H. Effect of calcitonin gene-related peptide on the cyclic AMP level of isolated mouse diaphragm. Jpn J Pharmacol. 1986 Nov;42(3):345–350. doi: 10.1254/jjp.42.345. [DOI] [PubMed] [Google Scholar]
- Takami K., Kawai Y., Uchida S., Tohyama M., Shiotani Y., Yoshida H., Emson P. C., Girgis S., Hillyard C. J., MacIntyre I. Effect of calcitonin gene-related peptide on contraction of striated muscle in the mouse. Neurosci Lett. 1985 Sep 30;60(2):227–230. doi: 10.1016/0304-3940(85)90248-4. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]



