Abstract
Background
The pathogenesis of infection with Burkholderia cepacia complex (Bcc) organisms may be linked to its capacity to invade respiratory epithelium.
Methods
An antibiotic exclusion assay was used to study B. dolosa AU4459 and B. cenocepacia J2315 invasion into wild-type (WT) and CFTR-deficient respiratory epithelial cells. Inhibitors were used to evaluate Bcc invasion dependency on host microtubule (mt) and microfilament (mf) systems.
Results
B. dolosa entered WT-CFTR cells with 5-fold greater efficiency than CFTR deficient cells (25% vs 5%, respectively). Invasion dropped to <0.5% after either mf or mt inhibition. B. cenocepacia entered WT (0.05%) and CFTR-deficient cells (0.07%) with similarly low efficiencies, which significantly decreased with either mf or mt inhibition (0.008% and 0.002%, respectively).
Conclusion
B. dolosa and B. cenocepacia enter respiratory epithelial cells in a mf and mt dependent fashion. Mutated CFTR leads to less internalization of B. dolosa, but not B. cenocepacia.
Keywords: Burkholderia cenocepacia, Burkholderia dolosa, invasion, respiratory epithelium, LC3, LCFSN, CFTR, microfilaments, microtubules
INTRODUCTION
Burkholderia cepacia complex (Bcc) organisms have been recognized as opportunistic pathogens in cystic fibrosis (CF) since the 1980s and infection prevalence now approaches 30% in some CF centers (2). Approximately 20% of Bcc colonized patients develop a rapidly progressive necrotizing pneumonia (cepacia syndrome) (10, 12). However, other patients exhibit transient colonization or indolent disease illustrating the variability in interaction of bacterial and host factors that contribute to disease (11, 15, 17, 19). Part of this variability is due to differences in the organisms themselves. Fifteen genetically distinct genomovars have been identified with over 43 species in the genus Burkholderia (5, 12, 19, 26). Burkholderia dolosa (genomovar VI) (27) accounts for only 3% of US Bcc strains (10); however, in one treatment center, the prevalence of B. dolosa infection was greater than 10-fold the national average due to infection with an epidemic strain designated SLC-6 (10). Among Bcc organisms, B. dolosa appears to be the most antibiotic resistant (17). Apart from clinical observations, virtually nothing is known about the pathogenesis of infection with B. dolosa in comparison to a more widely studied species, B. cenocepacia, which accounts for the majority of Bcc infections in the United States CF population (8, 11). In particular, the pathogenesis of B. dolosa infection may involve invasion into respiratory epithelial cells.
Mechanisms of host cell invasion have been studied extensively in gram negative enteric pathogens and all involve rearrangement of host cell cytoskeletal components (6, 9, 25). Actin and microtubule polymerization may be required for uptake of intestinal pathogens (1, 6). Among respiratory pathogens, Pseudomonas aeruginosa (PA) enters epithelial cells in an actin microfilament-, but not microtubule-dependent fashion after binding to the cystic fibrosis transmembrane conductance regulator protein, CFTR (3). CFTR defects impair the entry and subsequent clearance of PA, suggesting that CFTR-dependent cell entry functions in host cell defense. Certain Bcc organisms invade host epithelial cells in an actin-dependent fashion (2, 9, 10), but few studies have examined the role that CFTR may play in Bcc invasion (5, 10). In the present study, the role that CFTR, microtubules and microfilaments play in the invasion of B. dolosa and B. cenocepacia into respiratory epithelial cells is examined.
MATERIALS AND METHODS
Bacterial strains and growth conditions
B. dolosa clinical isolates (strain designated SLC6) were examined for antibiotic susceptibility. The most sensitive isolate AU4459 was further studied in invasion assays. The B. cenocepacia clinical isolate J2315 (strain designated ET12) was studied. The PA studied was a laboratory strain designated PAO1-V (provided by the Dr. G. Pier laboratory, Harvard University, Boston, MA). The Burkholderia isolates were grown overnight on blood agar plates and PAO1 was grown on tryptic soy agar plates (TSA), followed by inoculation in LB for growth to mid-log phase, which was monitored by following the optical density at 650nm (OD650) on a Genesys 20 spectrophotometer (ThermoSpectronic).
Cell culture
CFT1 cells are respiratory epithelial cells derived from a ΔF508 homozygous patient and immortalized with the human papilloma virus 18 E6 and E7 genes (30). They were complemented with either a full-length wild type (WT)-CFTR gene introduced by a retrovirus and designated CFT1-LCFSN, or with the control gene encoding β–galactosidase, CFT1-LC3 (cells provided by Dr. J. Yankaskas, University of North Carolina, Chapel Hill). CFT1 cells were grown in Ham’s F12 medium supplemented with 10 μg/mL insulin, 1 mM hydrocortisone, 3.75 μg/mL endothelial cell growth supplement, 25 ng/mL epidermal growth factor, 30 nM triiodo-L-thyronine, 5 μg/mL transferrin, and 10 ng/mL cholera toxin (CF12 media). Selection for transfected cells was provided through addition of neomycin at 150 μg/mL, which was removed by washing the plates with phosphate-buffered saline (PBS) and replaced with neomycin-free CF12 media immediately prior to the invasion experiments with bacteria (30). 16HBE cells were isolated from an individual with two WT copies of the CFTR gene and immortalized using the simian virus-40 (provided by Dr. D. Gruenert, University of California, San Francisco, California), and grown using standard techniques (7). All cultured cell lines were serum-starved for 10 minutes before infection with bacteria. All reagents for media supplementation were obtained from Sigma Aldrich (St. Louis) unless indicated. To assess whether B. dolosa or B. cenocepacia exposure induced apoptosis and hence, loss of respiratory epithelial cells, confluent cultures of LCFSN and LC3 cells in 6 well plates were inoculated with bacteria and incubated for 1, 2, and 3 hours (3). The cells were then stained with FITC-labeled M30 antibody, which is a monoclonal antibody to a cytokeratin 18 cleavage product generated by caspase 6 activity during apoptosis. The M30 stained cells were evaluated by flow cytometry (FACS Calibur, BD Biosciences).
Antibiotic susceptibility assay
To enumerate live intracellular bacteria, target cells are incubated with bacteria followed, typically, by gentamicin to kill the extracellular bacteria, then washed, lysed and plated (2). Because Bcc organisms are resistant to numerous drugs including gentamicin, the susceptibility of several clinical isolates of the SLC6 strain of B. dolosa were tested with both double and triple combinations of other antibiotics to determine an antibiotic combination useful for a modified assay (Table 1). The combination of meropenem (NovaPlus produced for Astra Zeneca: stock 50 mg/mL), ceftazidime (SanDoz GMBH produced for NovaPlus: stock 100 mg/mL) and either amikacin (Sicor Pharmaceuticals Inc: stock 250 mg/mL) or tobramycin (Abraxis Pharmaceutical produced for Novaplus: stock 40 mg/mL) provided the highest killing efficiency of B. dolosa AU4459. A bacterial inoculum of 5–10 × 106 organisms was used. A killing efficiency, defined as ((initial inoculum − recovered inoculum)/initial inoculum) × 100%, greater than 99.99% (>4 log killing of an initial inoculum of 106 CFU) was deemed adequate to proceed with the invasion studies (2).
Table 1.
Susceptibility of clinical SLC6 isolates to killing by a triple antibiotic combination of meropenem, ceftazidime and amikacin. Each antibiotic concentration was 1 mg/mL and bacteria were exposed for 3 hours at 37°C.
| SLC6 isolate | Initial inoculum × 106 CFU | Recovered inoculum × 102 CFU | Killing efficiency (%) |
|---|---|---|---|
| AU4459 | 6 | 1 | 99.9983 |
| AU5404 | 5 | 3 | 99.9940 |
| AU4894 | 4 | 9 | 99.9775 |
| AU4750 | 12 | 30 | 99.9750 |
| AU3123 | 6 | 30 | 99.9500 |
| AU4881 | 7 | 60 | 99.9143 |
| AU3556 | 10 | 200 | 99.8000 |
| AU4298 | 7 | 500 | 99.2857 |
| AU9248 | 5 | 1000 | 98.0000 |
To mimic the conditions of the invasion assay, aliquots of meropenem, ceftazidime, amikacin and tobramycin were diluted in CF12 media without neomycin to create four separate 1 mg/mL antibiotic stocks, as well as 1:10, 1:100 and 1:1000 dilutions of each antibiotic. First, single antibiotic concentrations of 1 mg/mL were tested to determine their killing efficiency. Bacteria were removed from fresh overnight plates and grown in LB at 37 °C in a shaking incubator until the bacteria reached an OD650 of 0.4. After incubation in a cell culture incubator for 1 hour, the wells were serially diluted and plated on blood agar, incubated overnight, and counted.
Invasion assay
A 96-well plate was seeded with CFT1-LC3, CFT1-LCFSN or 16HBE cells (105 cells per well). Once confluent (~24 hours), the growth media was removed, the wells were washed with PBS and the neomycin-free media was added to the wells. Stocks of cytochalasin D (2 mM in DMSO; Sigma) and colchicine (10 mM in sterile water; Sigma) were stored in the dark at 4 °C. Final concentrations of cytochalasin D and colchicine in neomycin-free CF12 were 2 and 10 μM, respectively (~1 μg/mL cytochalasin D and ~4 μg/mL colchicine). Cytochalasin D, colchicine or neomycin-free CF12 media was added to the wells and the plates were incubated for 1 hour prior to a PBS wash followed by addition of bacteria (multiplicity of infection [MOI] confirmed by plating serial dilutions). The bacteria were approximated to the epithelial cells by centrifugation of the plates (5 minutes at ~330 × g) and then incubated at 37 °C for 1 hour to allow for invasion. The wells were washed X3 with PBS to remove extracellular bacteria and the antibiotic-free media replaced with media supplemented with 1 mg/mL each of meropenem, ceftazidime and amikacin to kill adherent extracellular bacteria. The cells were then incubated for 3 hour at 37°C, washed with PBS, and lysed (40 mM Tris-Cl pH 7.4, 2 mM EGTA, 0.1% Triton X100) to release the intracellular bacteria. Bacteria were quantified by serial dilution. The % invasion efficiency was calculated as (recovered organisms/inoculated organisms)/epithelial cell number × 100%. All experiments were run in triplicate.
Transmission electron microscopy (TEM)
Cells were washed with PBS, the media replaced with neomycin-free media and inoculated with B. dolosa (AU4459) at an MOI between 15 and 20 in three separate experiments. The cells were incubated with bacteria for 2, 4 and 6 hours, washed with PBS, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate for one hour at 4°C, washed again, then incubated with 1.25% osmium tetroxide in PBS for 90 minutes at 25°C. Samples were fixed in 4% uranyl acetate, thin-sectioned (90 nm) in Polybed 812 (Polysciences, Warrington, PA), post-stained in uranyl acetate and lead citrate, and then visualized using a Zeiss 902 microscope (Zeiss, Thornwood, NY).
Quantitative adherence assay
CFT1-LCFSN or CFT1-LC3 cells (106 cells per well) were seeded onto round coverslips within 24-well tissue culture plates (Corning) then grown to confluence. Overnight cultures of B. dolosa grown on blood agar plates were diluted into LB to an OD650 of 0.2 and then grown in a shaking incubator at 37 °C to O D650 of 0.8. The bacteria were plated to determine the MOI. The epithelial cells were washed twice with 500 μL PBS per well. An aliquot of 300 μL CF12 neomycin-free media and 8 μL of bacteria were added to each well. The plates were centrifuged (5 minutes, ~330 ×g, 21 °C) and incubated for 25 minutes (37°C, 5% CO2, humidified air). Each well was washed X4 with 500 μL PBS and cells were stained using a HEMA 3 staining kit (Hema 3 Manual Staining System, Fisher Diagnostics). The coverslips were dried and mounted (Permount) for examination under a 100× oil immersion objective of a Zeiss Axioscope microscope. Images were acquired using Axiovision 3.1 software (Carl Zeiss, Thornwood, NY).
Statistical analysis
All analyses were performed using Prism 4 (San Diego, CA). Multiple groups were compared by ANOVA and post hoc analysis with Bonferroni Multiple Comparison test. Comparisons between the two groups were made using two-tailed t-test and p values < 0.05 were considered significant.
RESULTS
Modification of the antibiotic exclusion assay
Incubation of B. dolosa AU4459 with a triple antibiotic combination of 1mg/mL of meropenem, ceftazidime and amikacin for 3 hours resulted in the benchmark killing efficiency of 99.99% (Table 1), while incubation with single antibiotics and for shorter time intervals did not (data not shown). Using the same antibiotic combination over the same time interval, 100% killing efficiency was obtained for both B. cenocepacia J2315 and PAO1. The possibility of antibiotic permeation into the cells, and thus antimicrobial killing of internalized bacteria, was explored through invasion studies of the sensitive strain of PAO1 as it invaded into CFT1-LCFSN cells (3 hour incubation, triple antibiotic combination). If significant concentrations of the antibiotics accumulated in the cells allowing for intracellular killing, we would see an apparent lack of invasion of PAO1 into these cells. Instead, PAO1 invaded with efficiencies of 0.4% at two hours and 0.5% at three hours.
Microfilament and microtubular dependent invasion
To evaluate B. dolosa AU4459 invasion into respiratory epithelial cells, and look for evidence of mf and mt involvement, TEMs of CFT1-LCFSN (WT-CFTR) cells were obtained. TEMs showed finger-like projections at the cell surface that aligned with invading bacteria (Figure 1A). Additional evidence for possible mt involvement in the invasion process included the perinuclear localization of bacteria-filled vacuoles (Figure 1B).
Figure 1. B. dolosa enters respiratory epithelial cells.
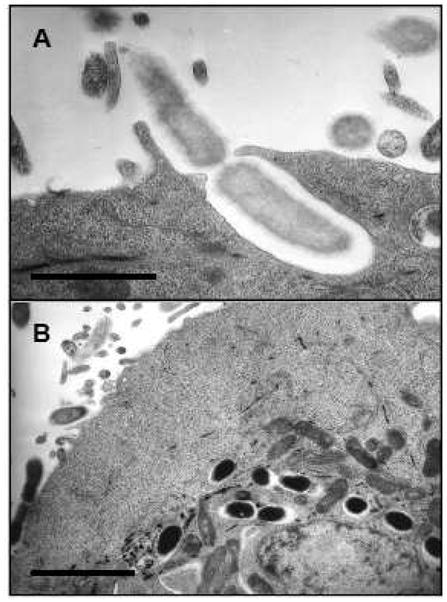
A Transmission electron micrograph (TEM) depicting entry of a clinical strain of B. dolosa bacterium into a CFT1-LCFSN, WT-CFTR expressing, respiratory epithelial cell. Note the alignment of the bacterium with a membrane protrusion which suggests microtubule and microfilament involvement. Scale bar indicates 1 μm. B. TEM depicting intracellular perinuclear vacuoles, which have been associated with microtubular trafficking, containing a clinical strain of B. dolosa in a CFT1-LCFSN cell. Both images were obtained after 6 hours of incubation with bacteria at an MOI of 15. Scale bar = 4 μm.
Using a modified antibiotic exclusion assay, B. cenocepacia invaded WT-CFTR expressing CFT1-LCFSN respiratory epithelial cells with an efficiency of 0.05%. Inhibition of mf and mt rearrangements significantly reduced the frequency of entry into CFT1-LCFSN to 0.006% (mf, cytochalasin D) and 0.011% (mt, colchicine), or 88% and 78% reduction of the control level, respectively (Figure 2A). In comparison, B. dolosa invaded CFT1-LCFSN cells with 25% efficiency. Invasion dropped to <0.5% after inhibition of either mf or mt, a 98% reduction of the control level (Figure 2B). Thus, both B. dolosa and B. cenocepacia are dependent on these pathways for entry into respiratory epithelial cells.
Figure 2. Affect of microfilament and microtubular inhibition on B. cenocepacia and B. dolosa entry into respiratory epithelial cells.
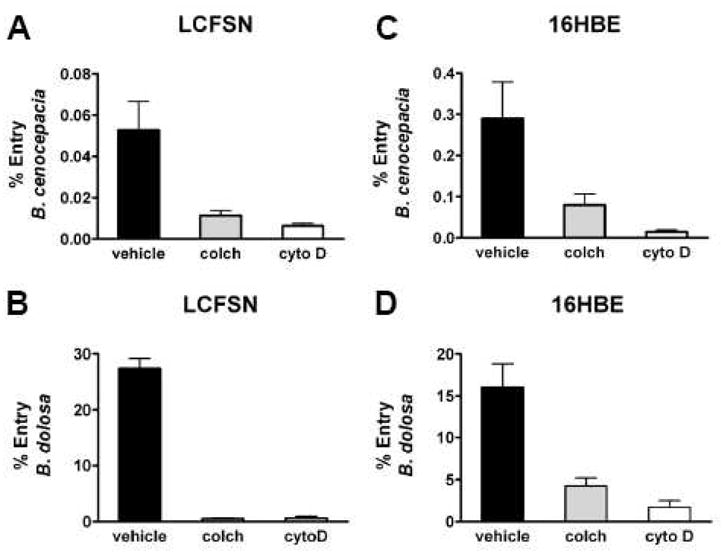
A Entry of B. cenocepacia into CFT1-LCFSN, WT-CFTR expressing cells incubated with either vehicle, the microtubule inhibitor colchicine (colch) or the actin filament inhibitor cytochalasin D (cytoD). B. Entry of B. dolosa into CFT1-LCFSN cells. C. Entry of B. cenocepacia into 16HBE, WT-CFTR expressing cells. D. Entry of B. dolosa into 16HBE cells. Bacterial entry is significantly reduced (p<0.05) with microtubule and actin filament inhibition in all cell lines with both bacterial isolates.
To determine if mt and mf invasion was cell line specific, the assay was repeated in WT-CFTR expressing 16HBE cells and the results mirrored those found with CFT1-LCFSN cells. B. cenocepacia invaded with 0.03% efficiency, which decreased to 0.008% efficiency (73% decrease) when colchicine was added (P<0.01), and 0.002% when cytochalasin D was added (93% decrease; P<0.001) (Figure 2C). B. dolosa invaded with 16% efficiency, which decreased to 4.4% with colchicine (73% decrease; P<0.01) and 1.7% with cytochalasin D (89% decrease; P<0.001) (Figure 2D).
The possibility of intracellular replication of bacteria during the three hour antibiotic incubation step of the modified invasion assay was explored. The standard gentamicin invasion assay used with gentamicin sensitive bacteria involves a one hour incubation step with the antibiotic to kill off extracellular bacteria. The B. dolosa studied in our invasion assay, however, required a three hour incubation with the triple antibiotic cocktail to obtain the 99.99% killing efficiency required for valid interpretation of the invasion assay. We compared the efficiency of invasion efficiency of B. dolosa and B. cenocepacia into CFT1-LCFSN cells at the same MOI after incubation with the triple antibiotic combination for either 2 or 3 hours. The invasion frequencies between the different time periods were comparable (B. dolosa invading at 16% efficiency at 2 hours and 18% at three hours, and B. cenocepacia invaded at 0.2% at 2 hours and 0.1% at 3 hours), suggesting that intracellular replication during the invasion assay was not significant. To ascertain whether the bacteria caused cell death, apoptosis studies were performed on the respiratory epithelial cells. No significant apoptosis was noted with incubation with P. aeruginosa, B. dolosa or B. cenocepacia over time periods up to 3 hours with varying MOIs (data not shown)
CFTR dependent invasion
To evaluate qualitative differences in invasion between the two different respiratory epithelial cell types due to CFTR function, TEMs of CFT1-LCFSN (WT-CFTR) and CFT1-LC3 (ΔF508-CFTR) cells were obtained after incubationB. dolosa with AU4459. Fewer finger-like projections were seen in CFT1-LC3 (ΔF508-CFTR) compared with CFT1-LCFSN (WT-CFTR) cells (Figure 3) suggesting that deficiency in CFTR altered the ability of epithelial cells to respond to bacteria with cell uptake mechanisms.
Figure 3. Cell ultrastructural change induced by B. dolosa in the presence and absence of functional CFTR.
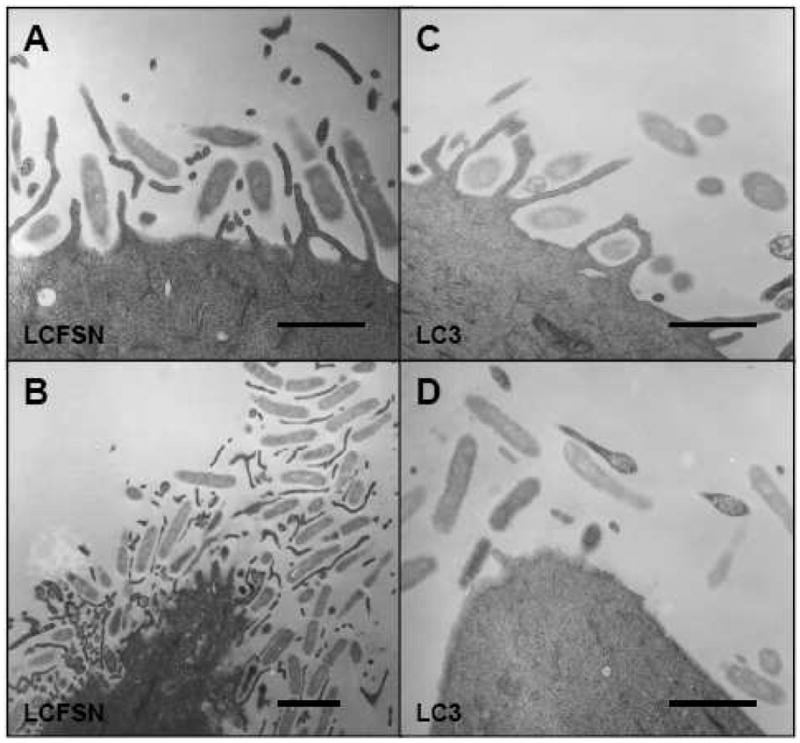
Entry of B. dolosa bacterium into WT-CFTR expressing CFT1-LCFSN cells (A. and B ) and ΔF508-CFTR expressing CFT1-LC3 cells (C. and D.). Note the more numerous cellular projections emanating from the CFT1-LCFSN surface and aligning with entering bacterium, as compared to the sparse cellular projections of the CFT1-LC3 cells. All scale bars = 1 μm.
CFT1-LCFSN (WT-CFTR) and CFT1-LC3 (ΔF508-CFTR) cells were used in invasion assays to quantitatively determine if CFTR function affected invasion. Invasion of B. cenocepacia into the WT-CFTR (CFT1-LCFSN) and the ΔF508-CFTR (CFT1-LC3) cells were not different (0.05% and 0.07% respectively; P>0.05; Figure 4A). For B. dolosa, however, a statistically significant difference was found between the invasion of the WT-CFTR (CFT1-LCFSN) and the ΔF508-CFTR (CFT1-LC3) respiratory epithelial cells (28% vs 5% respectively; P<0.001; Figure 4B). Thus, CFTR function appears to play a role in B. dolosa, but not B. cenocepacia invasion.
Figure 4. B. dolosa invasion efficiency changes with MOI and CFTR expression.
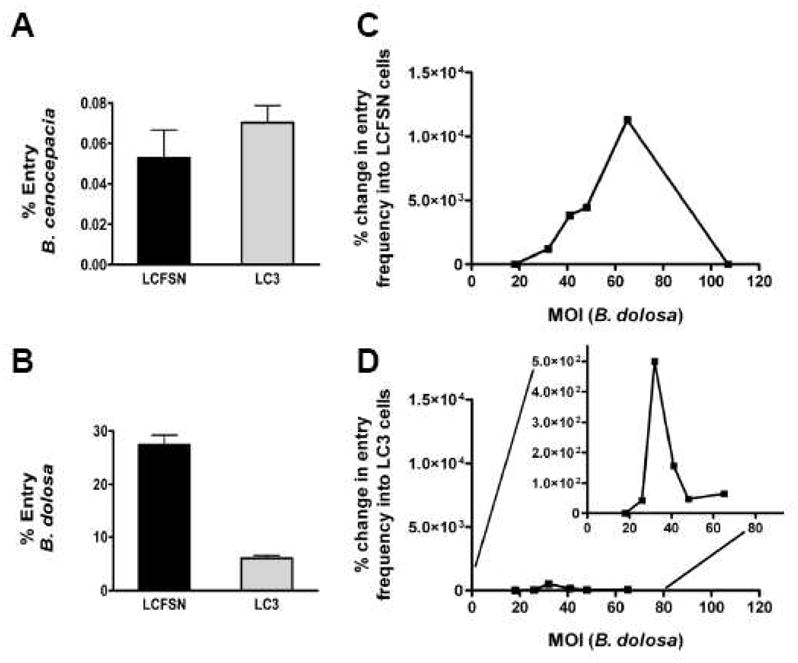
A Entry of B. cenocepacia into CFT1-LCFSN (WT-CFTR) and CFT1-LC3 (ΔF508-CFTR) cells (p>0.05). B. Entry of B. dolosa into CFT1-LCFSN and CFT1-LC3 cells (p<0.05). Per cent change in entry frequency of B. dolosa over differing MOIs in C. CFT1-LCFSN (WT-CFTR) and D. CFT1-LC3 (ΔF508-CFTR) respiratory epithelial cells. The insert in panel D. depicts the same information on an expanded Y axis.
Dependence of invasion efficiency on inoculum
Because bacterial invasion efficiency may depend on the initial inoculum, invasion assays using WT-CFTR (CFT1-LCFSN) respiratory epithelial cells exposed to a range of different MOI values were performed. The invasion efficiency of B. dolosa AU4459 increased to an MOI of approximately 35 and then declined, whereas invasion efficiency of B. cenocepacia J2315 peaked at an MOI of approximately 60 and then declined (Figure 4C-D). Because CFTR expression had been shown to affect B. dolosa invasion, we examined the MOI variation of invasion efficiency using ΔF508-CFTR (CFT1-LC3) cells. The efficiency of invasion of B. dolosa into the ΔF508-CFTR cells was less than that of the WT-CFTR cells at all MOIs studied.
Dependence of adherence on CFTR expression
To determine if there was a difference in bacterial adherence to respiratory epithelial cells with or without a functioning CFTR (WT-CFTR LCFSN or ΔF 508-CFTR LC3 cells, respectively), which could explain differences in bacterial invasion between the two cell types, an adherence assay was performed. Similar bacterial clumping occurred in both LCFSN and LC3 cell types (Figure 5A–D). A quantitative adherence assay did not reveal differences between the two groups (unpaired t-test, P=0.5075; Figure 5E). Accordingly, the observed difference in invasion efficiency of B. dolosa into WT- and ΔF508-CFTR expressing cells does not appear to be due to differences in adherence.
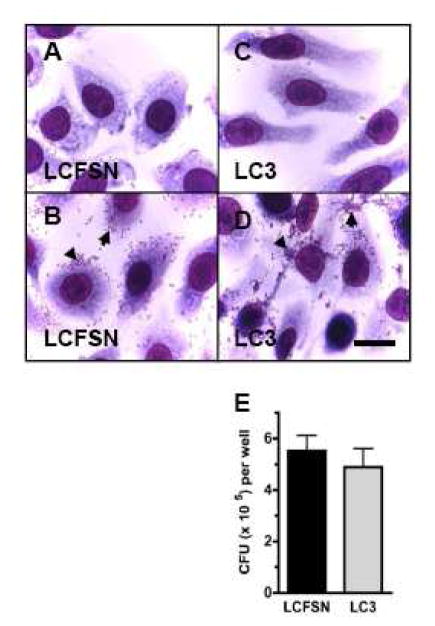
Figure 5. Similar adherence of B. dolosa to WT-CFTR and ΔF508-CFTR expressing epithelial cells.
CFT1-LCFSN (WT-CFTR, panels A. and B.) and CFT1-LC3 cells (ΔF508-CFTR, panels C. and D.) stained with Giemsa in order to visualize bacteria. Scale bar = 10 μm. Cells shown in panels A. and C are uninfected; cells in panels. B. and D. were incubated for 1 hour with 105 CFU of B. dolosa prior to washing and staining. Arrowheads point to clumps of adherent bacteria. Panel E. depicts the results of 3 separate quantitative adhesion assays performed in triplicate. Data are presented as mean and standard deviation. No statistical difference was found between the two groups (P>0.5).
DISCUSSION
Bacterial-host interactions have been studied extensively in invasive gram negative enteric bacterial species and involve mobilization of the target cell’s cytoskeletal components leading ultimately to bacterial phagocytosis, invasion and disease (9, 18). In comparison, bacterial-host interactions of invasive respiratory pathogens have not been as well documented. In the CF lung, bacteria residing in the airway lumens proliferate simultaneous with host attempts to clear them through macrophage and epithelial uptake, neutrophil killing and mucociliary clearance. In the case of P. aeruginosa, epithelial cell uptake is followed by epithelial cell apoptosis and clearance. Conversely, P. aeruginosa may also enter epithelial cells to replicate, thus avoiding rapid removal by macrophage phagocytosis or mucociliary clearance. P. aeruginosa has been shown to engage microfilaments, microtubules and CFTR protein in its interactions with respiratory epithelial cells (21). Similarly, some Bcc members invade host epithelial cells (2, 4), and microtubule involvement has been demonstrated for B. cenocepacia invasion (2). A role for microfilaments in Bcc invasion, however, has not been demonstrated and in particular, invasion strategies of B. dolosa, especially the highly successful and deadly epidemic strain SLC-6 (10), has not been studied. Due to their close kinship, a parallel to the pathogenesis of P. aeruginosa is probable. However, whether invasion of respiratory epithelial cells leads to progression of infection, as seen with enteric gram negative bacteria, or to accelerated clearance from the respiratory track is yet to be determined.
Our studies demonstrate that both B. cenocepacia and B. dolosa invade two different types of WT-CFTR expressing respiratory epithelial cells (CFT1-LSFCN and 16HBE). Electron micrographs of B. dolosa interacting with CFT1-LCFSN cells showed fingerlike projections from the plasma membrane of the respiratory epithelial cell surrounding invading bacteria. Once internalization occurred, bacteria were found in perinuclear locations, which is associated with microtubular trafficking (1). These observations prompted invasion assays that demonstrated both microtubule and microfilament involvement in B. cenocepacia and B. dolosa invasion.
Further electron microscopic studies of B. dolosa AU4459 invading both WT- and ΔF508-CFTR expressing cells showed many more fingerlike projections emanating from WT-CFTR cells exposed to B. dolosa compared with ΔF508 cells, suggesting that CFTR may be involved in invasion. WT-CFTR expression dramatically enhanced the uptake of B. dolosa, but not B. cenocepacia. It is possible that internalization of B. dolosa by respiratory epithelial cells may represent an important mechanism of bacterial clearance from the host with a functional CFTR. Patients with defective CFTR may not internalize inhaled bacteria as rapidly as control subjects with WT-CFTR. This time delay may allow the bacteria to replicate or communicate via quorum sensing and begin formation of biofilms. In support of this possibility, TEMs of B. dolosa interacting with ΔF508-CFTR cells showed clusters of extracellular bacteria in microcolonies that exhibited the coccobacilli morphology typical of gram negative rods in biofilms. These microcolonies were not seen in photomicrographs of B. dolosa interacting with WT-CFTR cells (data not shown).
The hypothesis that a functioning CFTR protein is required for maximal bacterial entry of B. dolosa into respiratory epithelial cells is further supported by the bacterial adherence and MOI data generated on B. dolosa in ΔF508 and WT-CFTR expressing cells. CFTR does not affect the capacity of the bacteria to adhere to the surface of the respiratory epithelial cells. As the bacteria number at the cell surface increases, the efficiency of invasion increases until it peaks and then falls, indicating that the system is saturable. B. dolosa reaches this saturation point around an MOI of 35 in ΔF508-CFTR cells as compared to WT-CFTR cells at an MOI of 60. Thus, B. dolosa invades WT-CFTR cells with much higher efficiency than ΔF508-CFTR cells. B. dolosa may bind directly to CFTR itself mirroring the WT-CFTR dependent mechanisms of internalization of P. aeruginosa into respiratory epithelial cells (3). The decreased internalization efficiency in the ΔF508-CFTR cells may reflect decreased receptor density at the cell surface. We propose that mutated CFTR protein leads to less internalization of B. dolosa, less clearance, and higher bacterial burden outside of the cell allowing for accelerated biofilm formation. However, a functioning CFTR protein is not required for all bacterial invasion since B. dolosa also invades the ΔF508 LC3 respiratory epithelial cells and microfilaments and microtubule inhibition affects invasion efficiency in all cell types regardless of CFTR functioning. In the case of P. aeruginosa, internalization of the organism triggers apoptosis of the cell (3) preventing spread of infection to neighboring cells. For B. dolosa, TEMs showed similar evidence of cellular apoptosis in invaded cells (data not shown) and staining of the infected respiratory epithelial cells over one, two and three hour time periods showed increasing numbers of cells undergoing apoptosis (2–3% apoptosis at three hours). While the cellular apoptosis rate does not significantly affect the invasion results over a three hour time period, apoptosis in infected respiratory epithelial cells over a longer time period (24hrs) has been shown to be significant (3). Further studies are needed to assess the role that CFTR and cellular apoptosis may play in the pathogenesis of infection with Bcc organisms. Also, more studies are warranted to determine the mechanism through which CFTR protein interaction with microfilaments and microtubules enhances B. dolosa invasion efficiency into respiratory epithelial cells and the role that that enhanced uptake may play in clearance of the organism.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-004277 to C. L. Cannon
The authors thank Drs. Khadijah M. Hindi and Dr. Thomas W. Ferkol, for their encouragement, critical review and thoughtful comments, and M. Levy for her expert assistance with the TEM imaging. This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-004277 to C. L. Cannon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infection and immunity. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns JL, Jonas M, Chi EY, Clark DK, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infection and immunity. 1996;64:4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon CL, Kowalski MP, Stopak KS, Pier GB. Pseudomonas aeruginosa- induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. American journal of respiratory cell and molecular biology. 2003;29:188–197. doi: 10.1165/rcmb.4898. [DOI] [PubMed] [Google Scholar]
- 4.Caraher E, Duff C, Mullen T, Mc Keon S, Murphy P, Callaghan M, McClean S. Invasion and biofilm formation of Burkholderia dolosa is comparable with Burkholderia cenocepacia and Burkholderia multivorans. J Cyst Fibros. 2007;6:49–56. doi: 10.1016/j.jcf.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Coenye T, Vandamme P, Govan JR, LiPuma JJ. Taxonomy and identification of the Burkholderia cepacia complex. J Clin Microbiol. 2001;39:3427–3436. doi: 10.1128/JCM.39.10.3427-3436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science (New York, NY) 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 7.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. American journal of respiratory cell and molecular biology. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig SM, Lee EJ, Wu C, Andika RC, Vallas V, Portoles M, Frank DW. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. Clao J. 1998;24:41–47. [PubMed] [Google Scholar]
- 9.Goosney DL, Knoechel DG, Finlay BB. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerging infectious diseases. 1999;5:216–223. doi: 10.3201/eid0502.990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govan JR, Brown AR, Jones AM. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future microbiology. 2007;2:153–164. doi: 10.2217/17460913.2.2.153. [DOI] [PubMed] [Google Scholar]
- 11.Kalish LA, Waltz DA, Dovey M, Potter-Bynoe G, McAdam AJ, Lipuma JJ, Gerard C, Goldmann D. Impact of Burkholderia dolosa on lung function and survival in cystic fibrosis. American journal of respiratory and critical care medicine. 2006;173:421–425. doi: 10.1164/rccm.200503-344OC. [DOI] [PubMed] [Google Scholar]
- 12.Kazachkov M, Lager J, LiPuma J, Barker PM. Survival following Burkholderia cepacia sepsis in a patient with cystic fibrosis treated with corticosteroids. Pediatr Pulmonol. 2001;32:338–340. doi: 10.1002/ppul.1127. [DOI] [PubMed] [Google Scholar]
- 13.LiPuma JJ. Burkholderia cepacia complex: a contraindication to lung transplantation in cystic fibrosis? Transpl Infect Dis. 2001;3:149–160. doi: 10.1034/j.1399-3062.2001.003003149.x. [DOI] [PubMed] [Google Scholar]
- 14.LiPuma JJ, Dasen SE, Nielson DW, Stern RC, Stull TL. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990;336:1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 15.Mahenthiralingam E, Simpson DA, Speert DP. Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J Clin Microbiol. 1997;35:808–816. doi: 10.1128/jcm.35.4.808-816.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia cepacia. Infection and immunity. 2000;68:24–29. doi: 10.1128/iai.68.1.24-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClean S, Callaghan M. Burkholderia cepacia complex: epithelial cell-pathogen confrontations and potential for therapeutic intervention. Journal of medical microbiology. 2009;58:1–12. doi: 10.1099/jmm.0.47788-0. [DOI] [PubMed] [Google Scholar]
- 18.Oelschlaeger TA, Guerry P, Kopecko DJ. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palfreyman RW, Watson ML, Eden C, Smith AW. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infection and immunity. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiniger N, Ichikawa JK, Pier GB. Influence of cystic fibrosis transmembrane conductance regulator on gene expression in response to Pseudomonas aeruginosa infection of human bronchial epithelial cells. Infection and immunity. 2005;73:6822–6830. doi: 10.1128/IAI.73.10.6822-6830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajjan U, Keshavjee S, Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infection and immunity. 2004;72:4188–4199. doi: 10.1128/IAI.72.7.4188-4199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajjan U, Wu Y, Kent G, Forstner J. Preferential adherence of cable-piliated burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. Journal of medical microbiology. 2000;49:875–885. doi: 10.1099/0022-1317-49-10-875. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerceker AA, Golan DE, Pier GB. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran Van Nhieu G, Bourdet-Sicard R, Dumenil G, Blocker A, Sansonetti PJ. Bacterial signals and cell responses during Shigella entry into epithelial cells. Cellular microbiology. 2000;2:187–193. doi: 10.1046/j.1462-5822.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, Mahenthiralingam E, Speert D, Dowson C, Vandamme P. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. International journal of systematic and evolutionary microbiology. 2008;58:1580–1590. doi: 10.1099/ijs.0.65634-0. [DOI] [PubMed] [Google Scholar]
- 27.Vermis K, Coenye T, LiPuma JJ, Mahenthiralingam E, Nelis HJ, Vandamme P. Proposal to accommodate Burkholderia cepacia genomovar VI as Burkholderia dolosa sp. nov. International journal of systematic and evolutionary microbiology. 2004;54:689–691. doi: 10.1099/ijs.0.02888-0. [DOI] [PubMed] [Google Scholar]
- 28.Vermis K, Vandamme PA, Nelis HJ. Burkholderia cepacia complex genomovars: utilization of carbon sources, susceptibility to antimicrobial agents and growth on selective media. J Appl Microbiol. 2003;95:1191–1199. doi: 10.1046/j.1365-2672.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- 29.Yabuuchi E, Kosako Y, Oyaizu Hk, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiology and immunology. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 30.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA, Jr, Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. The American journal of physiology. 1993;264:C1219–1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]