Abstract
Introduction
The purpose of this in vitro study was to determine whether para-chloroaniline (PCA) is formed through the reaction of mixing sodium hypochlorite (NaOCl) and chlorhexidine (CHX).
Methods
Initially commercially available samples of chlorhexidine acetate (CHXa) and PCA were analyzed with 1H NMR spectroscopy. Two solutions, NaOCl and CHXa, were warmed to 37°C and when mixed they produced a brown precipitate. This precipitate was separated in half and pure PCA was added to one of the samples for comparison before they were each analyzed with 1H NMR spectroscopy.
Results
The peaks in the 1H NMR spectra of CHXa and PCA were assigned to specific protons of the molecules, and the location of the aromatic peaks in the PCA spectrum defined the PCA doublet region. While the spectrum of the precipitate alone resulted in a complex combination of peaks, upon magnification there were no peaks in the PCA doublet region which were intense enough to be quantified. In the spectrum of the precipitate, to which PCA was added, two peaks do appear in the PCA doublet region. Comparing this spectrum to that of precipitate alone, the peaks in the PCA doublet region are not visible prior to the addition of PCA.
Conclusions
Based on this in vitro study, the reaction mixture of NaOCl and CHXa does not produce PCA at any measurable quantity and further investigation is needed to determine the chemical composition of the brown precipitate.
Keywords: Sodium hypochlorite, chlorhexidine, para-chloroaniline, nuclear magnetic resonance (NMR)
Introduction
The goal of root canal therapy is to remove inflamed or necrotic pulp tissue from within the root canal system through both chemical and mechanical debridement. The chemical debridement can take the form of intracanal medicaments, irrigants, or lubricants which facilitate the removal of the organic and inorganic components within the root canal system. Antimicrobial rinses are used to decrease the microbial loads within the system prior to obturation and reduce the potential for failure in the future.
Sodium hypochlorite (NaOCl) is an endodontic irrigant used at varied concentrations and has been shown to have broad spectrum antimicrobial action and tissue dissolving properties (1, 2). While effective against microorganisms, NaOCl has also been shown to have cytotoxic effects which can cause the irritation and necrosis of periapical tissues (3, 4). It was also suggested by Oncag that NaOCl lacks antimicrobial substantivity in root dentin (5). These possible shortcomings of NaOCl have led researchers and clinicians to explore additional irrigants which would complement or replace NaOCl.
Chlorhexidine (CHX) (Fig. 1A) has been used in many ionic forms (acetate, gluconate, and hydrochlorate) as an intraoral antimicrobial (6). Compared to NaOCl, CHX has been shown to be as efficacious against endodontic microbes and it lacks the cytotoxic effects of NaOCl (7, 8). CHX has also been shown to have antimicrobial substantivity in root dentin for up to 12 weeks (9, 10, 11). A possible disadvantage to CHX is that it lacks the tissue dissolving properties of NaOCl. The use of CHX in sequence with NaOCl, can possibly offer antimicrobial substantivity and tissue dissolution, respectively (12, 13). The mixture of NaOCl and CHX, however, produces a precipitate that stains the walls of the pulp chamber and has been reported to be difficult to remove (14). In addition, previous researchers have reported that this precipitate contains para-chloroaniline (PCA) (Fig. 1B), a toxin which can produce methemoglobin and is possibly carcinogenic over time (15, 16, 17).
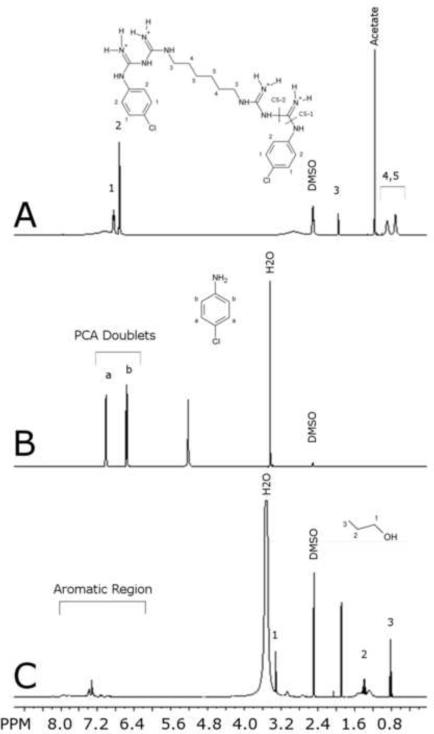
Figure 1.
1H NMR spectrum of (A) chlorhexidine acetate with characteristic peaks at 6.85 ppm and 6.71 ppm (labels 1–5 correspond to labeled chemical structure of chlorhexidine and cleavage sites are labeled CS-1 and CS-2) (B) p-chloroaniline with characteristic peaks at 7.01 ppm and 6.56 ppm (labels a and b correspond to labeled chemical structure of PCA). (C) Reaction precipitate sampled at 60 minutes with n-propanol added at 0.4 mg/ml as an internal standard (labels 1–3 corresponding to labeled chemical structure of n-propanol). All spectra were taken with 400-MHz Varian NMR System at 25°C, acquiring 32 scans, in d6-DMSO solvent.
Nuclear Magnetic Resonance (NMR) spectroscopy facilitates structural determinations by exposing a sample to a strong magnetic field and then to radiofrequencies which act upon atomic nuclei. Spin-active nuclei have specific spins which are acted upon by this radiation, creating a transition between energy levels which can be measured (18). This energy change is characteristic for each nucleus in a molecule, and this method of spectroscopy uses energy levels so low that even the most fragile chemical bonds remain intact. Following a chemical reaction, the presence or absence of specific molecules in a mixture can be determined by comparing the mixture's spectra to the spectra of pure compounds. The quantity of compounds present can also be determined by introducing an internal standard of a known concentration into the sample being analyzed.
The purpose of this in vitro study was to use Nuclear Magnetic Resonance (NMR) spectroscopy to determine whether para-chloroaniline (PCA) is formed through the reaction of mixing sodium hypochlorite and chlorhexidine.
Material and Methods
A commercially available sample of chlorhexidine acetate (CHXa) (Fischer Scientific, Pittsburgh, PA) and p-chloroaniline (Aldrich Chemical, St. Louis, MO) were analyzed with 1H NMR spectroscopy (400-MHz Varian NMR System acquiring 32 scans/spectrum) with perdeuterated DMSO (d6-DMSO) as a solvent.
A 2% (0.32 M) aqueous solution of CHXa was prepared by dissolving 1.0 g solid CHXa in 50 ml of deionized H2O. This solution was warmed to 37°C and mixed with 50 ml of 5.25 % NaOCl and stirred continuously. The brown precipitate formed immediately and a 2.0 ml sample was taken from the reaction at 60 minutes. This sample was divided in half and placed in two 1.5 ml microfuge tubes and centrifuged at 14,000 rpm for 2.5 minutes. The precipitate solid (37.6 mg) was removed from the supernatant liquid and dissolved in 1.0 ml of d6-DMSO. An internal standard of 0.5 μl of neat 1-propanol was added to each sample as an internal standard for 1H NMR experiments, resulting in a 0.4 mg/ml concentration. This solution was then divided in half, and 0.1 mg/ml of pure PCA was added to one of the two samples and 1H NMR spectra were collected for each of the samples at 25°C.
Results
The 1H NMR spectra were assigned for the aromatic ring protons of CHXa with peaks at 6.71 ppm and at 6.85 ppm (Fig. 1A) each exhibiting characteristic “doublet” patterns, and corresponding to protons in position 1 and 2 on the structure of CHX. A peak at 2.10 ppm corresponds to the methylene protons adjacent to the guanidine nitrogen (position 3 in Fig. 1A), and the 2 peaks at 1.38 ppm and 0.80 ppm correspond to the additional 8 methylene protons in the hexane diamine linker (positions 4 and 5 in Fig. 1A).
The 1H NMR spectrum for PCA was also assigned, identifying peaks at 6.56 ppm and at 7.01 ppm, which comprise what is hereinafter referred to as the PCA doublet region (Fig. 1B). These peaks also each had a characteristic “doublet” splitting, and correspond to the protons labeled a and b on the structure of PCA in Figure 1B.
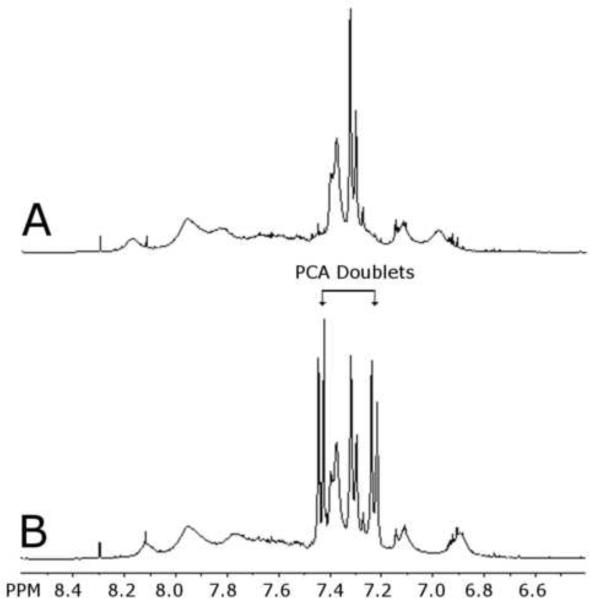
The NMR spectra of the isolated precipitate samples were analyzed, and a complex series of peaks were found in the 6.5–8.0 ppm region (Fig. 1C and Fig. 2A). The characteristic peaks in the PCA doublet region were not present in this spectrum, although the presence of a pair of doublets in Figure 2A indicates some type of p-substituted benzene. There was also no growth of the expected PCA doublet peaks over time, which indicated that PCA was not present and was not being produced in this reaction (Fig. 1C). It was confirmed that PCA should give a signal in the expected PCA doublet region under these conditions by spiking 0.1 mg/ml of pure PCA into the sample (Fig. 2B). The characteristic peaks for PCA are labeled in Figure 2B and it is clear they were not present in the spectrum before the PCA spike (Fig. 2A).
Figure 2.
(A) Expanded aromatic region of the 1H NMR spectrum of precipitate sampled at 60 minutes. Characteristic peaks in the PCA doublet region are not present. (B) Expanded aromatic region of the 1H NMR spectrum of the precipitate sampled at 60 minutes with 0.1 mg PCA added. Characteristic peaks for PCA in the PCA doublet region are labeled. Spectra were taken with 400-MHz Varian NMR System at 25°C, acquiring 32 scans, in d6-DMSO solvent.
Discussion
This study analyzed the mixture of products formed by the reaction of mixing a chlorhexidine salt (CHX acetate) and NaOCl using NMR spectroscopy to determine whether PCA was produced. It has been previously shown that the degradation of CHX occurs through the cleavage of the molecule along the hexane diamine linker between the aromatic rings at either end of the molecule (19, 20). Such cleavage results in smaller degradation products, each of which contains an aromatic ring. Over time one possible degradation product could result in the formation of PCA, if the chain is separated from the aromatic ring at cleavage site-1 (CS-1) in Figure 1A. If PCA is produced in the reaction of CHX and NaOCl, the two peaks in the PCA doublet region should also show up in the spectra of the precipitate and appear as a doublet pattern, but this is not what was observed. While the 1H NMR spectrum of the reaction mixture resulted in a complex combination of peaks from 6.5–8.0 ppm (where peaks from aromatic compounds appear), upon magnification it is clear that there are no peaks in the PCA doublet region which are intense enough to be quantified. Only upon adding pure PCA to the 60 minute reaction sample do two peaks appear in the PCA doublet region. Comparing this spectrum (Fig. 2B) to the original 60 minute spectrum (Fig. 2A) demonstrates that the peaks in the PCA doublet region are not present prior to the addition of PCA. These data clearly demonstrate that this breakdown product is produced at levels far less than the 0.1 mg/ml of PCA that was added to the sample. This would mean that in a worst case scenario <0.5 % of chlorhexidine is decomposed to PCA. Although the pair of doublets in Figure 2A do indicate some type of p-substituted benzene is produced, it is not PCA.
This finding is in conflict with Basrani, which reported that PCA was found in the precipitate formed through the interaction of CHX and NaOCl (15). Caution was suggested when using NaOCl and CHX in a final rinse because PCA is a component of the precipitate, but assessing the risk of exposure of patients to PCA during root canal therapy had not been explored (15). This previous research used a form of mass-spectrometry which relies on gas phase ionization that can fragment molecules. This technique is, therefore, not a conclusive method for determining the presence of degradation products. There is no way to determine whether the CHX molecule was broken through chemical degradation or through the mass spectrometry process itself. NMR spectroscopy analyzes molecules present in a sample at the time the spectrum is taken in a noninvasive and nondestructive manner. If a molecule is present in solution, then the spectrum of a mixture of molecules can be compared with a standard sample, and the resulting peaks will appear with the same pattern (singlet, doublet, triplet etc.) at the same chemical shift. If a peak does not share the same pattern and chemical shift, the corresponding molecule is not present in the mixture. The absence of the expected molecule can be confirmed by adding back the pure form of the expected molecule and comparing the spectra, as in Figure 2.
This study indicates that a number of breakdown products are produced when NaOCl and CHXa are mixed. These breakdown products contain an aromatic ring that is related to PCA and is para-substituted. Based on previous studies with amine-containing molecules, it can be predicted that chlorination from NaOCl should occur at one or more of the nitrogen atoms in CHX, producing chloramines (N-Cl bonds) (21, 22). Therefore, it is not surprising that there is no cleavage at CS-1 (Fig. 1A) to produce PCA, since the aniline amine is not as good of a leaving group as the guanidinium group would be (pKa=14) if cleavage occurred at CS-2 (Fig. 1A) (23). Based on this in vitro study, the reaction mixture of NaOCl and CHXa does not produce PCA at any measurable quantity. Further research should be conducted to determine the chemical composition of the mixture of products in the brown precipitate and their effects on teeth and periapical tissues.
Acknowledgements
The authors deny any financial affiliations related to this study or its sponsors.
The authors thank Dr. Sheng Cai for his assistance with the Nuclear Magnetic Resonance Spectroscopy.
This research was supported, in part by the National Institutes of Health/National Science Foundation Instrumentation Grants S10 RR019012 and CHE-0521323.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gomes BPFA, Ferraz CCR, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 2.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 3.Ehrich DG, Brian JD, Walker WA. Sodium hypochlorite accident: inadvertent injection into the maxillary sinus. J Endod. 1993;19:180–2. doi: 10.1016/S0099-2399(06)80684-9. [DOI] [PubMed] [Google Scholar]
- 4.Becking AG. Complication in the use of sodium hypochlorite during endodontic therapy. Oral Surg Oral Med Oral Pathol. 1991;71:346–8. doi: 10.1016/0030-4220(91)90313-2. [DOI] [PubMed] [Google Scholar]
- 5.Oncag O, Hosgor M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 6.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 7.Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20:276–8. doi: 10.1016/s0099-2399(06)80815-0. [DOI] [PubMed] [Google Scholar]
- 8.Ercan E, Ozekinci T, Atakul F, Gul K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J Endod. 2004;30:84–7. doi: 10.1097/00004770-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal S, Spangberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2004;98:488–92. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 10.White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23:229–231. doi: 10.1016/S0099-2399(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 11.Komorowski R, Grad H, Wu XY, Friedman S. Antimicrobial substantivity of chlorhexidine-treated bovine root dentin. J Endod. 2000;26:315–317. doi: 10.1097/00004770-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472–476. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 13.Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008;34:850–4. doi: 10.1016/j.joen.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bui T, Baumgartner J, Mitchell J. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod. 2008;34:181–5. doi: 10.1016/j.joen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Basrani BR, Manek S, Sodhi RNS, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33:966–9. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . International Agency for Research on cancer: IARC monography on the evaluation of carcinogenic risks to human. Vol. 57. Lyon, France: 1997. pp. 38–39. [Google Scholar]
- 17.Burkhardt-Holm P, Oulmi Y, Schroeder A, Storch V, Braunbeck T. Toxicity of 4-chloraniline in early life stages of Zebrafish (Danio rerio): II. Cytopathology and regeneration of liver and gills after prolonged exposure to waterborne 4 chloraniline. Arch Environ Contam Toxicol. 1999;37:85–102. doi: 10.1007/s002449900493. [DOI] [PubMed] [Google Scholar]
- 18.Crews P, Rodriguez J, Jaspars M. Organic Structure Analysis. Oxford University Press; New York: 1998. pp. 23–48. [Google Scholar]
- 19.Tanaka T, Murayama S, Tuda N, Nishiyama M, Nakagawa K, Matsuo Y, Isohama Y, Kido Y. Microbial degradation of disinfectants. A new chlorhexidine degradation intermediate (CHDI), CHDI-C, produced by Pseudomonas sp. strain No. A-3. J Health Sci. 2005;51:357–361. [Google Scholar]
- 20.Tanaka T, Ishii M, Mori Y, Yano Y, Iijima T, Takeda K, Kido Y. Microbial degradation of disinfectants: two new aromatic degradation products of chlorhexidine, chlorhexidine aromatic degradation product (CHADP)-4 and CHADP-6, produced by Pseudomonas sp. strain No. A-3. J Health Sci. 2006;52:58–62. [Google Scholar]
- 21.Thomas EL, Jefferson MM, Grisham MB. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochem. 1982;21:6299–308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- 22.Grisham MB, Jefferson MM, Melton DF, Thomas EL. Chlorination of endogenous amines by isolated neutrophils. Ammonia- dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984;259:10404–13. [PubMed] [Google Scholar]
- 23.Vickery B, Kaberia F. Reactions of sodium hypochlorite with some compounds having reactive methylene groups. Cell Mol Life Sci. 1979;35:299. [Google Scholar]