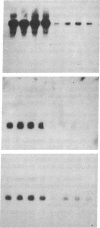
Abstract
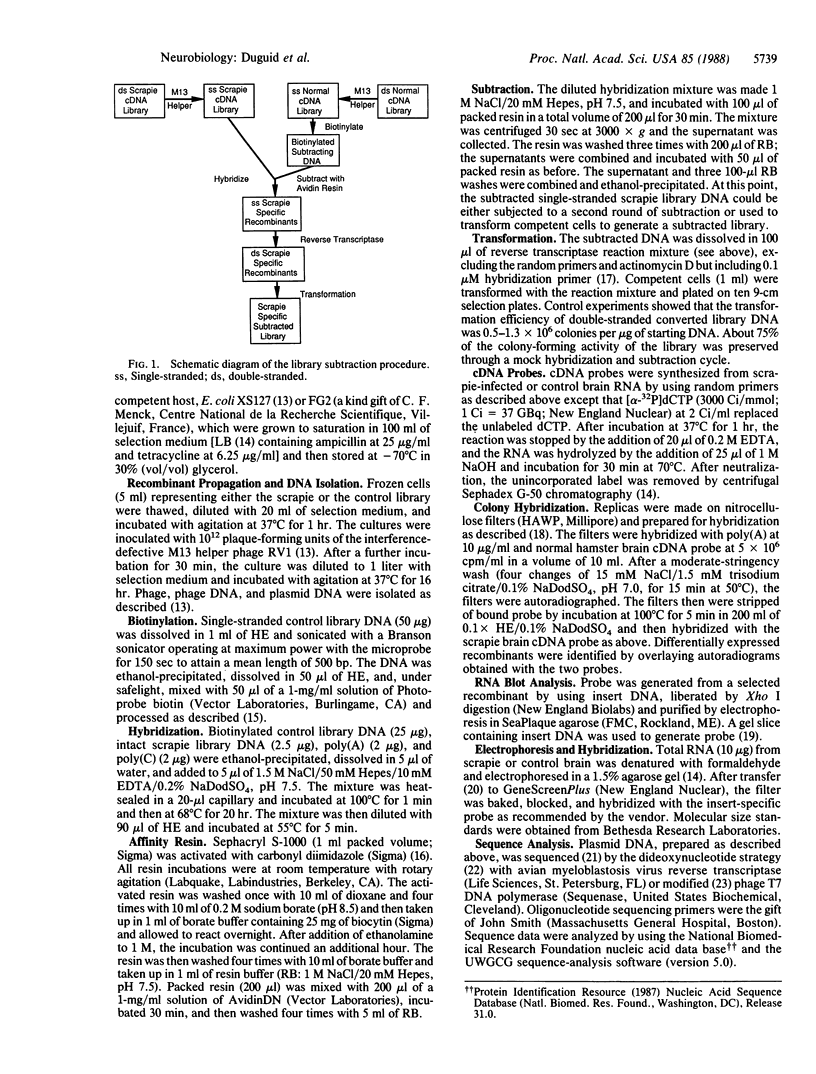
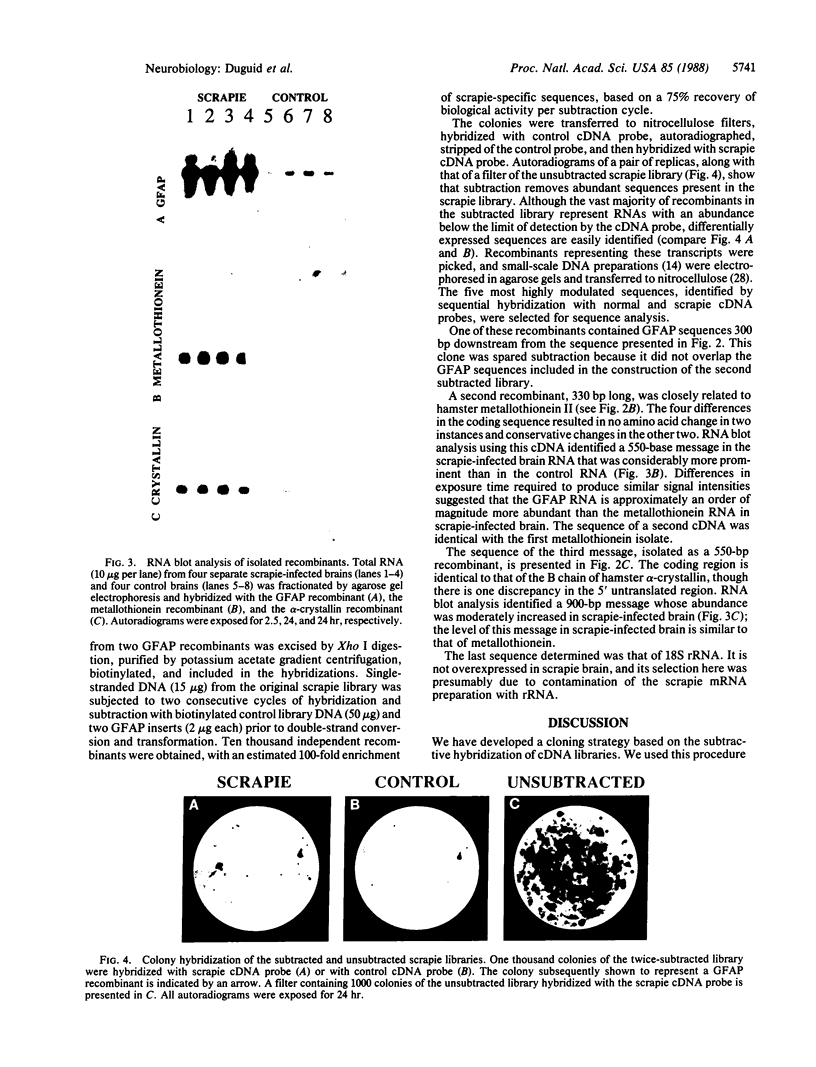
We have developed a subtractive cloning procedure based on the hybridization of single-stranded cDNA libraries constructed in pi H3M, a vector containing the phage M13 origin of replication. We have used this strategy to isolate three transcripts whose abundance is increased in scrapie-infected brain. DNA sequence analysis showed that they represent glial fibrillary acidic protein, metallothionein II, and the B chain of alpha-crystallin; the latter two may represent a response to stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell G. S., Ayers J. S., Hancock W. S., Hearn M. T. A novel method of activation of cross-linked agaroses with 1,1'-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J Biol Chem. 1979 Apr 25;254(8):2572–2574. [PubMed] [Google Scholar]
- Carp R. I., Merz P. A., Kascsak R. J., Merz G. S., Wisniewski H. M. Nature of the scrapie agent: current status of facts and hypotheses. J Gen Virol. 1985 Jul;66(Pt 7):1357–1368. doi: 10.1099/0022-1317-66-7-1357. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zumstein P., Zullo J., Rollins B., Mercola M., Stiles C. D. Differential colony hybridization: molecular cloning from a zero data base. Methods Enzymol. 1987;147:64–85. doi: 10.1016/0076-6879(87)47099-7. [DOI] [PubMed] [Google Scholar]
- Crapper D. R., Krishnan S. S., Dalton A. J. Brain aluminum distribution in Alzheimer's disease and experimental neurofibrillary degeneration. Science. 1973 May 4;180(4085):511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Garruto R. M., Fukatsu R., Yanagihara R., Gajdusek D. C., Hook G., Fiori C. E. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1875–1879. doi: 10.1073/pnas.81.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith B. B., Walters R. A., Enger M. D., Hildebrand C. E., Griffith J. K. cDNA cloning and nucleotide sequence comparison of Chinese hamster metallothionein I and II mRNAs. Nucleic Acids Res. 1983 Feb 11;11(3):901–910. doi: 10.1093/nar/11.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Levinson A., Silver D., Seed B. Minimal size plasmids containing an M13 origin for production of single-strand transducing particles. J Mol Appl Genet. 1984;2(6):507–517. [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A. Immunohistochemical demonstration of glial fibrillary acidic protein in scrapie. J Comp Pathol. 1983 Apr;93(2):251–259. doi: 10.1016/0021-9975(83)90012-9. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Tesin D. M., Sklaviadis T., Manuelidis E. E. Astrocyte gene expression in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5937–5941. doi: 10.1073/pnas.84.16.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl D. P., Gajdusek D. C., Garruto R. M., Yanagihara R. T., Gibbs C. J. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science. 1982 Sep 10;217(4564):1053–1055. doi: 10.1126/science.7112111. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions causing degenerative neurological diseases. Annu Rev Med. 1987;38:381–398. doi: 10.1146/annurev.me.38.020187.002121. [DOI] [PubMed] [Google Scholar]
- Quax-Jeuken Y., Quax W., van Rens G., Khan P. M., Bloemendal H. Complete structure of the alpha B-crystallin gene: conservation of the exon-intron distribution in the two nonlinked alpha-crystallin genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5819–5823. doi: 10.1073/pnas.82.17.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K. Use of benzoylated cellulose columns for the isolation of poly(adenylic acid) containing RNA and other polynucleotides with little secondary structure. Biochemistry. 1974 Aug 27;13(18):3677–3682. doi: 10.1021/bi00715a009. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness J., Maxwell I. H., Hahn W. E. Complex population of nonpolyadenylated messenger RNA in mouse brain. Cell. 1979 Dec;18(4):1341–1349. doi: 10.1016/0092-8674(79)90244-7. [DOI] [PubMed] [Google Scholar]
- Welcher A. A., Torres A. R., Ward D. C. Selective enrichment of specific DNA, cDNA and RNA sequences using biotinylated probes, avidin and copper-chelate agarose. Nucleic Acids Res. 1986 Dec 22;14(24):10027–10044. doi: 10.1093/nar/14.24.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietgrefe S., Zupancic M., Haase A., Chesebro B., Race R., Frey W., 2nd, Rustan T., Friedman R. L. Cloning of a gene whose expression is increased in scrapie and in senile plaques in human brain. Science. 1985 Dec 6;230(4730):1177–1179. doi: 10.1126/science.3840915. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hoekman W. A., Mulders J. W., Bloemendal H. Heat shock response of the rat lens. J Cell Biol. 1986 Jan;102(1):104–111. doi: 10.1083/jcb.102.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]