Abstract
The brain does not learn and remember in a unitary fashion. Rather, different circuits specialize in certain classes of problems and encode different types of information. Damage to one of these systems typically results in amnesia only for the form of memory that is the affected region's specialty. How does the brain allocate a specific category of memory to a particular circuit? This question has received little attention. The currently dominant view, Multiple Memory Systems Theory, assumes that such abilities are hard-wired. Using fear conditioning as a paradigmatic case, I propose an alternative model in which mnemonic processing is allocated to specific circuits through a dynamic process. Potential circuits compete to form memories with the most efficient circuits emerging as winners. However, alternate circuits compensate when these “primary” circuits are compromised.
What is the Origin of Memory Systems?
Neuroscientists recognize that there is no single form of memory. Different aspects of a memory and different kinds of learning are handled by different brain regions. With such recognition, it becomes critical to understand why certain problems are solved with one circuit and not another. Current approaches assume that this distribution of information processing reflects the hardwired organization of the brain. Here I wish to challenge that view and argue that the data suggests that this organization reflects a far more dynamic process. Instead, circuits actively compete during learning such that the most efficient path to solving a particular problem gains control of the necessary information processing and memory formation.
The dominant view of the neural systems responsible for learning and memory is Multiple Memory Systems Theory (MMST), which states that there are specific circuits that serve specific classes of learning and memory problems[1]. The view was initially stimulated by findings with Patient H.M. who lost “declarative” memory but, equally important, retained “nondeclarative” memory after removal of most of his medial temporal lobe[2]. MMST points to the hippocampus for spatial learning and episodic memory, the cerebellum for learning reflexive movements, and the striatum for habit learning[3]. According to MMST, these regions each constitute a critical junction essential for processing, storing and retrieving the information necessary for the category of memory they serve.
Fear learning is typically taken as a perfect exemplar for MMST. Fear serves the critical biological function of defense[4]. The consequences to reproductive fitness are greater for a single failure to defend than a single failure to mate or eat. This urgency of defense resulted in the evolution of a near perfect fear learning circuit that has rapid and potent plasticity. Significant Pavlovian Fear Conditioning occurs with a single trial and is not forgotten over the adult lifespan[5,6].
Discrete vs Contextual Fear Cues
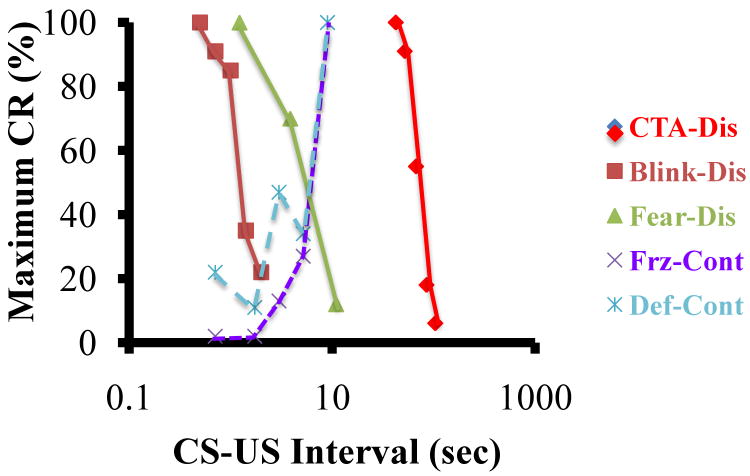
The most familiar examples of conditioning focus on discrete conditional stimuli(CS—e.g., Pavlov's bell), which are present briefly and immediately before the unconditional stimulus(US-typically an electric footshock for fear conditioning). But there are also static contextual cues that are present continually throughout the entire conditioning experience. Discrete and contextual CSs share the ability for one-trial permanent conditioning[6, 7]. However, contextual cues do not seem to play by the same rules as discrete CSs. A ubiquitous finding in the conditioning literature is that while the best conditioning occurs with the CS starting before the US, the shorter the interval between CS onset and US onset the better the conditioning (Figure 1). While the time constant differs for different types of conditioning, it is very short for eyeblink conditioning[9] and long for taste aversion conditioning[8], the rule is the same. The longer the time between the CS and the US the worse conditioning is, and this rule certainly applies to fear conditioning with discrete CSs[10]. Contextual conditioning violates this rule[11].
Figure 1. CS-US Interval.
The maximum possible Conditional Rresponse (CR) is plotted for three types of conditioning with discrete trials (conditioned taste aversion in rats—CTA-Dis [8], eyeblink conditioning in rabbits—Blink-Dis [9], and fear conditioning in rats—Fear-Dis [10]) using solid lines. Dashed lines present two measures of context fear: freezing—Frz-Cont [11] and defecation—Def-Cont [11]. Because of the tremendous range of CS-US intervals between eyeblink and taste aversion learning, the abscissa plots the square root of the number of seconds between CS and US onset on a log scale. Note that the time range for discrete and contextual fear conditioning overlaps but the direction of the functions is opposite.
I named this violation the immediate shock deficit(ISD), although it has been documented with other aversive USs such as loud noise[12] and several CRs[11-14]. Simply put, the greater the time between placement in a novel context and the delivery of the US (placement-to-shock interval) the greater the conditioning. While simultaneous presentation of tone and shock produces robust one-trial conditioning[7], a delay of at least 20 seconds between placement in a context and shock is needed for even minimally detectable conditioning[5]. I proposed that this deficit arises because contexts are made of many stimulus elements that “would not be experienced until the animal engages in some exploration” and that “the pattern of stimulation would change as the animal explores the chamber”[11]. Therefore, “the subject must learn to treat the complex compound of stimuli that make up a context as a whole …or Gestalt…before such a stimulus can enter into association as a CS”[11]. Note that none of these requirements are present for a discrete CS—one does not need to explore a brief, simple stimulus like a tone, and all of its elements are immediately present at the time of reinforcer delivery.
The slope of the CS-US interval (placement-to-shock interval) function provides a diagnostic for elemental vs configural learning (Figure 1). If that slope is negative, with conditioning degrading as CS-exposure increases, learning about the CS occurs in an elemental manner. However, if this slope is positive, where learning increases with CS exposure, learning about the CS occurs in a configural fashion. Figure 1 shows that the positive slope is unique to contexts. Thus we have an experimental tool for probing the nature of learning about stimuli. Later, in this article I will put this tool to use.
Further, support for this view of the context as a configuration comes from the finding that allowing the animal to form this unitary representation prior to conditioning, by simply giving it an opportunity to explore the environment, mitigates the ISD[5]. Importantly, this pre-exposure must consist of all the context elements being present together; separately exposing the elements does not attenuate the ISD[15]. The fact that context pre-exposure facilitates conditioning is again strikingly different than what happens with discrete CSs, where pre-exposure leads to a reduction in conditioning termed latent inhibition[16]. Note that the context pre-exposure design is really a variant of the placement-to-shock interval experiment. Both context pre-exposure and increasing the placement-to-shock interval increases experience with the CS prior to shock. Both facilitate the formation of a configural representation of the context through experience.
If the polymodal features of the context must be linked together as a unified representation, a brain structure that processes polymodal stimuli is a likely place. Nowhere else in the brain is there a compression of multi-sensory information that rivals the hippocampus[17]. The prediction that arises is that the hippocampus will be especially important for conditioning to contextual as opposed to discrete stimuli, because contextual but not discrete stimuli are a configuration of multisensory elements. Lesion studies confirm this prediction[18-20].
If the hippocampus forms the contextual configure, there should be some neural signature that reflects this process. Hippocampal neurons prefer to respond in specific locations and it is thought that the relationship of firing patterns between these place neurons allows animals to distinguish one context from another[21]. These hippocampal place fields require a period of exploration to become stable and estimates of the time needed for stability are similar to the time needed to overcome the ISD[22]. Furthermore, just as conditioning occurs with less time between placement and shock in a pre-exposed context, hippocampal place fields stabilize more rapidly when rats are replaced in a familiar environment[22]. Thus there is at least a rough correspondence between the temporal pattern of hippocampal place neuron activity and contextual fear conditioning.
Fear conditioning requires labeling a cue with emotional content but formation of a contextual representation by the hippocampus is independent of emotional valence. In the pre-exposure experiments, formation of the representation occurs in the absence of the US[5,23]. The amygdala is a structure that has long been linked to emotion and fear conditioning[24-28]. Hippocampal inputs to the basolateral amygdaloid complex (lateral and basal nuclei-BLA) support synaptic plasticity and damage to these hippocampal-BLA projections attenuates context but not tone conditioning[26]. However, damage to neurons in the BLA attenuates both[26].
Figure 2 shows a well-accepted view of a circuit for contextual fear that is uniquely capable of executing the requisites of this form of learning[27-28]. A contextual representation is assembled in the hippocampus and this representation, like that of a simpler discrete CS, is associated with negative affect at the BLA. This gives the CS the ability to activate the BLA on its own. BLA activation descends to generate the many overt responses that constitute a fear reaction via the central nucleus. Importantly, the ventral periaqueductal gray organizes a freezing response. I say, “organize” because the initial component of freezing is to retreat to the nearest good location to freeze (e.g., the closest dark corner) and then arrest visible motor activity[29].
Figure 2.
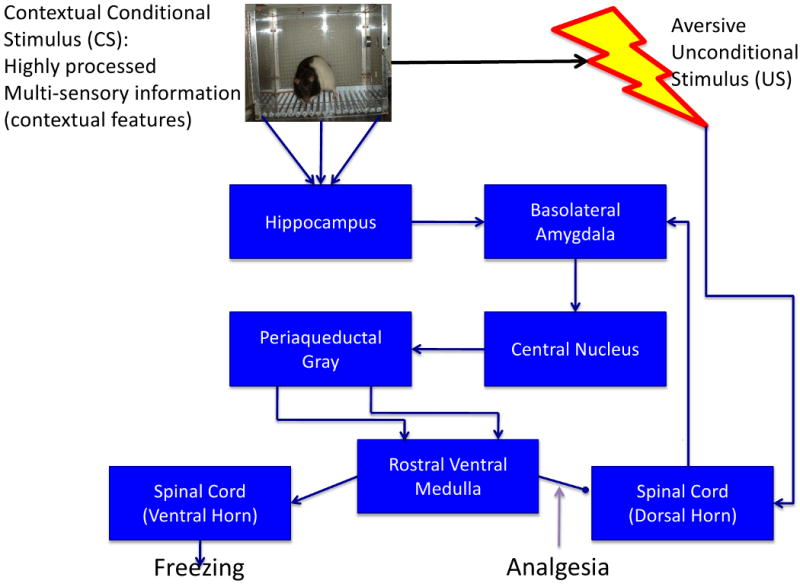
Multimodal sensory information constituting the context is integrated at the hippocampus and associated with shock in the basolateral amygdala. Descending circuitry from there generates conditional responses such as freezing and analgesia. The analgesic effects on ascending pain information from the dorsal horn constitute the error-correcting negative feedback arm of the circuit. See [27-28 for reviews].
Multiple Memory Systems Theory (MMST)
Contextual fear conditioning fits elegantly within MMST. There are a number of unique attributes to this learning process and there are specific neural circuits that carry out the necessary work. Damage to the circuit and pharmacological blockade of systems required for synaptic plasticity and its consolidation all cause a loss in conditioning[30].
MMST suggests that the circuits underlying a specific form of learning should be essential in carrying out that form of learning. According to MMST damage to an essential circuit before or after training will have similar effects; anterograde amnesia and retrograde amnesia should be proportional[31]. Early studies were consistent with just that. Both pretraining and post-training hippocampal lesions produced what appeared to be complete deficits in contextual fear conditioning[18-20, 32-33]. The one caveat, hippocampal lesions made a month or more after training had little effect on context fear[18], had already been built into MMST. As in H.M., whose older presurgery memories were preserved, the hippocampus was expected to play an initially important role that gradually faded over time as memory consolidated into cortical networks[1-2, 31, 34]. The retrograde amnesia produced by post-training hippocampal lesions was temporally graded in contextual fear conditioning just like medial temporal lobe amnesia in the patients that stimulated development of MMST.
So what is wrong with this picture?
We revealed a fly in the ointment by explicitly testing whether anterograde and retrograde amnesia were proportional[35]. Despite identical training parameters, retrograde amnesia was profound but there was no indication of anterograde amnesia. Why were these results discrepant with earlier studies? In our first retrograde amnesia study we used very strong training parameters (15 trials) to be assured of strong conditioning to both tones and contexts to allow direct comparison. Our initial anterograde amnesia studies used a more typical context conditioning preparation with just a few unsignaled shocks[32-33]. Philips and LeDoux[19], who also observed anterograde amnesia, specifically titrated their conditioning parameters to be the minimal amount that supported context conditioning. The fact that context conditioning was abolished in all these cases suggested an invariant dependence on the hippocampus. But the conclusion was confounded by the use of weak training parameters in the pretraining lesion studies and strong training parameters in the retrograde amnesia study. Maren et al[35], using a moderate set of parameters revealed that anterograde amnesia and retrograde amnesia were clearly not proportional. Our results in rats were soon replicated in mice by Frankland et al[36].
What should we make of this surprising result? We already knew that “elemental” conditioning to discrete CSs occurred without the hippocampus. Perhaps, while contexts were normally learned as a configuration, when the hippocampus was not available during acquisition, some contextual element conditioned much like the tone[35-37]. Configural cues, being more salient than the weak elements of the context, normally overshadowed elemental conditioning. Pavlovian overshadowing refers to a form of associative competition where a CS that supports conditioning when paired with a US on its own, fails to condition when it is reinforced in compound with a more salient stimulus[38]. The hippocampal configure normally overshadows any individual elements in the context. Without the configure to compete with, some element of the context acquires associative strength. We do not see anterograde amnesia because pretraining lesions do not weaken the net magnitude of conditioning. Rather, they switch the nature of what is learned from configural to elemental. Since animals with an intact hippocampus learn configurally, and the hippocampus is necessary to retrieve the configure, post-training lesions are strongly amnestic[35-36].
Recently, this hypothesis has been developed more fully by Rudy(37). He suggests that the hippocampal system learns through a conjunctive or configural process that operates through pattern completion, where sampling one aspect of the configure retrieves the entire configure. But there is also a neocortical system that learns in a feature-based manner where learning changes the value of elements individually. According to Rudy, contextual fear is mediated by this neocortical/elemental system when the hippocampus is not available.
If pretraining lesions shift the nature of what is learned during contextual fear conditioning from configural to elemental it should be fairly easy to find fundamental differences in how learning progresses. A one trial context conditioning experiment by Wiltgen et al[39] went right to the heart of the matter. All the arguments suggesting that contextual learning is a configural or Gestalt process rest on the ISD and the inverse function between CS onset and US onset between context and discrete cue conditioning. Therefore, we used the placement-to shock-interval function to diagnose between elemental and configural learning in rats with hippocampal ablations. If a pretraining lesion switches the nature of learning, it should switch the direction of the function and make context learning follow the principles of elemental conditioning. We generated placement-to-shock interval functions in intact and lesioned rats[39]. What we discovered was quite contrary to the prediction that hippocampal lesions turn rats into elemental information processors. Two sets of parallel curves emerged with the lesioned rats showing a rightward shift in the effects of increasing delay between context placement and shock. The function's slope was still positive; lesioned animals just needed more time to explore to condition comparably.
The Pavlovian literature is very clear that elemental conditioning will suffer when there is a delay between placement in the context and US delivery. While the context is made of many cues, from a temporal perspective they fall into two classes. Some cues are statically present throughout the entire interval (e.g., background noise from a ventilation fan) others are phasic, present only when attended to (e.g., tactile cues from a whisker stroking a corner). Longer periods to explore should hurt conditioning to static cues by lengthening the CS-US interval. Phasic cues have multiple onsets and terminations before they have the opportunity to be paired with shock. The longer the time to explore the context, the more CS presentations prior to the conditioning event occur. These CS presentations should reduce conditioning through latent inhibition[16] and they should also reduce the element-shock contingency[40]. Both factors would be detrimental to elemental conditioning. Since animals without a hippocampus, just like intact animals, profit from more time to explore, they cannot be learning elementally. They must be using this time to learn configurally. For certain, lesioned animals are less efficient than intact animals, but the nature of the learning is similar. This inefficiency of lesioned animals in configural learning is further illustrated by the finding that increasing the number of conditioning trials eliminates any anterograde amnesia[39].
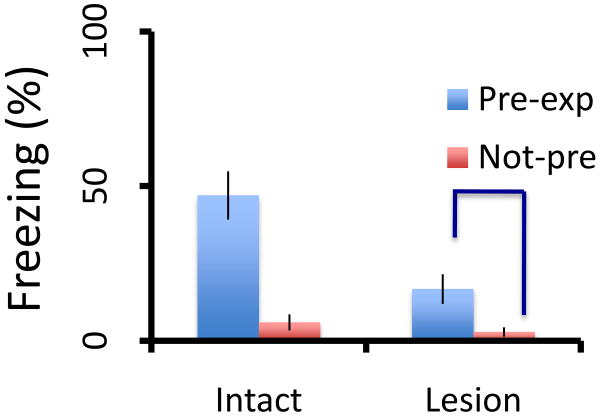
The suggestion that contextual fear learning in the absence of the hippocampus is configural is a major point of departure from both my previous view [35] and those of others [36-37, 41-42]. Rudy[37] suggests that the critical test between elemental (feature-based) and configural (conjunctive) learning is whether or not contextual pre-exposure can benefit animals trained with immediate shock. I agree on the importance of this test. Relevant data from the Rudy lab[23] are presented in Figure 3. The critical comparison is between lesioned animals with and without context pre-exposure. It is clear that lesioned rats with pre-exposure freeze more than their lesioned counterparts without pre-exposure. Thus like the positively sloped placement-to-shock interval function, the pre-exposure data support the conclusion that both the hippocampal system and the alternate system learn in a configural manner. Lesioned rats clearly benefit from context pre-exposure, they just do so to a lesser degree than intact animals (fig 3). Remember, that pre-exposure to discrete CSs produces a LOSS in conditioning—the classic latent inhibition effect[16]. Together these data support the hypothesis that hippocampal-lesioned animals use a quantitatively (i.e., less efficient), not qualitatively (i.e., configural vs elemental) different process than intact animals.
Figure 3.
Rudy et al [42] pre-exposed intact and hippocampus-lesioned rats to a context without shock on one day. The next day the rats received a shock immediately upon placement in either the same chamber (Pre-exp) or a different chamber (Not-pre) as pre-exposure. The data measure freezing in the shocked chamber during a test given on the third day. The immediate shock deficit is illustrated by the lack of freezing in both No-pre groups. Pre-exposure increased freezing in the lesioned rats, albeit to a lesser extent than the unlesioned rats. The critical contrast showing lesioned rats profited from pre-exposure is indicated by the dark blue brackets. Based on Rudy et al [42] with permission.
A Pattern Emerges
There are 3 key observations from these hippocampal lesion studies. First, configural learning occurs with or without a hippocampus. Second, with weak conditioning parameters hippocampal lesions produce anterograde amnesia, but with less challenging parameters no anterograde amnesia is found. Finally, with post-training lesions there is a profound retrograde amnesia even with generous training parameters. These observations can be easily explained with the hypotheses in Box 1.
Box 1. Hypotheses accounting for hippocampal effects on context conditioning
Animals normally learn about contexts by using the hippocampus to form a configural representation of the context.
Animals without a hippocampus can use some alternative structure (or pathway) to form a similar contextual representation but they do so less efficiently (they need more time to explore the context or more training trials). This explains the parametric dependence of anterograde amnesia.
When the hippocampus learns, the alternative structure does not. Therefore, losing the hippocampus shortly after learning produces a parameter-independent retrograde amnesia.
These hypotheses lay to rest several troubling findings in the hippocampal literature. For example, Sutherland and Rudy[43] suggested that the hippocampus is necessary for learning a discrimination when the solution requires the use of configural cues. They proposed negative patterning, where two stimuli are reinforced when presented separately but not reinforced when presented together, as the critical test of the theory. In order to withhold responding to the compound, the learner must use the compound as a unique configural stimulus[44]. If the hippocampus is critical for configural memory then learners without a hippocampus should be unable to solve this problem. While the prediction was initially confirmed[45], other experiments found that animals with lesions could learn the problem[46]. This was taken as an insurmountable problem for the theory. However, one of the experiments taken as a disconfirmation is particularly instructive. Richardson et al[47] trained intact rats on a negative patterning task and found that post-training hippocampal lesions devastated the discrimination. However, with retraining the animals acquired negative patterning. This is directly analogous to one of our experiments with contextual fear conditioning[39]. Rats were first trained to fear a context. While complete lesions of the hippocampus eliminated this memory, retraining resulted in normal fear. In both experiments rats appear to normally learn with a hippocampus, but when the hippocampus is not available some other region(s) can compensate.
Pharmacological manipulations of the hippocampus
Direct infusion of drugs into the hippocampus offers a way of temporally manipulating the hippocampus around the time of learning but leaving it normal during testing. Of particular interest is a comparison of substances that inactivate the hippocampus and those that block synaptic plasticity while preserving synaptic transmission. Inactivating the hippocampus with a local anesthetic (lidocaine) or a drug mimicking the inhibitory transmitter GABA (muscimol) immediately prior to or after contextual fear learning cause, at most, a small deficit in context fear learning unless a demanding contextual fear conditioning task is used (23, 48-49). This is quite consistent with the pattern just described with permanent pretraining lesions, when the hippocampus is inactivated other structures compensate but they have trouble when the task is particularly demanding.
A very different picture emerges with infusion of an NMDA antagonist (APV) into the hippocampus prior. NMDA-blockade during training is devastating to context fear learning even with robust training procedures (50). Importantly, hippocampal place fields form and place cells fire under NMDA-receptor blockade. However, place cell activity induces long-term plasticity only when NMDA-receptors are normally activated (51). Under an NMDA antagonist the hippocampus is processing context during training so the alternate is not engaged, but since the hippocampus forms no long-term memory there is a profound anterograde amnesia.
Retrieval and the Hippocampus
Quite surprisingly, inactivation of the hippocampus during testing causes no loss of fear in an animal given standard training (48-49). This lack of effect on retrieval does not fit easily into any of the current views of context conditioning including the current one. One key to this puzzle is Ji & Maren's (53) suggestion that the hippocampus is important to retrieval only when the situation consists of ambiguous information. In standard contextual fear conditioning the context is not ambiguous; the only previous experience with the context was when the animal received shock. Hippocampal inactivation does prevent retrieval of contextual fear when animals learn with context pre-exposure and subsequent immediate shock (23). In that design animals receive a safe exposure to the context on one day and a shock in the context on another. In that sense the context is ambiguous at the time of testing. The hippocampus' role in resolving ambiguity during retrieval may be quite different from its role in learning about contexts. While the hippocampus is not involved in cued fear when straightforward tone-shock training is used (18-19) it does play a role when cued fear is rendered ambiguous through extinction (53), minimal training (54), when the cue and reinforcer are separated in time (55) or when the tested cue is different from the trained cue (56).
Beyond the Hippocampus
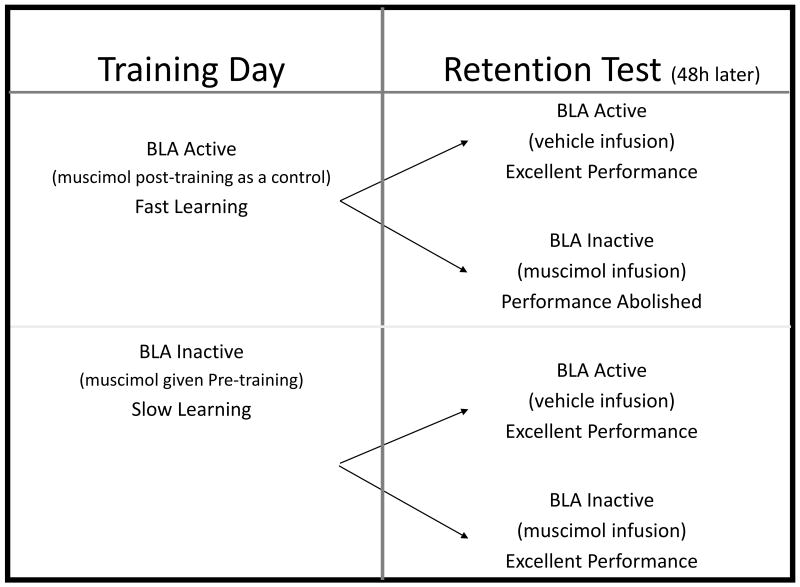
The general pattern present in these three observations about hippocampus can be recognized for the BLA's role in contextual fear conditioning. The BLA is considered essential for fear conditioning and to be sure interfering with BLA function has very pronounced effects on fear learning. However, following large BLA lesions rats can acquire fear if given an extensive regimen of overtraining[57]. Importantly, animals overtrained with the BLA intact lose their fear if given post-training lesions[57]. We[58] extended Maren's finding using muscimol to temporally inactivate the BLA[Figure 4]. Rats normally acquire fear very rapidly with some learning after a single trial. When we inactivated the BLA we saw no one-trial learning. However, the rats did begin to learn after about 10 trials. Learning was slow; it took 50 trials for the inactivated rats to catch up. During a subsequent memory test all groups tested with the BLA intact show similar levels of fear. While pretest inactivation of the BLA abolished memory recall in the rats trained with the BLA functional; animals that learned fear without the BLA were unaffected by BLA inactivation at the time of test. Fear can occur with or without the BLA, but without the BLA learning is very inefficient. If learning occurred with the BLA, then this structure is needed to express fear regardless of the training regime (e.g., normal or overtraining). But if learning occurred without the BLA, then the BLA is not needed in order to express fear.
Figure 4.
The design and summary of results of an overtraining experiment [58] showing that rats learn context fear, albeit slowly, when the BLA is shutdown by direct infusions of the GABA agonist muscimol. Despite overtraining, rats that learn with a functional BLA needed the BLA to express fear. However, rats that learned without the BLA did not need the BLA to express fear.
The pattern is present not only for the BLA complex as a whole, but also for nuclei within the BLA. Using auditory conditioning, Anglada-Figueroa & Quirk found that pretraining lesions of just the basal nuclei had no effect on acquisition of fear[59]. However, if the same lesions were made after conditioning, tone fear was abolished. Normally rats use the basal nuclei for auditory conditioning, but if they are not available, alternative routes support learning.
Let one more example, this one from auditory cortex, suffice to make the generality of the point. Auditory information arrives at the BLA from two routes. One directly from the medial geniculate nucleus, the other from auditory cortex[60]. Pretraining lesions one of these pathways does not affect fear conditioning to a tone, but lesions of both pathways impair learning. These findings lead to the conclusion that both pathways are equally capable of auditory fear conditioning but left open the question of what NORMALLY happens during learning. Subsequently, Boatman & Kim[61] found that post-training lesions of the auditory cortex abolished tone fear memory. While both pathways have the potential for learning, normally the cortical input mediates acquisition.
A Dynamic Model
Together, the first four assumptions in Box 2 can account for this entire data set[58]. Normally we learn by using the most efficient circuit for producing learning, so if that circuit is unavailable, learning is possible but it requires a more forgiving training regimen. We will see deficits with pretraining lesions but only if the training regimen is demanding. Post-training lesions in the primary pathway are devastating because when this pathway was strengthened it prevented learning in the alternate pathway.
Box 2. The Dynamic Origins of Memory Systems
There are primary and alternate pathways capable of mediating fear behavior.
The alternate pathways are less efficient than the primary pathway.
The more efficient primary pathway dominates the learning, and simultaneously prevents significant learning in the alternate pathway(s).
The alternate pathways compensate when the dominant pathway is compromised.
Plasticity in these circuits may be regulated by the same mechanisms responsible for competitive learning between stimuli and described by Pavlovian principles.
This model provides a set of rules for how the brain can select specific and efficient circuits for production of specific adaptive behaviors.
How does the primary pathway down-regulate learning in the alternate? Why is the alternate not strengthened when the primary learns? An appeal to basic Pavlovian processes provides an answer. It has long-been recognized that potential CSs compete for the acquisition of associative strength[44] and such competition could be achieved by a circuit that detects differences between obtained and expected outcomes (error detection) and then uses this difference to reinforce changes in the predictive value of the CS (error correction) [62, Fig.1]. If a US is unpredicted it reinforces learning, if the US is predicted it does not reinforce. Again an important example is overshadowing[38]. Mackintosh[63] described an experiment where a not particularly salient light conditioned well on its own but failed to condition when presented in compound with a more salient tone. The effect occurs because salient (more intense) stimuli condition more quickly and rapidly come to predict the US[44]. Since the more salient tone predicts the US before the less efficiently learned about light acquires much associative strength, the light never has a chance to condition well. When the light is alone, nothing else predicts the US, so learning proceeds, albeit inefficiently. It should be clear that this pattern for conditioning to stimuli is analogous to what the first 4 assumptions in Box 2 say about neural pathways.
While traditional models of Pavlovian conditioning spoke of increasing the associative strength of stimuli, in reality a circuit is strengthened. When light is conditioned alone, visual inputs to the amygdala undergo synaptic strengthening, but when the light is reinforced together with a tone only the auditory input to the amygdala is strengthened. Thus associative competition selectively strengthens a more efficient (dominant) circuit over a less efficient (secondary) circuit. This leads to postulate 5 of the model; associative competition rules explain how the brain selects specific circuits for learning and why these circuits are particularly effective at the type of learning they mediate. Since learning is driven by error signals arising from the failure to predict significant events, loss of a region that normally accomplishes the learning results in continued error signals, and this continued reinforcement eventually strengthens the next best alternative. Obviously there are some limits to this compensation. When the amygdala was deprived of both thalamic and cortical auditory input no tone conditioning occurred[60]. In this case we expect that continued error signals would have driven context fear to an exceptionally high level, as it served as the only predictor of shock. While Romanski and LeDoux[60] never measured context fear, context fear is greater under unsignaled than signaled shock conditions[64].
Implications for Multiple Memory Systems Theory (MMST)
MMST views the neural circuit that serves a particular class of learning as hard-wired and essential. The model described in Box 2 suggests a far more dynamic process. The origin of a “memory system” reflects an ontogenetic, not phylogenetic, process where through experience, and the rules of associative competition, the brain selects the most efficient circuits for solving a particular problem. A memory system is not “essential” but rather the brain tends to use a particular circuit for a particular class of problems because this circuit is the best it has available to solve the problem. While the evidence may be most developed for the case of contextual fear conditioning, postulate 6 suggests that these principles are applicable to memory systems in general. Our model is certainly consistent with both the generalities and specifics in food reinforced negative patterning studies[47].
Future Directions
To further develop this approach, future work will need to detail precisely these alternate pathways and determine where compensatory plasticity occurs. This search should be guided by neuroanatomy. Furthermore, the exact neural mechanisms responsible for down-regulation of plasticity in potential pathways needs to be revealed. Some predictions of the model with respect to alternate pathways are described in Box 3. What is known about error-correction circuits should provide keys to that research. Hopefully, the dynamic approach in Box 2 not only provides some insight into previously troubling experimental data but will also result in new approaches to those who, because of brain damage to the circuits normally responsible for memory formation, must learn to use alternative pathways.
Box 3. Predictions About Alternate Pathways
What Are the Alternate Pathways: The model postulates the existence of alternate neural pathways for solving particular problems but those regions/circuits are currently unknown. The model suggests an anatomical and a functional strategy for identifying potential candidates. Anatomically, an area that compensates for the amygdala's role in contextual fear conditioning needs to get information about context from the hippocampus and project to areas that generate fear behaviors such as freezing. The bed nuclei of the stria terminalis (BST) makes a strong candidate (66-68). The functional strategy asks what areas contribute to similar behaviors. Because of its role in mediating some types of anxiety responses, the BST is again a good candidate (69).
Place Cells: The alternate region for hippocampus in processing contexts as a configure should have place fields but these place fields should be slower to form and this time course should match the longer placement-to-shock interval function of lesioned rats. To the extent that anything is known about place fields outside the hippocampus, the time for stabilization is longer (22).
Preferential Training: It is possible that certain types of parameters will favor learning in the alternate system over the primary system. With such a set of parameters post-training lesions to the primary system should have no effect—but post-training lesions to the alternate pathway should be devastating. Recently, Lehman et al. (70) confirmed the first part of this prediction; giving multiple reinforced context exposures spaced a day apart renders context conditioning immune to posttraining hippocampal lesions.
Acknowledgments
This article profited from discussions with Brian Wiltgen and Moriel Zelikowski. Supported by NIMH grant MH62122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squire LR, et al. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–95. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- 4.Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- 5.Fanselow MS. Factors governing one trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 6.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahoney W, Ayres J. One-trial simultaneous and backward fear conditioning as reflected in conditioned suppression of licking in rats. Anim Learn Behav. 1976;4:357–362. [Google Scholar]
- 8.Garcia J, et al. Learning with prolonged delay of reinforcement. Psychon Sci. 1966;5:121–122. [Google Scholar]
- 9.Schneiderman N, Gormezano I. Conditioning of the nictitating membrane of the rabbit as a function of CS-US interval. J Comp Physiol Psychol. 1964;57:188–195. doi: 10.1037/h0043419. [DOI] [PubMed] [Google Scholar]
- 10.Kamin LJ. Temporal and intensity characteristics of the conditioned stimulus. In: Prokasy WF, editor. Classical Conditioning. Appleton-Century-Crofts; 1965. pp. 118–147. [Google Scholar]
- 11.Fanselow MS. Associative vs. topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species specific defensive reactions. Learn Motiv. 1986;17:16–39. [Google Scholar]
- 12.Kiernan M, Cranney J. Immediate-startle stimulus presentation fails to condition freezing responses to contextual cues. Behav Neurosci. 1992;106:121–124. doi: 10.1037//0735-7044.106.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Kiernan M, et al. Immediate shock, passive avoidance, and potentiated startle: Implications for the unconditioned response to shock. Anim Learn Behav. 1995;23:22–30. [Google Scholar]
- 14.Fanselow MS, et al. The immediate shock deficit and postshock analgesia: Implications for the relationship between the analgesic CR and UR. Anim Learn Behav. 1994;22:72–76. [Google Scholar]
- 15.Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–80. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 16.Lubow RE. Problems in the behavioural sciences. Cambridge University Press; 1989. Latent inhibition and conditioned attention theory; p. 324. [Google Scholar]
- 17.Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40:220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear following hippocampal lesions. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland RJ, McDonald RJ. Hippocampus, amygdala, and memory deficits in rats. Behav Brain Res. 1990;37:57–79. doi: 10.1016/0166-4328(90)90072-m. [DOI] [PubMed] [Google Scholar]
- 21.Leutgeb S, et al. Fast rate coding in hippocampal CA3 cell ensembles. Hippocampus. 2006;16:765–774. doi: 10.1002/hipo.20201. [DOI] [PubMed] [Google Scholar]
- 22.Frank LM, et al. Hippocampal and cortical place cell plasticity: implications for episodic memory. Hippocampus. 2006;16:775–784. doi: 10.1002/hipo.20200. [DOI] [PubMed] [Google Scholar]
- 23.Matus-Amat P, et al. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 25.Russo NJ, et al. Passive avoidance and amygdala lesions: Relationship with pituitary-adrenal system. Physiol Behav. 1976;16:191–199. doi: 10.1016/0031-9384(76)90304-8. [DOI] [PubMed] [Google Scholar]
- 26.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 28.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 29.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Erlbaum; 1988. pp. 185–211. [Google Scholar]
- 30.Fanselow MS, LeDoux JE. Why We Think Plasticity Underlying Pavlovian Fear Conditioning Occurs in the Basolateral Amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 31.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–77. doi: 10.1016/0959-4388(95)80023-9. 1995. [DOI] [PubMed] [Google Scholar]
- 32.Kim JJ, et al. Effects of amygdala, hippocampus and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 33.Young SL, et al. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: Immunization against amnesia by contextual preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990;250:288–90. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- 35.Maren S, et al. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 36.Frankland PW, et al. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 37.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlov I. Conditioned reflexes. Oxford University Press; 1927. [Google Scholar]
- 39.Wiltgen BJ, et al. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rescorla R. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- 41.Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn Mem. 2008;16:38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudy JW, et al. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiol. 1989;17:129–144. [Google Scholar]
- 44.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 45.Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- 46.Davidson TL, et al. Hippocampal lesions do not impair negative patterning: A challenge to configural association theory. Behav Neurosci. 1993;107:227–234. doi: 10.1037//0735-7044.107.2.227. [DOI] [PubMed] [Google Scholar]
- 47.Richmond MA, et al. Effects of scopolamine and hippocampal lesions on negative patterning dis- crimination performance in rats. Behav Neurosci. 1997;111:1217–1227. doi: 10.1037//0735-7044.111.6.1217. [DOI] [PubMed] [Google Scholar]
- 48.Daumas S, Halley H, Francés B, Lassalle JM. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem. 2005;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- 50.Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–667. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- 51.Isaac JT, et al. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29:6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–6. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 54.Quinn JJ, et al. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008;18:640–654. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- 55.Chowdhurry N, et al. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 56.Quinn JJ, et al. Post-training excitotoxic lesions of the dorsal hippocampus attenuate generalization in auditory delay fear conditioning. Eur J Neurosci. 2009;29:1692–1700. doi: 10.1111/j.1460-9568.2009.06727.x. [DOI] [PubMed] [Google Scholar]
- 57.Maren SJ. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. Neuroscience. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ponnusamy RP, et al. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–5. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–9. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- 62.Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci. 1980;3:291–301. [Google Scholar]
- 63.Mackintosh N. Overshadowing and stimulus intensity. Anim Learn Behav. 1976;4:186–192. doi: 10.3758/bf03214033. [DOI] [PubMed] [Google Scholar]
- 64.Fanselow MS. Signaled shock-free periods and preference for signaled shock. J Exp Psychol: Anim Behav Process. 1980;6:65–80. [Google Scholar]
- 65.Fanselow MS. Pavlovian conditioning, negative feedback, and blocking: Mechanisms that regulate association formation. Neuron. 1998;20:625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 66.Dong HW, Swanson LW. J Comp Neurol. Vol. 494. 2006. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance; pp. 142–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006;494:108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker DL, et al. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychia. 2009 doi: 10.1016/j.pnpbp.2009.06.022. 2009 Jul 10. PMID: 19595731 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann H, et al. Making context memories independent of the hippocampus. Learn Mem. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]