Abstract
Certain sickness behaviors occur consistently in influenza-infected humans and mice. These include body temperature changes, somnolence, and anorexia. Several cytokines serve as mediators of the influenza acute phase response (APR), including these sickness behaviors, and one likely inducer of these cytokines is dsRNA produced during viral replication. TLR3 is known to be one of the host cellular components capable of recognizing dsRNA and activating cytokine synthesis. To determine the role of TLR3-detected viral dsRNA in the causation of viral symptoms, TLR3-deficient mice (TLR3 knockouts, or KOs) were infected with a marginally lethal dose of mouse-adapted X-31 influenza virus. TLR3 KOs and their wild-type (WT) controls were monitored for baseline body temperature, locomotor activity, and sleep profiles prior to infection. Both mouse strains were then infected and monitored for changes in these sickness behaviors plus body weight changes and mortality for up to 14 days post-infection. Consistent with the observations that influenza pathology is reduced in TLR3 KOs, we showed that hypothermia after post-infection day 5 and the total loss of body weight were attenuated in the TLR3 KOs. Sleep changes characteristic of this infection model [particularly increased non-rapid-eye-movement sleep (NREMS)] were also attenuated in TLR3 KOs and returned to baseline values more rapidly. Locomotor activity suppression was similar in both strains. Therefore virus-associated dsRNA detected by TLR3 appears to play a substantial role in mediating several aspects of the influenza syndrome in mice.
Keywords: Sickness Behavior, Acute Phase Response, Toll-like Receptor 3, dsRNA, Virus, Influenza, Hypothermia, Locomotor Activity, Sleep Changes, Body Weight Changes
1. Introduction
The viral acute phase response, or `flu syndrome,' is commonly experienced in varying degrees of severity following acute respiratory and intestinal viral infections. The most common symptoms of acute viral infections in humans are fever, somnolence, headache, and a general feeling of malaise. These symptoms are usually indistinguishable from those associated with bacterial infections, and are thought to be mediated by some of the same cytokines that are induced by bacteria, such as IL-1β and TNF-α (Leon, 2004).
All viruses produce double-stranded (ds)RNA during replication (Majde, 2000), regardless of the form of nucleic acid borne by the virions. DsRNA associated with single-stranded (ss)RNA viruses, such as influenza, is thought to be derived primarily from annealing of ssRNA replication intermediates (Majde, 2000), though other sources of intracellular dsRNA may be increased by viral infection such as small interfering RNAs (Matskevich and Moelling, 2007). Synthetic dsRNA (polyriboinosinic:polyribocytidylic acid, or pI:C) or virus-derived dsRNA stimulates sickness behaviors indistinguishable from influenza virus itself (Carter and De Clercq, 1974; Fang et al., 1999; Kimura-Takeuchi et al., 1992; Majde et al., 1991; Majde, 2000; Traynor et al., 2006). In addition, pI:C can substitute for influenza virus in the blockade of the acute phase response (APR) (Kimura-Takeuchi et al., 1992), suggesting that dsRNA alone can induce APR regulatory mediators similar to those induced by the virus. [Details of the mouse sickness, cytokine and endocrine responses to pI:C were recently described (Cunningham et al., 2007; Gandhi et al., 2007).] Virus-associated dsRNA is thought to be an important mediator of viral cytokine induction and contribute to the influenza APR (Guillot et al., 2005).
In the last decade much has been learned about a pathogen recognition system termed the Toll-like receptors (TLRs). These receptors play a prominent role in the initiation of cytokine induction by microbes (Kawai and Akira, 2006). Cytokine induction by bacteria is known to be initiated by bacterial cell-wall products such as endotoxins/lipopolysaccharides that are recognized by TLR4 (Akira and Hemmi, 2003), or lipopeptides (Hashimoto et al., 2007) recognized by TLR2 (Kawai and Akira, 2005). One group of TLRs detects microbial nucleic acids (Akira and Hemmi, 2003), which have subtle structural differences from mammalian nucleic acids. Specifically, unmethylated microbial DNA is recognized by TLR9 (Akira and Hemmi, 2003). Intracellular viral ssRNA, on the other hand, is recognized by either TLR7 (in the mouse) or TLR8 (in the human) (Diebold et al., 2006). Extracellular viral dsRNA is detected primarily by TLR3 (Gantier and Williams, 2007), while intracellular dsRNA made during viral replication is also detected by cytoplasmic helicases such as RIG-I and MDA-5 (Eisenacher et al., 2008; Pichlmair and Reis e Sousa, 2007) as well as by vesicular TLR3 (Vercammen et al., 2008). Intracellular viral ssRNA and dsRNA also activate the inflammasome Nod-like receptor family (NLRP)3, which is important in host defense against influenza virus (Allen et al., 2009). Different dsRNA-recognition factors are expressed in different cell types (Kawai and Akira, 2006) and recognize different viruses (Kato et al., 2006).
TLR3s recognize both synthetic and virus-derived dsRNA (Vercammen et al., 2008). They are found on CD8+ T lymphocytes (Salem et al., 2009), influenza-infected epithelial cells (Guillot et al., 2005), macrophages (Miettinen et al., 2001), myeloid dendritic cells (Schroder and Bowie, 2005), glial cells (Carpentier et al., 2007) and other immunocompetent cells. Binding of dsRNA to TLR3 induces apoptosis and autophagy of immune and cancer cells as well as cytokines and chemokines (Seya, T. and Matsumoto, M.,2009).
The protective/pathogenic roles of TLR3 receptors have been analyzed using TLR3 knockout (KO) mice in several viral models (Vercammen et al., 2008), including influenza. However, the role of TLR3 signaling in the induction of the APR to a virus has not been explored. In this study we analyze several acute phase sickness behaviors in TLR3 KOs and their wild-type (WT) controls challenged intranasally with a marginally-lethal dose of mouse-adapted influenza A virus.
2. Materials and Methods
2.1. Animals
Two mouse strains were employed: B6;129S1-Tlr3tm1Flv/J (TLR3 KOs) and B6:129SF1/J (WT) strain controls. Three to four month old male mice of each strain were purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were quarantined in AAALAC-approved animal quarters and housed in filter-top cages throughout the study to prevent intercurrent infections or spread of X-31. For body temperature/locomotor activity/body weight studies, mice were housed individually at 22–23° C ambient. During sleep data collection the mice were housed individually in a sound-attenuated environmental chamber maintained at a temperature of 29 ± 1° C to which they were adapted for at least a week. All mice were maintained on a 12:12 h light-dark cycle with lights on at 0900 PDT. Food and water were available ad libitum. All experiments were approved by the Washington State University Animal Care and Use Committee and conformed to National Institutes of Health guidelines.
2.2. Virus purification and titration
The influenza strain employed was mouse-adapted X-31 influenza A, a reassortant between A/PR/8/34 (H1N1) (PR8) and A/Aichi/68 (H3N2) (Lee et al., 2001). X-31 expresses H3N2 surface genes but contains the internal genome segments of PR8, a strain of influenza that is highly pathogenic for mice. About 10,000-fold more X-31 virus is required to kill mice in the same time-frame as PR8, largely due to the retention of glycosylated surface proteins on X-31 that are targeted by circulating host lectins (Reading et al., 1997). The X-31 virus grown in specific pathogen-free chick embryos was purified and tested for possible contaminants as previously described (Chen et al., 2004). Viral titrations were performed in Madin-Darby canine kidney cells as previously described (Chen et al., 2004) and expressed as median tissue culture infectious doses (TCID50). The starting titer of the purified X-31 virus was 1 × 106 TCID50/mL.
2.3. Virus inoculation
The purified virus was diluted in Dulbecco's PBS containing magnesium and calcium (Invitrogen, Carlsbad, CA) and mice were inoculated intranasally using a 100 μl micropipette with 200 TCID50 X-31 in a volume of 50 μl/mouse (25 μl each nostril) under light methoxyfluorane (Metofane, Pitman-Moore, Inc., Mundelein, IL) inhalation anesthesia. All inoculations were performed within 10 minutes of light onset at 0900 h PDT. The dose of virus used was approximately 2 median lethal doses (LD50) as determined by titration in 129 SvEv mice maintained at 23° C.
2.4. Surgeries
All surgeries were conducted in mice anesthetized with intraperitoneal ketamine-xylazine (87 and 13 mg/kg, respectively). For temperature and locomotor activity studies, mice (8 TLR3 KO and 15 WT) were implanted intraperitoneally with chemically sterilized biotelemetry transmitters (Minimitter, Bend, OR) as previously described (Traynor et al., 2006). For sleep studies, mice (n = 8 for TLR3 KO and n = 8 for WT controls) were implanted with two electromyogram (EMG) electrodes and three EEG electrodes (Plastics One, Roanoke, VA) as described in reference (Chen et al., 2004). Electrodes for EEG recordings were placed over the frontal and parietal cortices and over the cerebellum. Electrodes for EMGs were placed in the dorsal neck muscles. Following surgery, mice were allowed to recover for 7 days prior to sleep recording. Baseline wake (W), non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) characteristics were established by recording for 24–48 h prior to infection. Mice were allowed to recover for at least a week following surgeries before viral challenge.
2.5. Body temperature and locomotor activity analyses
Body temperature and locomotor activity measurements were performed using Mini Mitter (Bend, OR) biotelemetry as described in (Traynor et al., 2006). Temperature and locomotor activity data were recorded at 6-minute intervals and data points representing those values were averaged over 6-h intervals. The mice were kept at 23°C for these experiments because body temperature responses to influenza challenge are larger at this ambient temperature (Jhaveri et al., 2007).
2.6. Sleep scoring
EEG and EMG signals were digitized (128 Hz sampling rate) and stored on digital media. W, NREMS and REMS were scored by hand in 10-second epochs by defining NREMS as high amplitude EEG slow waves and low-tone muscle activity, REMS as highly regular theta EEG activity and loss of muscle tone with occasional twitches, and W as EEG activities similar to, but often less regular and with lower amplitude than, those in REMS and high EMG activity. Time spent in each state was tabulated into 2-h intervals and graphed. In infected mice sleep was analyzed over 24-h intervals prior to infection (baseline) or three days (Virus 3), 5 days (Virus 5) or 8 days (Virus 8) following intranasal X-31 challenge. These scoring days were selected based on body temperature changes and on previous studies in this model (Traynor et al., 2006), and represent the acute phase (Virus 3 and 5) and the early recovery phase (Virus 8) of the infection, respectively.
2.7. EEG delta (1/2-4Hz) wave power analysis
EEG delta-wave activity during NREMS is also called the slow-wave activity (SWA) of the EEG and often regarded as a measure of NREMS intensity. To measure SWA, on-line fast Fourier transformation was performed on EEG data in 2-second epochs and averaged for every 10 seconds. For each artifact-free 10-second NREMS epoch, power values in the 0.5 to 4 Hz (delta wave) range were integrated and then averaged in 2-h blocks. For each animal, raw SWA values were averaged across a 24-h baseline day to calculate a reference value. SWA was then collapsed in 2-h blocks was then expressed as the percentage of this reference value (arbitrary units). Furthermore, on the baseline day and on day 8 after inoculation, total EEG power was computed separately for NREMS and REMS episodes for the entire 24-h periods. EEG power was then expressed in 0.5 Hz bins in the 0.5 – 20 Hz frequency range as percent of total power.
2.8. Body weights
Mice of both strains (n = 15 for WT and n = 14 for KOs) were weighed on the day of inoculation (day 0) and at the termination of the infection studies (12–14 d post infection) when the mice were euthanized. Weights were adjusted for Mini Mitters or electrode carriers when present.
2.9. Statistics
For the comparison of NREMS, REMS, SWA, motor activity and body temperature between the two genotypes on the baseline day, 2-way ANOVA was used on 2-h data blocks (independent factor: genotype, repeated factor: time). For the analysis of NREMS, REMS, and SWA after virus treatment, 2-way ANOVA (repeated factors: treatment and time) was performed on 2-h data blocks between the baseline and the given post-infection day across 24 h separately in the WT and KO groups. For the analysis of body temperature and motor activity responses to virus inoculation across the baseline and 8-day post-infection period, 3-way ANOVA was used on 6-h data blocks [independent factor: genotype, repeated factors: day (virus treatment) and time (within the day)]. Power spectrum data between genotypes and between baseline and day 8 post-inoculation were compared by using 3-way ANOVA [independent factors: genotype and frequency, repeated factor: day (virus treatment)]. Body weight changes over time were analyzed using a t-test. In all statistical tests p values less than 0.05 were considered significantly different from WT controls.
3. Results
3.1. Body temperature changes
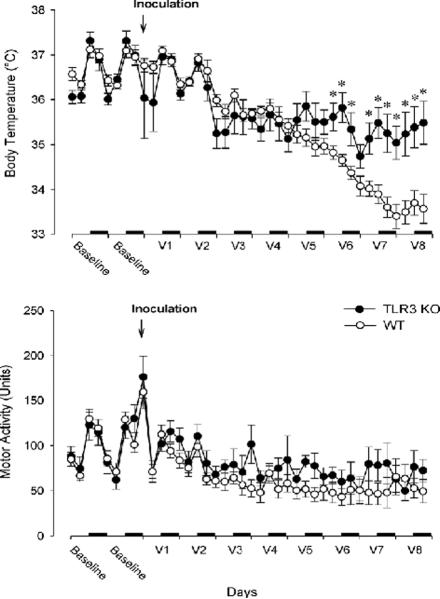
Baseline body temperatures showed a similar diurnal rhythm in both WT and TLR3 KOs [time effect: F(11,198) = 47.8, p < 0.001; genotype effect: F(1,18) = 0.5, n.s.; genotype × time interaction: F(11, 198) = 1.2, n.s.]. No differences in baseline temperatures were seen between the two strains during the 48 h baseline studies [time effect: F(11,198) = 47.8, p < 0.001; genotype effect: F(1,18) = 0.5, n.s.; genotype × time interaction: F(11, 198) = 1.2, n.s.]; baseline temperatures of both strains over 48 h are shown in Fig. 1 (top graph, left side).
Fig. 1.
Body temperature (top panel) and locomotor activity (bottom panel) of WT (white circles, n = 15) and TLR3 KO (black circles, n = 8) 48 h prior to infection and for 8 days following virus inoculation (V1 – V8). Horizontal dark bars: dark phase of the day. Asterisks: significant difference between the two genotypes (Student's t-test, p < 0.005). Data are expressed as 6-h averages (± SE).
Virus inoculation led to an overall decrease in body temperature in both genotypes [day effect: F(8,144) = 63.5, p < 0.001], but the extent and time-course of this decrease significantly differed between groups [genotype × day interaction: F(8,144) = 13.1, p < 0.001]. During the first two days after infection, both WT and KO mice showed similar hypothermic responses (Fig. 1, upper panel). Thereafter, while body temperature in the WT animals decreased continuously until day 8, it stabilized at a higher, but still below baseline, level in KOs starting at day 3 post-infection. Daily comparisons indicate that KO animals, in fact, had higher body temperature than WTs on the last three days of recording (p < 0.05 for all three days). WT temperatures reached a nadir of 33.3°C on day 7 at light onset (Fig. 1, upper panel), while mean KO body temperatures never fell below 34.5°C (Fig. 1).
3.2. Locomotor activity changes
On the baseline day, both WTs and KOs showed pronounced diurnal rhythm in locomotor activity [time effect: F(11, 198) = 7.28]; there was no significant difference between the two genotypes [genotype effect: F(1,18) = 1.20, n.s.; genotype × time interaction: F(11,198) = 1.4, n.s.] (Fig. 1). Viral infection elicited similar and significant changes in the locomotor activity of the two groups [day effect: F(8,144) = 14.4, p < 0.001; genotype effect: F(1,18) = 1.4, n.s.; day × genotype interaction: F(8, 144) = 0.4, n.s.] (Fig. 2, lower panel). On the first post-inoculation day, there was a significant increase in locomotor activity in both groups due to the handling of the animals during the inoculation. Locomotor activity returned to baseline on the second day and from the third post-inoculation day on, locomotor activity dropped below baseline level and remained suppressed until the end of the recording period. As depicted in Fig. 1, lower panel, the WT mice showed a slight trend towards lower activity levels with less diurnal variation compared to TLR3 KOs, but these differences were not statistically significant.
Fig. 2.
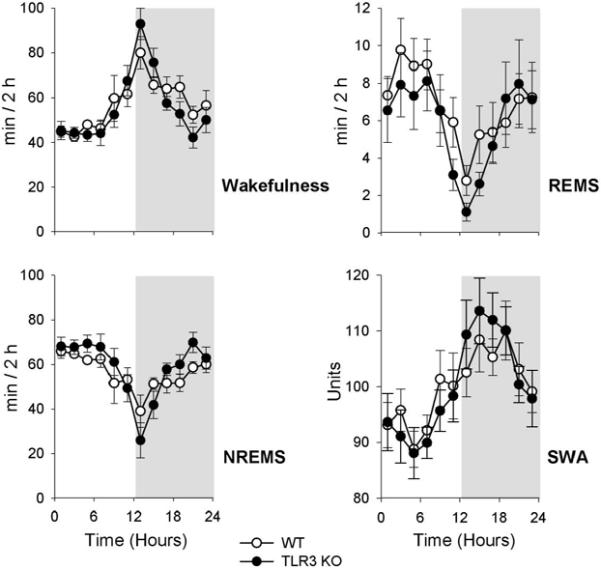
Wakefulness, non-rapid-eye movement sleep (NREMS), rapid-eye movement sleep (REMS) and EEG slow-wave activity (SWA) in uninfected WT (white circles, n = 8) and TLR3 KO mice (black circles, n = 8). Data are expressed in 2-h blocks (average ± SE). The unshaded area represents the light period and the shaded area represents the dark period.
3.3. Sleep changes
There was no significant difference between the baseline sleep-wake activities of the WT and KO animals [genotype effect for W: F(1,14) = 0.2, n.s.; NREMS: F(1,14) = 0.4, n.s.; REMS: F(1,14) = 0.4, n.s.] (Fig. 2). All three vigilance states and the SWA showed clear and similar diurnal rhythms in both uninfected animal groups [time effect for W: F(11,154) = 13.0, p < 0.001; NREMS: (11, 154) = 11.6, p < 0.001; REMS: F(11,154) = 7.8, p < 0.001; SWA: F(11,154) = 5.1, p < 0.001].
Virus inoculation induced significant increases in NREMS in both genotypes but the effects in KO mice were shorter lasting and confined to the dark periods (Fig. 3b). NREMS was elevated on the third [treatment effect WT: F(1,7) = 17.4, p < 0.01; KO: F(1,7) = 6.6, p < 0.05] and fifth post-virus day [treatment effect WT: F(1,7) = 32.3, p < 0.001; treatment × time KO: F(11,77) = 2.8, p < 0.05] in both WT (Fig. 3a) and KO mice (Fig. 3b) but NREMS sleep values were significantly lower in KOs than in WT at several time points on both post-infection days 3 and 5 (Fig 4). On day 8 after inoculation, NREMS remained elevated in WTs (Fig. 3a), but it returned to baseline in KO animals (Fig. 3b) [WT treatment effect: F(1,7) = 12.3, p < 0.01; KO treatment effect: F(1,7) = 4.5, n.s., treatment × time interaction: F(11,77) = 1.2, n.s.]. There was a tendency towards increased EEG SWA in the WT group on post-virus days 5 and 8 (Fig. 3a), but due to the high variability caused by one mouse, these changes were not statistically significant (p = 0.07 and p = 0.11, respectively); no such tendency was observed in the KO group (Fig. 3b).
Fig. 3.
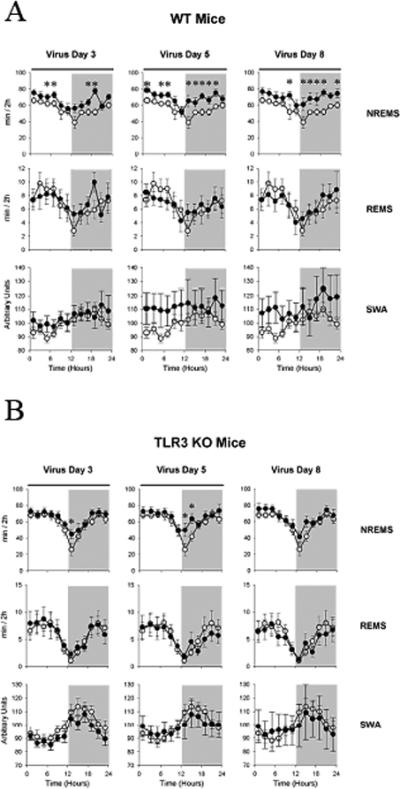
A. NREMS, REMS and SWA responses to viral infection in WT mice (n = 8). White circles represent baseline sleep responses prior to infection and black circles represent WT sleep changes following X-31 infection. Horizontal dark bar: significant treatment effect or treatment x time interaction by ANOVA across 24 h. Asterisks: significant difference between baseline and virus day, Student's t-test, p < 0.05.
B. NREMS, REMS and SWA responses to viral infection in TLR3 KO mice (n = 8). White circles represent baseline sleep responses prior to infection and black circles represent TLR3 KO sleep changes following X-31 infection. Horizontal dark bar: significant treatment effect or treatment × time interaction by ANOVA across 24 h. Asterisks: significant difference between baseline and virus day, Student's t-test, p < 0.05.
Fig. 4.
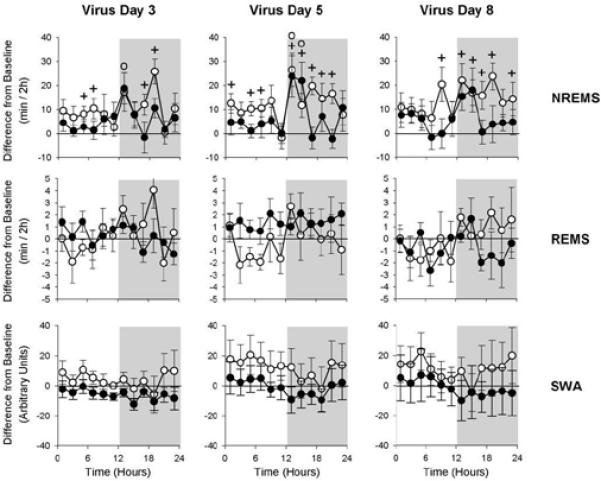
Comparison of the effects of viral infection on NREMS, REMS and SWA in TLR3 KO (black circles, n = 8) and WT (white circles, n = 8) mice. Data are expressed as difference from baseline. Significant differences from baseline are indicated by crosses in WT and by open circles in TLR3 KO mice; Student's t-test, p < 0.05.
A direct comparison of sleep responses in infected WT and KO mice is shown in Fig. 4. The major difference between the two strains is seen in the NREMS values, which are significantly different at several time points during the dark period. This difference is more marked on days 5 and 8, where significant differences from baseline are seen in the KOs only at the beginning of the dark period while NREMS elevation in WT mice is seen throughout the dark period.
The EEG during NREMS showed the typical power distribution in both genotypes with the predominant activity in the slow-wave range; there was no significant difference between the two infected groups. Virus inoculation significantly affected the power distribution on day 8 [ANOVA, treatment × frequency interaction: F(39,560) = 1.9, p < 0.01]; this effect was significantly different between WT and KO mice [ANOVA, treatment × frequency × genotype interaction: F(39,560) = 1.8, p < 0.01]. There was an increase in the power in the 1.5 to 6 Hz range in response to virus in the WT group while in KOs, power in the slowest frequency band (0.5–1 Hz) was below baseline (Fig. 5). EEG slow wave power is an indictor of NREMS intensity (38); the data thus indicate that the KO mice had less intense NREMS after the viral challenge. EEG power distribution during REMS was not different between genotypes and virus inoculation did not have a significant effect on it [ANOVA, treatment × frequency interaction: F(39,560) = 1.3, n.s.; treatment × frequency × genotype interaction: F(39,560) = 0.8, n.s.].
Fig. 5.
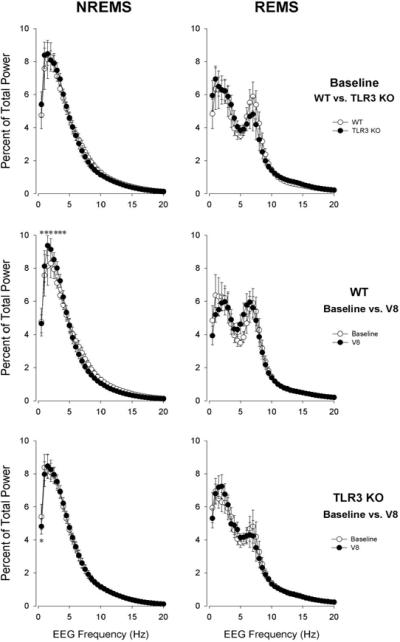
EEG power spectra during NREMS (left) and REMS (right) in uninfected mice (n = 8) (white circles) and on day 8 (black circles) after virus inoculation. Asterisks: significant difference between uninfected and infected (univariate tests of significance for planned comparison, p < 0.05); error bars represent the standard error of the mean.
3.4. Body weight changes
Body weight changes were acquired as a cumulative measure of illness (specifically anorexia) by subtracting the animal weights on the day of euthanization from the weights taken on the day of inoculation. WT mice (n = 15) showed an average weight loss of 2.3 ± 0.6 g, while the TLR3 KOs (n = 14) lost only 0.7 ± 0.2 g (p < 0.04) over 12–14 days PI.
3.5. Mortality
None of the mice maintained at 29°C died over the course of the experiments. In the body temperature/locomotor activity studies conducted at 23°C, one WT mouse died and no KO mice died.
4. Discussion
Influenza in the mouse is associated with hypothermia, reduced locomotor activity, anorexia and reduced water intake leading to a loss of body weight (Conn et al., 1995). Also stereotypic sleep changes [increased time spent in NREMS, decreased time spent in REMS, and increased slow wave amplitudes (Fang et al., 1995; Toth et al., 1995)] found in many infections also occur in mouse influenza. These virus-associated sickness behaviors are similar regardless of whether a highly lethal dose of virus (Conn et al., 1995; Fang et al., 1995; Toth et al., 1995) or a marginally lethal dose similar to that employed in these studies is used (Conn et al., 1995; Traynor et al., 2007). The primary difference seen in the illness induced by different viral doses and viral strains is the time of onset of the symptoms (Conn et al., 1995) (ranging from 13 to 60 h), suggesting a threshold phenomenon. Proinflammatory cytokines are thought to trigger these viral symptoms (Conn et al., 1995; Leon, 2004; Traynor et al., 2007). Cytokines demonstrated to play a role in the hypothermia response to moderate doses of influenza in mice include IL1β (Kozak et al., 1995) and IL6 (Kozak et al., 1997), but not TNFα (Kapas et al., 2008).
In this report TLR3 KO mice showed attenuation (but not abolition) of several APR parameters (body temperature changes, NREMS EEG slow wave power, and body weight loss) compared to WT controls (Figs. 1, 3–5). Time spent in REMS tended to be lower in the WT mice on day 5 during the light period compared to KO values, though REMS was not consistently suppressed in the WT mice as it is in more severe influenza infections (Fang et al., 1995). As depicted in Fig 1, lower panel, there was a trend towards increased locomotor activity during the day in the KO mice relative to WT (Fig 1, lower panel), but the differences were not significant and both strains showed reduced activity following infection compared to uninfected mice. Collectively these data suggest that virus-associated dsRNA bound to the TLR3 receptor plays a substantial role in activation of the sickness behaviors determined in this influenza model. Our findings are consistent with those reported in the influenza-infected lung where reduced inflammation and proinflammatory cytokine levels were seen in TLR3 KOs compared to WT controls (Le Goffic, R. et al., 2006).
The time course of the hypothermia response to X-31- infected WT (C57BL/6) mice was comparable to published values in this model (Kapas et al., 2008). In this study similar hypothermia responses occurred in both infected mouse strains until day 6 post infection. On days 3–8, KO mice began to show a stabilization of body temperature with some hints of a diurnal rhythm, while WT mice became increasingly hypothermic. Stabilization of body temperature and a gradual rise to normal levels reflects recovery from the infection, which occurs about day 15 in this model. The hypothermic response is thought to represent a semi-hibernating state (torpor) that protects energy reserves in small mammals (Geiser, 2004); if influenza-infected mice are forced to warm to “normal” body temperatures (and raise their metabolic rate) they die in larger numbers (Klein et al., 1992).
Interestingly, the time course and outcome of the hypothermia response in infected TLR3 KOs resembled that seen in IFN type I receptor KOs on a 129 SvEv background (Traynor et al., 2007). Binding of dsRNA to TLR3 on the myeloid dendritic cell membrane results in a strong IFNβ response (Matsumoto et al., 2004). Reduced IFNβ expression can, in turn, result in reduced IFNα release by plasmacytoid dendritic cells (Phipps-Yonas, H. et al., 2008). Therefore TLR3 KOs may demonstrate a reduced type I IFN response with similar effects on sickness behaviors as a deficiency in the type I IFN receptor. Because type I IFNs play an important role in dampening proinflammatory cytokine-driven inflammation (Amadori, 2007), the absence of a functional IFN receptor (or reduced IFN expression) may protect the mice by allowing greater expression of proinflammatory cytokines (and a more substantial APR) early in the infection when their protective roles may be critical (Traynor et al., 2007). The enhanced sickness behaviors in the WT mice overall may mirror increased proinflammatory cytokine production in this strain.
It should be noted that the primary anti-influenza effect of type I IFN in mice is to induce the guanosine triphosphatase myxovirus-1 (Mx1), which inhibits influenza transcription and prevents mortality (Haller et al., 2007). However, the Mx1 gene is defective in our mice and in all commercially available inbred mice (Haller et al., 2007). Therefore innate immune response(s) to influenza, such as IFN-independent natural lectins (Reading et al., 2007) are likely to play a protective role in our X-31 model (Traynor et al., 2007) and substitute for IFN-induced antiviral activity.
Two recent publications have asserted that influenza virus does not produce dsRNA (Pichlmair and Reis e Sousa, 2007; Weber et al., 2006). However, our data (Majde et al., 1998) and that of others (Guillot et al., 2005; Guo et al., 2007) suggest that functional dsRNA is produced during influenza replication and could activate TLR3 in the WT mice either extracellularly (Gantier and Williams, 2007; Majde et al., 1998) or intracellularly (Guillot et al., 2005; Matsumoto et al., 2004). Just a single molecule of viral dsRNA per cell will initiate interferon synthesis (Marcus, 1983), and only molecular amplification methods would be sensitive enough to detect such low quantities (Majde et al., 2007). It is likely that influenza virus produces levels of dsRNA undetectable by ELISA (Pichlmair and Reis e Sousa, 2007) or immunohistochemistry (Pichlmair and Reis e Sousa, 2007; Weber et al., 2006) but which are still functional in the mice.
The virus-associated dsRNA involved in APR induction by influenza virus could come from several sources, including viral replication intermediates (Majde, 2000), small interfering RNAs (Kariko et al., 2004a), t-RNAs (Wang et al., 2006) and certain forms of host mRNA from dead cells (Kariko et al., 2004b)]. All of these sources could be recognized by intracellular TLR3 (see below) or released into the extracellular environment when influenza-infected cells die (Majde et al., 1998). TLR3 appears to bind extracellular dsRNA primarily (Dogusan et al., 2008), which probably would include that released from neighboring dying cells. This extracellular dsRNA could play a role in amplifying the effects of viral dsRNA on the APR by binding to and activating neighboring cells such as macrophages to produce cytokines (Majde et al., 1998). It is not possible to distinguish the source (or sources) of the TLR3-bound dsRNA that is amplifying the WT APR in our model, but the findings reported here are consistent with the extracellular dsRNA hypothesis.
Intracellular dsRNA derived from viral replication processes can activate cells via cytoplasmic TLR3 and other intracellular receptors or enzymes (Dogusan et al., 2008). These factors include two cytoplasmic RNA helicases RIG-I and melanoma differentiation-associated gene 5 (MDA-5) (Kato et al., 2006)], the mitochondrial protein interferon-promoter stimulator 1 (IPS-1) (Leavy, 2006), 2',5'-oligoadenylate synthase (Baas et al., 2006), and the protein kinase R (Bose and Banerjee, 2003). While TLR3 activates NF-κB and primarily regulates a proinflammatory response, RIG-I mediates both a type I IFN response and proinflammatory mediators in influenza infections (Le Goffic et al., 2007). Influenza ssRNA also activates cytokine responses via cytoplasmic TLR7/8 (Diebold et al., 2004), and influenza genomic ssRNA bearing 5'polyphosphates binds to RIG-I and induces interferons and other cytokines (Pichlmair et al., 2006). Therefore intracellular viral ssRNA could also contribute to the residual sickness behaviors seen in our TLR3 KOs.
Many viruses, including influenza (Garcia-Sastre, 2004), have evolved strategies to block dsRNA activity within the infected cell (Levy and Garcia-Sastre, 2001). The influenza gene product NS1 plays a substantial role in viral pathogenicity (Fernandez-Sesma et al., 2006), and an effective isoform of NS1 blocks RIG-I/dsRNA responses (Pichlmair et al., 2006). The X-31 strain of influenza used in these studies expresses an effective NS1 protein [derived from the highly pathogenic PR8 strain (Reading et al., 1997)] that could suppress dsRNA responses initiated by RIG-I. A consequence may be a greater contribution of TLR3-dsRNA responses to the APR in this model than would occur with a viral strain expressing a less potent NS1.
The exact role of TLR3 in host defense has not been established. Minimal effects of deleting the TLR3 gene are seen on development of acquired immunity to experimental viral infections (Edelmann et al., 2004). Where mortality or pathology have been examined in animal models, functional TLR3 appeared detrimental to the outcome of most viral infections examined (Vercammen et al., 2008), including influenza. Lung inflammation and proinflammatory cytokines are reduced in TLR3 KOs infected with influenza {Le Goffic, 2006 8762 /id}. West Nile virus invades the brain and causes fatal encephalitis in TLR3 WT mice but not TLR3 KO mice (Wang et al., 2004). These findings are compatible with ours, in that APR components were less severe in mice lacking the TLR3. While no animal model has demonstrated benefits from a functional TLR3 receptor [with the exception of mouse cytomegalovirus (Boehme and Compton, 2004)], this may be a result of using virus doses that overwhelm the protective effects of activated TLR3, or an unnatural route of infection in these models.
In contrast to findings in animal models, TLR3 appears to protect against selected clinical viral encephalopathies. A patient with a missense mutation of TLR3 developed influenza encephalopathy (Hidaka et al., 2006). Specific TLR3 polymorphisms are associated with measles-induced sub-acute sclerosing panencephalitis (Ishizaki et al., 2008) and selected inflammatory ocular diseases (Ueta, 2008) in Japanese individuals. Children with a dominant-negative TLR3 allele appear to be more susceptible to herpes simplex encephalitis (Zhang et al., 2007b), and it has been suggested that TLR3 may play a more prominent role in host defense against neurotropic viruses than viruses in other body compartments (Zhang et al., 2007a). Interestingly, individuals with selected IFN subtype deficiencies are also more susceptible to herpes encephalitis (Sancho-Shimizu et al., 2007; Zhang et al., 2008). While influenza virus is generally thought to be restricted to the upper respiratory tract, we have shown that it rapidly invades the brain following intranasal infection and up-regulates proinflammatory cytokine (and IFN-induced enzyme) mRNAs at the time of illness onset (Majde et al., 2007). Data from our laboratory (Leyva-Grado et al., 2009) indicate that IL1β is up-regulated in temperature regulating nuclei in the hypothalamus at illness onset and thus these virus-induced brain cytokines could contribute to the viral APR. Whether the APR is modulated in TLR3 KOs via altered brain expression of cytokines is not known.
In conclusion, our studies suggest that TLR3 receptors (and putatively, virus-associated dsRNA) play a substantial, but not exclusive, role in regulating several behavioral aspects of the influenza APR. The absence of a functional TLR3 resulted in attenuation of most of the APR responses examined.
Acknowledgements
This research was supported by the NIH Institute of Child Health and Development Grant No. HD36520 and the National Institute of Neurological Disorders and Stroke NIH Grant Nos. NS25378 and NS31453.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, Elvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immun. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori M. The role of IFN-alpha as homeostatic agent in the inflammatory response: A balance between danger and response? J. Interferon Cytokine Res. 2007;27:181–190. doi: 10.1089/jir.2006.0110. [DOI] [PubMed] [Google Scholar]
- Baas T, Baskin CR, Diamond DL, Garcia-Sastre A, Bielefeldt-Ohmann H, Tumpey TM, Thomas MJ, Carter VS, Teal TH, Van Hoeven N, Proll S, Jacobs JM, Caldwell ZR, Gritsenko MA, Hukkanen RR, Camp DG, II, Smith RD, Katze MG. Integrated molecular signature of disease: Analysis of influenza virus-infected macaques through functional genomics and proteomics. J. Virol. 2006;80:10813–10828. doi: 10.1128/JVI.00851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J. Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Banerjee AK. Innate immune response against nonsegmented negative strand RNA viruses. J. Interferon Cytokine Res. 2003;23:401–412. doi: 10.1089/107999003322277810. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- Carter WA, De Clercq E. Viral infection and host defense. Science. 1974;186:1172–1178. doi: 10.1126/science.186.4170.1172. [DOI] [PubMed] [Google Scholar]
- Chen L, Duricka D, Nelson S, Mukherjee S, Bohnet SG, Taishi P, Majde JA, Krueger JM. Influenza virus-induced sleep responses in mice with targeted disruptions in neuronal or inducible nitric oxide synthases. J. Appl. Physiol. 2004;97:17–28. doi: 10.1152/japplphysiol.01355.2003. [DOI] [PubMed] [Google Scholar]
- Conn CA, McClellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 1995;268:R78–R84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav. Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur. J. Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- Dogusan Z, Garcia M, Flamez D, Alexopoulou L, Goldman M, Gysemans C, Mathieu C, Libert C, Eizirik DL, Rasschaert J. Double-stranded RNA induces pancreatic β-cell apoptosis by activation of the Toll-like receptor 3 and interferon regulatory factor 3 pathways. Diabetes. 2008;57:1236–1245. doi: 10.2337/db07-0844. [DOI] [PubMed] [Google Scholar]
- Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virol. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Eisenacher K, Steinberg C, Reindl W, Krug A. The role of viral nucleic acid recognition in dendritic cells for innate and adaptive antiviral immunity. Immunobiol. 2008;212:701–714. doi: 10.1016/j.imbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Bredow S, Taishi P, Majde JA, Krueger JM. Synthetic influenza viral double-stranded RNA induces an acute phase response in rabbits. J. Med. Virol. 1999;57:198–203. [PubMed] [Google Scholar]
- Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc. Soc. Exp. Biol. Med. 1995;210:242–252. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 2006;80:6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: Moderation by social stressors. Brain Behav. Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gantier MP, Williams BRG. The response of mammalian cells to double-stranded RNA. Cytokine & Growth Factor Reviews. 2007;18:363–371. doi: 10.1016/j.cytogfr.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 2004;283:249–280. doi: 10.1007/978-3-662-06099-5_7. [DOI] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, Fujita T, Katz JM, Donis RO, Sambhara S. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 2007;36:263–269. doi: 10.1165/rcmb.2006-0283RC. [DOI] [PubMed] [Google Scholar]
- Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochim. 2007;89:812–818. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect. Immun. 2007;75:1926–1932. doi: 10.1128/IAI.02083-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka F, Matsuo S, Muta T, Takeshige K, Mizukami T, Nunoi H. A missense mutation of the Toll-like receptor 3 gene in a patient with influenza-associated encephalopathy. Clin. Immunol. 2006;119:188–194. doi: 10.1016/j.clim.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Takemoto M, Kira R, Kusuhara K, Torisu H, Sakai Y, Sanefuji M, Yukaya N, Hara T. Association of toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J. Neurovirol. 2008;14:486–491. doi: 10.1080/13550280802298120. [DOI] [PubMed] [Google Scholar]
- Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav. Immun. 2007;21:975–987. doi: 10.1016/j.bbi.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapas L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNFα double-receptor deficient mice. J. Appl. Physiol. 2008;105:1187–1198. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through Toll-like receptor 3. J. Immunol. 2004a;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004b;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kimura-Takeuchi M, Majde JA, Toth LA, Krueger JM. The role of double-stranded RNA in the induction of the acute phase response in an abortive influenza viral infection. J. Infect. Dis. 1992;166:1266–1275. doi: 10.1093/infdis/166.6.1266. [DOI] [PubMed] [Google Scholar]
- Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol. Behav. 1992;52:1133–1139. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- Kozak W, Poli V, Soszynski D, Conn CA, Leon LR, Kluger MJ. Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. Am. J. Physiol. 1997;272:R621–R630. doi: 10.1152/ajpregu.1997.272.2.R621. [DOI] [PubMed] [Google Scholar]
- Kozak W, Zheng H, Conn CA, Soszynski D, Van der Ploeg LHT, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- Leavy O. Sensing viruses. Nat. Rev. Immunol. 2006;6:492–492. [Google Scholar]
- Lee KH, Youn JW, Kim HJ, Seong BL. Identification and characterization of mutations in the high growth vaccine strain of influenza virus. Arch. Virol. 2001;146:369–377. doi: 10.1007/s007050170181. [DOI] [PubMed] [Google Scholar]
- Leon LR. Hypothermia in systemic inflammation: role of cytokines. Front. Biosci. 2004;9:1877–1888. doi: 10.2741/1381. [DOI] [PubMed] [Google Scholar]
- Levy DE, Garcia-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12:143–156. doi: 10.1016/s1359-6101(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Leyva-Grado V, Churchill L, Wu M, Williams TJ, Majde JA, Taishi P, Krueger JM. Influenza virus- and cytokine-immunoreactive cells in the murine olfactory and central autonomic nervous systems before and after illness onset. J. Neuroimmunol. 2009;211:73–83. doi: 10.1016/j.jneuroim.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA. Viral double-stranded RNA, cytokines and the flu. J. Interferon Cytokine Res. 2000;20:259–272. doi: 10.1089/107999000312397. [DOI] [PubMed] [Google Scholar]
- Majde JA, Bohnet SG, Ellis GA, Churchill L, Leyva-Grado V, Wu M, Szentirmai E, Rehman A, Krueger JM. Detection of mouse-adapted human influenza virus in the olfactory bulb of mice within hours after intranasal infection. J. Neurovirol. 2007;13:399–409. doi: 10.1080/13550280701427069. [DOI] [PubMed] [Google Scholar]
- Majde JA, Brown RK, Jones MW, Dieffenbach CW, Maitra N, Krueger JM, Cady AB, Smitka CW, Maassab HF. Detection of toxic viral-associated double-stranded RNA (dsRNA) in influenza-infected lung. Microb. Pathogen. 1991;10:105–115. doi: 10.1016/0882-4010(91)90071-h. [DOI] [PubMed] [Google Scholar]
- Majde JA, Guha-Thakurta N, Chen Z, Bredow S, Krueger JM. Spontaneous release of stable viral double-stranded RNA into the extracellular medium by influenza virus-infected MDCK epithelial cells: Implications for the viral acute phase response. Arch. Virol. 1998;143:2371–2380. doi: 10.1007/s007050050467. [DOI] [PubMed] [Google Scholar]
- Marcus PI. Interferon induction by viruses: One molecule of dsRNA as the threshold for interferon induction. Interferon. 1983;5:115–180. [PubMed] [Google Scholar]
- Matskevich AA, Moelling K. Dicer is involved in protection against influenza A virus infection. J. Gen. Virol. 2007;88:2627–2635. doi: 10.1099/vir.0.83103-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol. Immunol. 2004;48:147–154. doi: 10.1111/j.1348-0421.2004.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-β pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immun. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Tate MD, Pickett DL, Brooks AG. Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv. Exp. Med. Biol. 2007;598:279–292. doi: 10.1007/978-0-387-71767-8_20. [DOI] [PubMed] [Google Scholar]
- Salem ML, Diaz-Montero CM, EL-Naggar SA, Chen Y, Moussa O, Cole DJ. The TLR3 agonist poly(I:C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naive recipient mice. Vaccine. 2009;27:549–557. doi: 10.1016/j.vaccine.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova JL. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr. Opin. Allergy Clin. Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Seya T, Matsumoto M. The extrinsic RNA-sensing pathway for adjuvant immunotherapy of cancer. Cancer Immunol. Immunother. 2009 Jan 31; doi: 10.1007/s00262-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J. Neuroimmunol. 1995;58:89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- Traynor TR, Majde JA, Bohnet SG, Krueger JM. Sleep and body temperature responses in an acute viral infection model are altered in interferon type I receptor-deficient mice. Brain Behav. Immun. 2006;20:290–299. doi: 10.1016/j.bbi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Traynor TR, Majde JA, Bohnet SG, Krueger JM. Interferon type I receptor-deficient mice have altered disease symptoms in response to influenza virus. Brain Behav. Immun. 2007;21:311–322. doi: 10.1016/j.bbi.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M. Innate immunity of the ocular surface and ocular surface inflammatory disorders. Cornea. 2008;27(Suppl 1):S31–S40. doi: 10.1097/ICO.0b013e31817f2a7f. [DOI] [PubMed] [Google Scholar]
- Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin. Microbiol. Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xiang L, Shao J, Yuan Z. The 3' CCACCA sequence of tRNAAla(UGC) is the motif that is important in inducing Th1-like immune response, and this motif can be recognized by Toll-like receptor 3. Clin. Vaccine Immunol. 2006;13:733–739. doi: 10.1128/CVI.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol. Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Zhang, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunological Reviews. 2007a;S.Y.220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007b;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]