Abstract
Objectives
To investigate the expression of matrix metalloproteinases (MMPs) in apical periodontitis and during the periapical healing phase following root canal treatment.
Methods
Apical periodontitis was induced in dog teeth and root canal treatment was performed in a single visit or using an additional calcium hydroxide root canal dressing. One hundred and eighty days following treatment the presence of inflammation was examined and tissues were stained to detect bacteria. Bacterial status was correlated to the degree of tissue organization, and to further investigate molecules involved in this process, tissues were stained for MMP-1, MMP-2, MMP-8, and MMP-9. Data were analyzed using one-way ANOVA followed by Tukey test or Kruskal-Wallis followed by Dunn.
Results
Teeth with apical periodontitis that had root canal therapy performed in a single visit presented an intense inflammatory cell infiltrate. Periapical tissue was extremely disorganized, and this was correlated with the presence of bacteria. Higher MMP expression was evident, similar to teeth with untreated apical periodontitis. In contrast, teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide presented a lower inflammatory cell infiltrate. This group had a moderately organized connective tissue, a lower prevalence of bacteria, and a lower number of MMP-positive cells, similar to healthy teeth submitted to treatment.
Conclusion
Teeth treated with calcium hydroxide root canal dressing exhibited a lower percentage of bacterial contamination, a lower MMP expression, and a more organized ECM, unlike those treated in a single visit. This suggests that calcium hydroxide may be beneficial in tissue repair processes.
Keywords: apical periodontitis, root canal treatment, single visit, calcium hydroxide, matrix metalloproteinases
INTRODUCTION
Periapical disease represents a local immune response to the progression of microorganisms from the dental pulp to the apical foramen that results in bone resorption (1–4). The periapical reaction consists of a mixed inflammatory cell infiltrate, including large numbers of T cells, B cells, neutrophils, macrophages and plasma cells (5–7).
The importance of bacteria in periradicular lesion development has been demonstrated following mechanical pulp exposure in germ-free rats compared with conventional bacteria-harboring rats. Germ-free animals do not develop periradicular lesions and they show evidence of repair at pulpal exposure sites. However, in conventional rats, necrosis of pulpal tissue and development of periradicular lesions occurs 15 days after exposure of dental pulp to the oral environment. Bacterial components evoke an intense inflammatory response (8) and mediate activation of destructive enzymes (9), which leads to severe tissue destruction.
Matrix metalloproteinases (MMPs) are an important family of metal-dependent endopeptidases that represent the major class of enzymes responsible for degradation of extracellular matrix (ECM) components. MMPs are classified into five main classes (collagenases, gelatinases, stromelysins, membrane-type and others) on the basis of their putative substrate specificity and internal homologies (10–12).
Several pieces of evidence support the fundamental role of MMPs during the development, remodeling and destruction of oral tissues (13–15). MMPs are the major players in collagen breakdown during periodontal tissue destruction (12, 16–19). High levels of MMPs in periodontal tissues promote an imbalance between production and degradation of collagen, causing tooth attachment loss (20, 21).
Interstitial collagenase (MMP-1) has been implicated as a key enzyme in the initiation of the bone resorptive phase of periapical lesions (10, 12, 22, 23). In addition, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13 have been described in periapical lesions from humans (24–28). Higher MMP-8 levels measured in the fluid inside the root canals of necrotic teeth were markedly reduced following root canal therapy with a calcium hydroxide paste (26) suggesting that MMP analysis from periapical exudate could be used to monitor inflammatory activity, and potentially the success of treatment in teeth with apical periodontitis. Since no evaluation of the involvement of other MMPs during and after root canal treatment has been performed, we sought to investigate the expression of this group of proteases in periapical disease and during the periapical healing phase following root canal treatment in teeth with apical periodontitis.
MATERIAL & METHODS
All animal procedures performed in this study conformed to protocols reviewed and approved by the Animal Care Committee of the University of São Paulo (Protocol #2007.1.191.53.0). The third and fourth mandibular premolars of 15 mongrel dogs (12 months-of-age, weighing 10 to 15 kg) were selected for various treatment protocols for a total of 60 treated teeth. Three animals were randomly assigned to each experimental group. All endodontic procedures were performed aseptically with sterile instruments under a rubber dam which was surface-disinfected with 2% chlorhexidine.
Table 1 delineates the treatment protocols tested. In general, Group 1 had root canal treatment performed on healthy teeth (HT-RCT). Group 2 had root canal treatment performed in a single visit on teeth with apical periodontitis (SV-RCT). Group 3 had root canal treatment plus application of a calcium hydroxide dressing (Calen; SS White Artigos Dentários Ltda, Rio de Janeiro, Brazil) for 15 days on teeth with apical periodontitis (CH-RCT). Group 4 had no root canal treatment but teeth had apical periodontitis (AP). Group 5 had healthy teeth without any treatment, which were used as controls (HT).
Table 1.
Percentage of PMN, mononuclear and fibroblastic cells in teeth submitted to root canal treatment using different clinical protocols. Inflammatory activity index depicts the ratio between PMN plus mononuclear inflammatory cells over fibroblasts
| Groups |
|||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| HT-RCT | SV-RCT | CH-RCT | AP | HT | |
| Apical periodontitis | − | + | + | + | − |
| Calcium Hydroxide Dressing | − | − | + | − | − |
| Gutta Percha Root Canal Filling | + | + | + | − | − |
| Inflammatory Cell Infiltrate | |||||
| Polymorphonuclear | 1.2 ± 0.9 | 9.6 ± 6.6 | 4.3 ± 3.2 | 9.5 ± 2.4 | 0.17 ± 0.7 |
| Mononuclear | 36.7 ± 9.8 | 59.5 ± 9.1* | 48.7 ± 9.5* | 61.7 ± 4.8* | 13.9 ± 7.6 |
| Fibroblast | 62.1 ± 9.6* | 30.9 ± 4.4 | 46.9 ± 11.2* | 28.7 ± 3.8 | 85.8 ± 7.6* |
| Inflammatory Activity Index | 0.68 | 2.31#,& | 1.23& | 2.53#,& | 0.17 |
most prevalent cell type;
compared to CH-RCT;
compared to HT-RCT or HT;
p < 0.05
For the root canal treatment and apical periodontitis groups (HT-RCT, SV-RCT, CH-RCT, AP), after coronal pulp exposure, the pulp tissue was extirpated and the apical cementum layer was perforated with the sequential use of size #15 to #30 K-files, thus creating standardized apical openings as previously described (29, 30). Following this, all roots in HT-RCT were instrumented to ISO K-file size 60 and root canal filling was performed with gutta-percha cones and AH Plus Jet Mix (Dentsply De Trey, Konstanz, Germany) using a lateral condensation technique. In SV-RCT, CH-RCT and AP, the root canals were left exposed to the oral cavity for 7 days to allow microbial contamination, and then access openings were sealed with a quick-setting zinc oxide-eugenol cement (IRM; Dentsply Indústria e Comércio Ltda, Petrópolis, Brazil). After 45 days, development of apical periodontitis was radiographically confirmed. Then, in groups SV-RCT and CH-RCT, root canal instrumentation and root canal filling were performed as described above for the HT-RCT group, except the CH-RCT group received a calcium hydroxide dressing. The crown openings were permanently restored with silver amalgam (Velvalloy; SS White Artigos Dentários Ltda), which was condensed on a glass ionomer cement base (Vitremer; 3M/ESPE, Saint Paul, MN).
The animals were euthanized by anesthetic overdose 180 days following root canal treatment. The mandibles were removed, and the anatomic segments containing the teeth were sectioned using water-cooled diamond disks.
Histological processing and analysis
Tissue blocks containing the roots were fixed in 10% buffered-formalin and demineralized in a 20% ethylenediaminetetraacetic acid (EDTA) solution in a microwave oven. Five-micrometer-thick sections were obtained and stained by Hematoxilin and Eosin, Mallory Trichrome and according to the Brown & Brenn technique. Serial sections were analyzed under conventional light microscopy for morphological evaluation of the apical third of each dental root.
The intensity of the periapical inflammatory response was determined by counting the number of inflammatory cells in the periapical region. The number of inflammatory cells was counted in three representative microscopic fields-of-view around the root apex in three orientations, including the longitudinal axis, through the center of the apical opening, and at 45 degrees to this long axis on each side (100 × magnification). Data distribution was normal and were analyzed using one-way ANOVA followed by Tukey test (α = 0.05).
The presence or absence of periodontal ligament fibers attached to the apical third was recorded and the degree of collagen fiber breakdown and disorganization was classified as (1) absent, (2) mild, (3) moderate, or (4) severe to indicate the level of apical periodontal ligament destruction. These scores were based on a previous broad survey of the slides without knowledge of group designation. The presence of microorganisms was evaluated by optical microscopy under oil immersion (100 × magnification). The 2-mm apical region of the root canal system was evaluated and tissues were classified according to the percentage of specimens with bacterial contamination in following areas: (1) absence of bacteria, (2) presence of bacteria in the main root canal, (3) presence of bacteria inside the dentinal tubules, (4) presence of bacteria in areas of cementum resorption, (5) presence of bacteria in the apical periodontitis area. Microscopic analyses were performed by an examiner in a blinded manner on at least 15 sections per dental root. The prevalence of microorganisms at different sites on the roots and apical periodontitis were analyzed statistically using the Fisher Exact Test (α = 0.05). The groups were compared for extracellular matrix disorganization and distribution of bacteria by means of Kruskal-Wallis followed by Dunn non-parametric tests (α = 0.05).
Immunohistochemistry
Tissue sections were quenched in 1.5 % H2O2 in methanol for 30 min, and antigen retrieval performed by boiling sections in 10 mM sodium citrate (pH 6.0) at 93°C for 15 min. Nonspecific binding was blocked by treating sections with 1% bovine serum albumin (Sigma) for 60 min, then sections were incubated for 1 hr with primary antibodies for MMP-1 (5 µg / ml; IM35; Calbiochem, San Diego, CA), MMP-2 (5 µg / ml; MAB3308, Chemicon, Temecula, CA), MMP-8 (5 µg / ml; MAB3316, Chemicon, Temecula, CA), and MMP-9 (5 µg / ml; MAB3309, Chemicon), specific MMPs known to be associated with apical periodontitis. Next, sections were incubated with secondary antibody followed by Streptavidin HRP and 3,3’-Diaminobenzidine as the enzyme substrate (DAB500 Chromogen System, Biocare Medical). Tissues were counterstained with Mayer’s Hematoxylin and mounted using standard protocols. Negative controls consisted of replacing the primary antibody with mouse IgG. The number of positive cells was calculated for each antibody in three representative fields-of-view (100 × magnification) in the periapical region. Normal data were analyzed using one-way ANOVA followed by Tukey test (α = 0.05).
RESULTS
Periapical inflammatory response is abrogated in teeth subjected to calcium hydroxide-enhanced root canal therapy
The most prevalent cells found in teeth with apical periodontitis (AP) were mononuclear inflammatory cells followed by fibroblastic and polymorphonuclear cells (PMNs) (Table 1). Teeth submitted to root canal treatment using calcium hydroxide as a dressing presented a diminished percentage of mononuclear and PMN cells, whereas teeth filled in a single visit with gutta percha did not (p < 0.05). Also, in calcium hydroxide-treated teeth, the percentage of fibroblastic cells was higher than that in teeth where root canal filling was performed in a single visit or in teeth with apical periodontitis without treatment (p < 0.05). Vital teeth submitted to root canal treatment presented a higher percentage of fibroblastic cells compared to the inflammatory cell component. Healthy teeth, almost exclusively, exhibited fibroblasts in the apical periodontal ligament with occasional mononuclear inflammatory cells.
Inflammatory activity, assessed as a ratio of inflammatory cellular components (PMNs plus mononuclear inflammatory cells) to fibroblast-like cell count, was highest in teeth with apical periodontitis without treatment and in teeth with apical periodontitis submitted to root canal filling in a single visit. In contrast, inflammatory activity was lower in teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as a root canal dressing, vital teeth submitted to root canal treatment, and healthy teeth (p < 0.05) (Table 1).
Calcium hydroxide therapy minimizes bacterial presence following root canal therapy
Teeth with apical periodontitis that did not receive root canal treatment exhibited the highest frequency of bacterially-infected roots (100%). Of all teeth with apical periodontitis, those which were filled with a calcium hydroxide paste as a root canal dressing exhibited a lower percentage of roots infected with bacteria compared to teeth subjected to root canal treatment in a single visit (42.8% versus 83.3%, respectively; p < 0.05), but higher than that observed in teeth submitted to root canal treatment that did not exhibit apical periodontitis (42.8% versus 18.75%, respectively; p < 0.05). However, teeth with apical periodontitis in which root canal treatment was performed in a single visit exhibited similar bacterial contamination to teeth with apical periodontitis not submitted to root canal therapy (p > 0.05). Bacteria were distributed in the main root canal, dentinal tubules and areas of cementum resorption (Figure 1 and Figure 2A).
Figure 1.
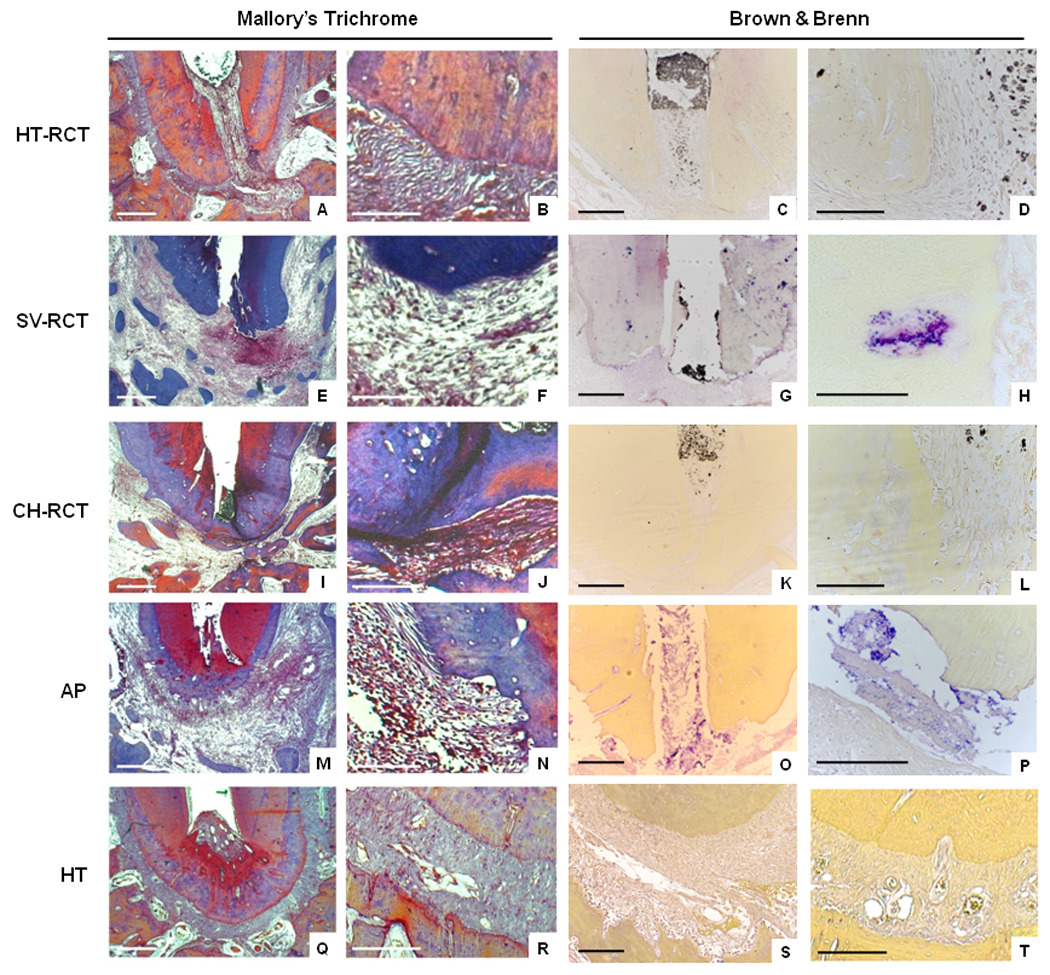
Collagen fibers stained with Mallory Trichrome to evaluate extracellular matrix organization in apical periodontitis and in teeth submitted to root canal treatment using different clinical protocols. Brown & Brenn Staining Technique indicates the presence or absence of bacteria. HT-RCT- healthy teeth submitted to root canal treatment (A–D), SV-RCT- teeth with apical periodontitis submitted to root canal treatment in a single visit (E–H), CH-RCT- teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as root canal dressing (I–L), AP- teeth with apical periodontitis not submitted to root canal treatment (M–P), HT- healthy teeth (Q–T). Bar = 500 µm (A, E, I, M, Q), 100 µm (B, C, D, F, G, H, J, K, L, N, O, P, R, S, T).
Figure 2.
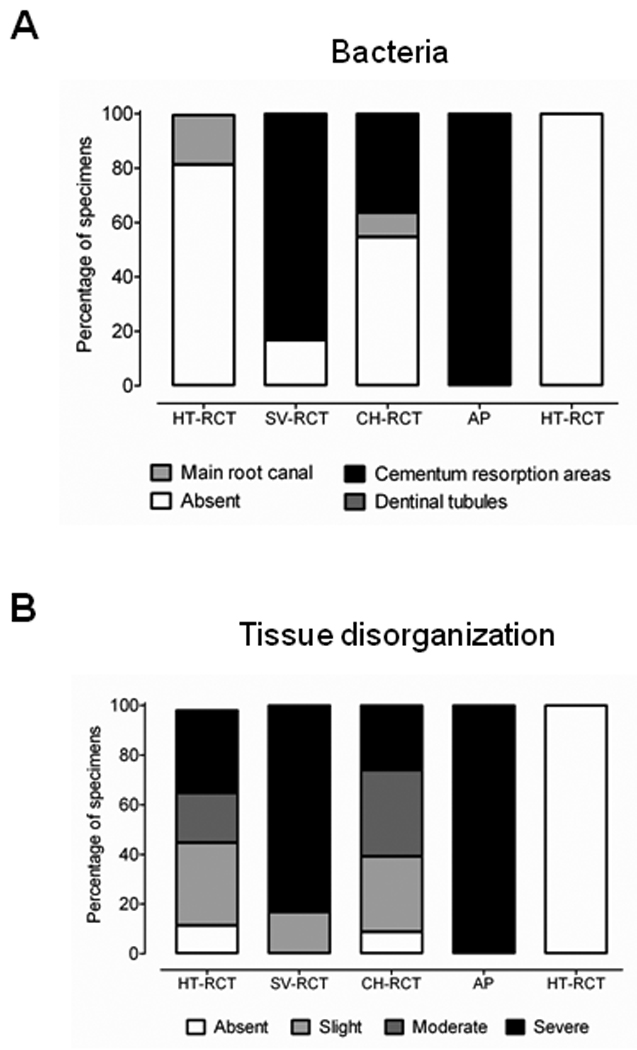
(A) Distribution of bacteria throughout the root canal system and (B) periapical tissue disorganization in teeth submitted of not to root canal treatment. HT-RCT- healthy teeth submitted to root canal treatment, SV-RCT- teeth with apical periodontitis submitted to root canal treatment in a single visit, CH-RCT- teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as root canal dressing, AP- teeth with apical periodontitis not submitted to root canal treatment, HT- healthy teeth.
Extracellular matrix destruction is mitigated under calcium hydroxide treatment
Teeth with apical periodontitis exhibited severe disorganization of the periodontal ligament connective tissue in the apical third of the tooth, and no insertion of Sharpey’s fibers into the root cementum. Teeth submitted to root canal treatment with a calcium hydroxide root canal dressing exhibited mild to moderate tissue disorganization, unlike those filled with gutta percha in a single visit, which exhibited a moderate to severe disorganization (p < 0.05). Likewise, higher frequency of dental roots with Sharpey’s fibers inserted into the cementum was observed in calcium hydroxide-treated teeth compared to those treated in a single visit (p < 0.05). Tissue disorganization and insertion of fibers in the apical third of teeth treated with calcium hydroxide were similar to teeth without apical periodontitis submitted to root canal treatment (p > 0.05). Tissue destruction in teeth with apical periodontitis submitted to root canal treatment in a single visit was similar to that observed in teeth with apical periodontitis without treatment (p > 0.05). The presence of bacteria was associated with the severity of tissue disorganization (p < 0.05).
Matrix metalloproteinase expression is lower under calcium hydroxide treatment
Since tissue disorganization in apical periodontitis was associated with the presence of bacteria, we hypothesized that bacterial contamination was mediating the concurrent ECM breakdown by the induction of MMPs in these tissues.
Higher percentages of MMP-1, MMP-2, MMP-8, and MMP-9 positively stained cells were observed in teeth with lesions that did not receive treatment. Root canal treatment using calcium hydroxide as the intracanal dressing led to a lower number of MMP positive cells compared to that in the single visit treatment group (p < 0.05). Root canal treatment in teeth without apical periodontitis did not induce MMP expression similar to that observed in healthy teeth (p > 0.05), except that MMP-9 was detected at low levels in healthy teeth.
MMP-1, MMP-2, MMP-8, and MMP-9 were preferentially expressed in mononuclear and fibroblastic cells with low expression noted in PMNs. More than 50% of fibroblasts expressed MMP-1, MMP-2, and MMP-8 in healthy teeth, which was higher than that observed in all treatment groups. In teeth with apical periodontitis submitted to root canal treatment in a single visit or teeth with apical periodontitis that did not receive treatment, mononuclear cells were predominantly responsible for MMP expression. In teeth submitted to root canal treatment using calcium hydroxide as the root canal dressing, MMP-2, MMP-8 and MMP-9 expression was similar in fibroblasts and mononuclear cells, and lower than that observed in teeth submitted to root canal treatment in a single visit (p < 0.05).
DISCUSSION
Apical periodontitis pathogenesis involves degradation of several ECM components due to bacterial infection within the root canal system. Collagen degradation is an important characteristic of apical periodontitis, and MMPs are likely candidates in this process. We demonstrated that the persistence of microorganisms following root canal therapy was associated with the presence of severe tissue disorganization and increased levels of MMPs in the periapical area.
Successful root canal treatment, indicated by absent or mild connective tissue disorganization in the periapical area, was achieved in teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as the root canal dressing. Lower percentages of roots contaminated with bacteria were found in this group, similar to that found in vital teeth submitted to root canal treatment. The use of calcium hydroxide dressing as an intracanal medication is widely accepted because it exhibits antimicrobial activity, neutralizes bacterial toxins, is biocompatible with the periapical tissues, inhibits inflammatory root and bone resorption and stimulates mineralization (31–33). Hydroxyl ions act directly and irreversibly on essential molecules important for bacterial metabolism and reproduction, such as structural proteins and enzymes, nucleic acids, phospholipids and unsaturated fatty acids. Additionally, physical filling of the root canal inhibits the influx of nutrients and microbial recolonization (34).
The persistence of bacteria and connective tissue degradation following root canal treatment indicated reduced periapical healing in teeth with apical periodontitis submitted to root canal therapy in a single visit. Persistent microbial contamination has been associated with maintenance of a chronic inflammatory infiltrate in the periapical area (35). The higher inflammatory activity index in the periapical area indicated delayed repair in an MMP positively-stained area for this group. Although MMP expression had been immunolocalized in human apical periodontitis previously (24–27, 36–38), this is the first study to compare MMP expression in teeth with apical periodontitis submitted to different clinical protocols of root canal therapy.
MMPs play an important role in dental pulp tissue destruction (25, 26) and it has been proposed that MMP-1, MMP-8, MMP-9, and MMP-13 together are involved in jaw cyst expansion (27, 36–38). In teeth submitted to root canal treatment using calcium hydroxide as the root canal dressing, a lower inflammatory index was observed, accompanied by an increased percentage of fibroblasts. In addition, MMP-2, MMP-8, and MMP-9 expression levels were lower than in teeth with apical periodontitis without treatment or in teeth submitted to root canal treatment in a single visit. Together, these findings indicate a reduced MMP synthesis in a calcium-rich environment. Although calcium hydroxide inhibition of MMP activity has not been addressed in vivo, in vitro, when an osteoblastic cell line (MG63) was stimulated with Ca(OH)2-pretreated Prevotella nigrescens LPS, MMP-1 gene expression was down-regulated. Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) expression was slightly up-regulated in the same condition (39). Thus, calcium hydroxide may act to inhibit MMP activity in vivo.
MMP-2 and MMP-8 expression levels were reduced upon calcium hydroxide treatment compared to the single visit treatment, yet in the HT or HT-RCT groups more than 50% of the cells (primarily fibroblasts) expressed those MMPs, indicating that these enzymes might play a role in periodontal ligament physiology. MMP-2 is expressed by PDL cells in physiological conditions (17) and MMP-8, long thought to be exclusively expressed by PMNs, can be expressed by a wide variety of cell types, including fibroblastic cells (40). Also, it has been reported that a resin-based sealer AH26 or a zinc oxide–eugenol–based sealer induce MMP expression in human osteoblastic cells (41), which could have influenced the MMP expression observed in groups HT-RCT, SV-RCT and CH-RCT. Although, whether AH Plus Jet Mix regulates MMP expression is not known.
The high levels of inflammation observed in this study following root canal treatment can be due to short term follow up (42). Previous studies have demonstrated that in dogs it is necessary to evaluate results 270–360 days following treatment to detect the absence of apical periodontitis (31, 43). In this study, we observed a reduction in the size and volume of the apical periodontitis lesion using calcium hydroxide as the root canal dressing compared to teeth filled in a single visit, although complete resolution of inflammatory response could not be achieved (29, 30).
Histological experimental studies have continuously demonstrated that the apical and periapical healing process is more advanced in teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as the root canal dressing compared to single visit treatment (31, 33, 42, 44–46). On the other hand, clinical trials have failed to demonstrate this difference (47–51), probably due to the small number of patients in those studies as well as the limited clinical and radiographic methods used to detect apical periodontitis (30, 52).
We demonstrated that bacterial persistence following root canal treatment was associated with collagen fiber disorganization and expression of higher levels of MMPs in teeth submitted to root canal treatment in a single visit. In contrast, teeth submitted to root canal treatment using calcium hydroxide as a root canal dressing exhibited a lower percentage of bacterial contamination, a lower MMP expression, and a more organized ECM, suggesting a beneficial role for calcium hydroxide in tissue repair processes.
Figure 3.
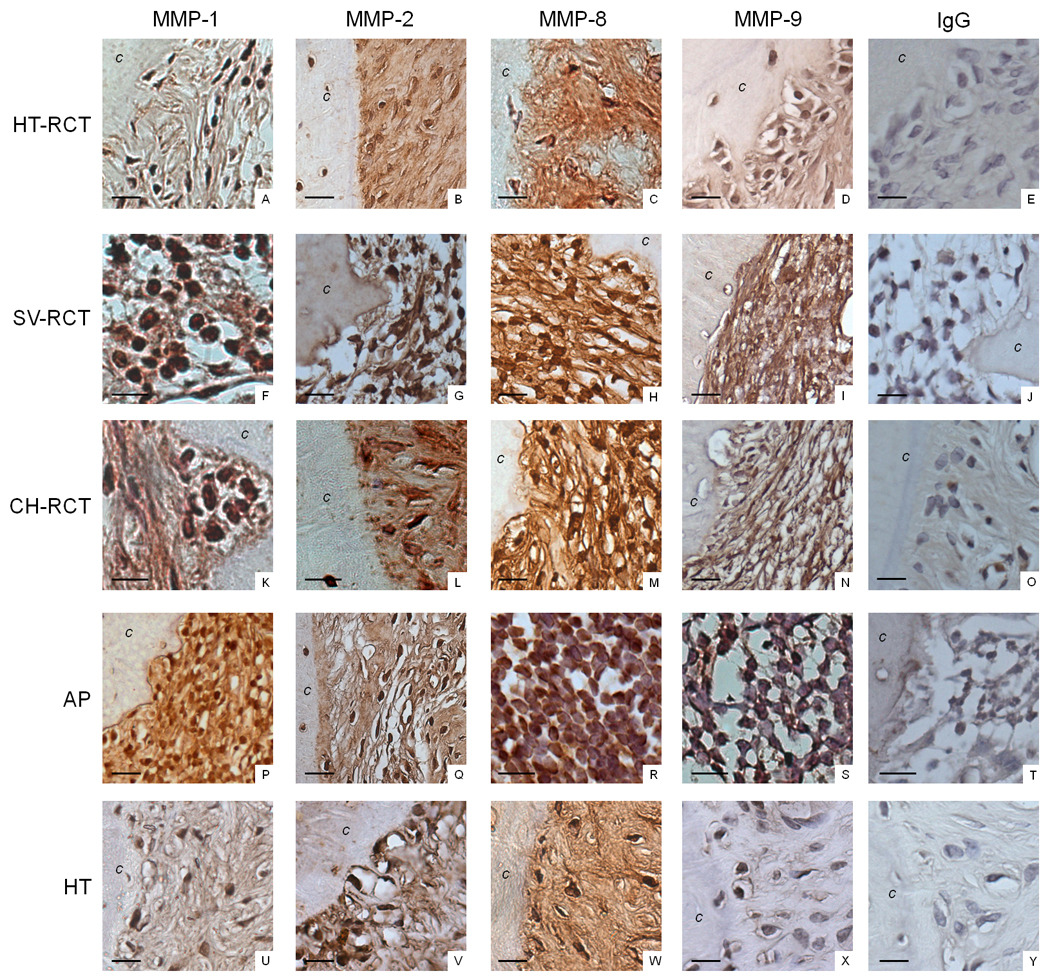
Immunostaining for MMP-1, MMP-2, MMP-8, and MMP-9 was performed to evaluate the profile of MMPs in teeth with apical periodontitis submitted to different root canal treatment protocols. HT-RCT- healthy teeth submitted to root canal treatment (A–E), SV-RCT- teeth with apical periodontitis submitted to root canal treatment in a single visit (F–J), CH-RCT- teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as root canal dressing (K–O), AP- teeth with apical periodontitis not submitted to root canal treatment (P–T), HT- healthy teeth (U–Y). Bar = 15 µm; (c) cementum.
Figure 4.
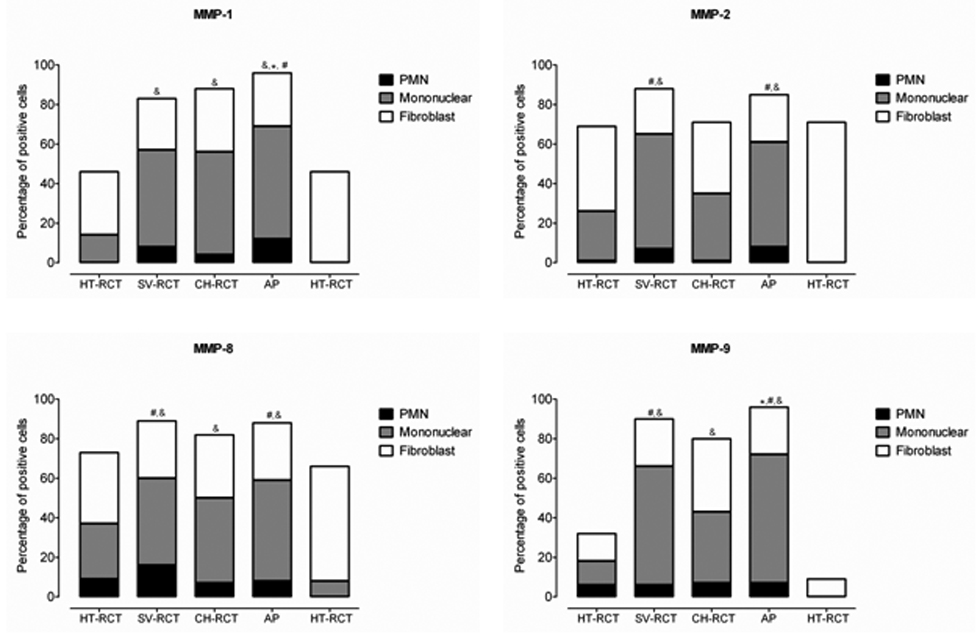
MMPs are differentially expressed by several cellular components in apical periodontitis lesions. Quantification of cell staining was performed by grouping cells into polymorphonuclear leukocytes (PMN), mononuclear or fibroblast-like cells. The percentage of cells expressing MMP-1, MMP-2, MMP-8, and MMP-9 was calculated in relation to the total amount of cells per field-of-view in three representative areas. HT-RCT- healthy teeth submitted to root canal treatment, SV-RCT- teeth with apical periodontitis submitted to root canal treatment in a single visit, CH-RCT- teeth with apical periodontitis submitted to root canal treatment using calcium hydroxide as root canal dressing, AP- teeth with apical periodontitis not submitted to root canal treatment, HT- healthy teeth. &, #, * p < 0.05; *compared to all groups, # compared to CH-RCT, & compared to HT-RCT or HT.
ACKNOWLEDGMENTS
This study was supported by Grant NIH-R01-DE013725 to YLK and Fellowships from CAPES Foundation (0668/07-9), FAPESP (06/51161-0) and National Council for Scientific and Technological Development (CNPq) to FWGPS. No financial affiliation exists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kawashima N, Stashenko P. Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch Oral Biol. 1999;44(1):55–66. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 2.Martón IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000;15(3):139–150. doi: 10.1034/j.1399-302x.2000.150301.x. [DOI] [PubMed] [Google Scholar]
- 3.Liapatas S, Nakou M, Rontogianni D. Inflammatory infiltrate of chronic periradicular lesions: an immunohistochemical study. Int Endod J. 2003;36:464–471. doi: 10.1046/j.1365-2591.2003.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Kabak SL, Kabak YS, Anischenko SL. Light microscopic study of periapical lesions associated with asymptomatic apical periodontitis. Ann Anat. 2005;187(2):185–194. doi: 10.1016/j.aanat.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Stashenko P, Yu SM. T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dent Res. 1989;65:830–834. doi: 10.1177/00220345890680051601. [DOI] [PubMed] [Google Scholar]
- 6.Wang CY, Stashenko P. The role of interleukin-1α in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993;8:50–56. doi: 10.1111/j.1399-302x.1993.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H. Effect of methotrexate-induced neutropenia on rat periapical lesion. Oral Surg Oral Med Oral Pathol. 1994;77(6):655–661. doi: 10.1016/0030-4220(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 8.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 9.Itoh T, Nakamura H, Kishi J, Hayakawa T. The activation of matrix metalloproteinases by a whole-cell extract from Prevotella nigrescens. J Endod. 2009;35(1):55–59. doi: 10.1016/j.joen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991:2145–2154. [PubMed] [Google Scholar]
- 11.Krane SM. Clinical Importance of metalloproteinases and their inhibitors. Ann NY Acad Sci. 1994;732:1–10. doi: 10.1111/j.1749-6632.1994.tb24719.x. [DOI] [PubMed] [Google Scholar]
- 12.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 13.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontologica Scandinavica. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in development and disease of oral tissues. Dent in Japan. 1998;34:167–177. [Google Scholar]
- 15.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Zee E, Everts V, Beertsen W. Cytokine-induced endogenous procollagenase stored in the extracellular matrix of soft connective tissue results in a burst of collagen breakdown following its activation. J Peridontol Res. 1996;3:483–488. doi: 10.1111/j.1600-0765.1996.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang YC, Yang SF, Lai CC, Liu JY, Hsieh YS. Regulation of matrix metalloproteinase production by cytokines, pharmacological agents and periodontal pathogens in human periodontal ligament fibroblast cultures. J Periodontal Res. 2002;37(3):196–203. doi: 10.1034/j.1600-0765.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa M, Yamaguchi Y, Yoshitake K, Saeki Y. Effects of TNF-alpha and prostaglandin E2 on the expression of MMPs in human periodontal ligament fibroblasts. J Periodontal Res. 2002;37(3):167–176. doi: 10.1034/j.1600-0765.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossa-Junior C, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK negatively regulates murine MMP-13 gene expression induced by IL-1beta and TNF-alpha in immortalized periodontal ligament fibroblasts. Matrix Biol. 2005;24(7):478–488. doi: 10.1016/j.matbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez M, Valenzuela MA, Lopez-Otin C, Alvarez J, Lopez JM, Vernal R, Gamonal J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J Periodontol. 2006;77(11):1863–1870. doi: 10.1902/jop.2006.050461. [DOI] [PubMed] [Google Scholar]
- 21.Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mantyla P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 22.Lin SK, Kok SH, Kuo MYP, Wang TJ, Wang JT, Yeh FTC, Hsiao M, Lan WH, Hong CY. Sequential expressions of MMP-1, TIMP-1, IL-6 and COX-2 genes in induced periapical lesions in rats. Eur J Oral Sci. 2002;110:246–253. doi: 10.1034/j.1600-0447.2002.11227.x. [DOI] [PubMed] [Google Scholar]
- 23.Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS, Wang TM, Chen CS, Lin LD, Wang JS. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med. 2004;33(3):162–169. doi: 10.1111/j.0904-2512.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin SK, Chiang CP, Hong CY, Lin CP, Lan WH, Hsieh CC, Kuo MYP. Immunolocalization of interstitial collagenase (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in radicular cysts. J Oral Pathol Med. 1997;26:458–463. doi: 10.1111/j.1600-0714.1997.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 25.Shin SJ, Lee J, Baek SH, Lin SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002;28:313–315. doi: 10.1097/00004770-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J. 2002;35:897–904. doi: 10.1046/j.1365-2591.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi R, Caltabiano R, Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: na immunohistochemical study. Int Endod J. 2005;38:297–301. doi: 10.1111/j.1365-2591.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Paula-Silva FWG, D’Silva NJ, Silva LAB, Kapila YL. High matrix metalloproteinase activity is a hallmark of periapical granulomas. J Endod. 2009;35(9):1234–1242. doi: 10.1016/j.joen.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paula-Silva FWG, Hassan B, Silva LAB, Leonardo MR, Wu MK. Outcome of root canal treatment in dogs determined by periapical radiography and cone-beam computed tomography scans. J Endod. 2009;35(5):723–726. doi: 10.1016/j.joen.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Paula-Silva FWG, Wu M-K, Silva LAB, Leonardo MR, Wesselink PR. Accuracy of periapical radiography and cone-beam computed tomography in diagnosing apical periodontitis using histopathological findings as a gold standard. J Endod. 2009;35(7):1009–1012. doi: 10.1016/j.joen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Leonardo MR, Almeida WA, Ito IY, Silva LAB. Radiographic and microbiologic evaluation of posttreatment apical and periapical repair of root canals of dogs’ teeth with experimentally induced chronic lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1994;78:232–238. doi: 10.1016/0030-4220(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 32.Silva LAB, Nelson-Filho P, Leonardo MR, Rossi MA, Pansani CA. Effect of calcium hydroxide on bacterial endotoxin in vivo. J Endod. 2002;28:94–98. doi: 10.1097/00004770-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Leonardo MR, Hernandez ME, Silva LA, Tanomaru-Filho M. Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: a histological study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:680–685. doi: 10.1016/j.tripleo.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 35.Siqueira JF., Jr Strategies to treat infected root canals. J Calif Dent Assoc. 2001;29:825–837. [PubMed] [Google Scholar]
- 36.Teronen O, Salo T, Konttinen YT, Rifkin B, Vernillo A, Ramamurthy NS, et al. Identification and characterization of gelatinase/ type IV collagenase in jaw cysts. J Oral Pathol Med. 1995;24:78–84. doi: 10.1111/j.1600-0714.1995.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 37.Teronen O, Salo T, Laitinen J, Törnwall J, Ylipaavalniemi P, Konttinen YT, et al. Characterization of interstitial collagenases in jaw cyst wall. Eur J Oral Sci. 1995;103(3):141–147. doi: 10.1111/j.1600-0722.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 38.Carneiro E, Menezes R, Garlet GP, Garcia RB, Bramante CM, Figueira R, et al. Expression analysis of matrix metalloproteinase-9 in epithelialized and nonepithelialized apical periodontitis lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):127–132. doi: 10.1016/j.tripleo.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang WK, Kim MR, Lee Y, Son HH, Lee W. Effect of calcium hydroxide-treated Prevotella nigrescens on the gene expression of matrix metalloproteinase and its inhibitor in MG63 cells. J Endod. 2006;32(12):1142–1145. doi: 10.1016/j.joen.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17(4):217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Huang FM, Yang SF, Chang YC. Up-regulation of gelatinases and tissue type plasminogen activator by root canal sealers in human osteoblastic cells. J Endod. 2008;34(3):291–294. doi: 10.1016/j.joen.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Katebzadeh N, Hupp J, Trope M. Histological periapical repair after obturation of infected root canals in dogs. J Endod. 1999;25:364–368. doi: 10.1016/S0099-2399(06)81173-8. [DOI] [PubMed] [Google Scholar]
- 43.Grecca FS, Leonardo MR, Silva LAB, Tanomaru Filho M, Borges MAG. Radiographic evaluation of periradicular repair after endodontic treatment of dog´s teeth with induced periradicular periodontitis. J Endod. 2001;27:610–612. doi: 10.1097/00004770-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Holland R, Otoboni-Filho JA, Souza V, Nery MJ, Bernabé PF, Dezan E., Jr A comparison of one versus two appointment endodontic therapy in dogs' teeth with apical periodontitis. J Endod. 2003;29:121–124. doi: 10.1097/00004770-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 45.De-Rossi A, Silva LAB, Leonardo MR, Rocha LB, Rossi MA. Effect of rotary or manual instrumentation, with or without a calcium hydroxide / 1% chlorhexidine intracanal dressing, on the healing of experimentally induced chronic periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:628–636. doi: 10.1016/j.tripleo.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Silveira AM, Lopes HP, Siqueira JF, Jr, Macedo SB, Consolaro A. Periradicular repair after two-visit endodontic treatment using two different intracanal medications compared to single-visit endodontic treatment. Braz Dent J. 2007;18:299–304. doi: 10.1590/s0103-64402007000400005. [DOI] [PubMed] [Google Scholar]
- 47.Trope M, Delano EO, Ørstavik D. Endodontic treatment of teeth with apical periodontitis: single vs. multivisit treatment. J Endod. 1999;25:345–350. doi: 10.1016/S0099-2399(06)81169-6. [DOI] [PubMed] [Google Scholar]
- 48.Weiger R, Rosendahl R, Löst C. Influence of calcium hydroxide intracanal dressing on the prognosis of teeth with endodontically induced periapical lesions. Int Endod J. 2000;33:219–226. doi: 10.1046/j.1365-2591.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 49.Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660–667. doi: 10.1046/j.1365-2591.2002.00541.x. [DOI] [PubMed] [Google Scholar]
- 50.Figini L, Lodi G, Gorni F, Gagliani M. Single versus multiple visits for endodontic treatment of permanent teeth. Cochrane Database Syst Rev. 2007;17 doi: 10.1002/14651858.CD005296.pub2. CD005296. [DOI] [PubMed] [Google Scholar]
- 51.Naito T. Single or multiple visits for endodontic treatment? Evid Based Dent. 2008;9:24. doi: 10.1038/sj.ebd.6400570. [DOI] [PubMed] [Google Scholar]
- 52.Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008;34:273–279. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]


