Abstract
Three to 5 days after a primary HSV-1 infection, macrophages infiltrate into the trigeminal ganglia (TG) and produce anti-viral cytokines to reduce viral replication. Previous research demonstrated that social disruption stress (SDR) enhances the trafficking of monocytes/macrophages from the bone marrow to the spleen and increases pro-inflammatory cytokine production in vitro and in vivo. The impact of SDR on the trafficking of these cells to loci of herpes simplex virus type 1 (HSV-1) infection and subsequent function has not been examined. The following studies were designed to determine whether SDR would enhance the innate immune response during a primary HSV-1 infection by increasing the number of macrophages in the cornea and TG, thus increasing anti-viral cytokine production and reducing viral replication. BALB/c mice were exposed to six cycles of SDR prior to ocular infection with HSV-1 McKrae virus. Flow cytometric analysis of cells from the TG revealed an increase in the percentage of CD11b+ macrophages in SDR mice compared to controls. Immune cell infiltration into the cornea, however, could not be determined due to low cell numbers. Although gene expression of IFN-β was decreased, SDR increased gene expression of IFN-α, and TNF-α, in the cornea and TG. Examination of viral proteins showed decreased expression of infected cell protein 0 (ICP0), glycoprotein B (gB), glycoprotein H (gH) and latency-associated transcript (LAT) in the TG, however, expression of ICP0 and gB were elevated in the cornea of SDR mice. These results indicate that the innate immune response to HSV-1 was altered and enhanced by the experience of repeated social defeat.
Keywords: Social stress, HSV-1 infection, Innate immunity, Cornea, Trigeminal ganglia, Ocular infection, Monocytes/macrophages
1. Introduction
Herpes simplex virus type 1 (HSV-1) is a common oral pathogen that has infected approximately 90% of the adult population worldwide (Raguin and Malkin, 1997). During a primary HSV-1 infection, the virus initially replicates in oro-facial epithelial cells. The virus establishes latency by gaining access to sensory neuronal cell bodies of the trigeminal ganglia (TG) via nerve termini that inner-vate the peripheral infected tissue. During latency, the virus is in a nonproductive state. However, certain stimuli, such as psychological stress or UV irradiation, can reactivate the virus and may result in the induction of productive replication of the latent virus and re-infection of tissue in the periphery (Padgett et al., 1998; Goade et al., 2001). Approximately 30% of those infected with HSV-1 will have recurrences throughout their lifetime (Siegel, 2002). Effective resolution of the primary infection can have a profound impact on the frequency and severity of future recurrences. It has been suggested that the likelihood of HSV-1 reactivation is influenced by genome copy number per neuron and/or the number of neurons that become latently infected after the primary infection (Sawtell, 1998).
Previous studies using a mouse model of HSV-1 corneal infection have established that leukocyte infiltration and cytokine production are associated with corneal infection and latency in the TG (Gebhardt and Hill, 1990; Liu et al., 1996; Shimeld et al., 1999). In the cornea, neutrophils, NK cells, and macrophages have been shown to be important immune cells during a primary infection that are essential for clearance of HSV-1 through the production of anti-viral cytokines, such as the type I interferons, TNF-α, and iNOS (Cheng et al., 2000; Inoue et al., 2001; Mistry et al., 2001; Stumpf et al., 2002). In the TG, macrophages have been shown to be the predominant infiltrating cell 3–5 days following primary infection (Bourcier et al., 1999). During infection, these cells surround the infected neuronal cell bodies and produce cytokines to control viral replication (Kodukula et al., 1999). When macrophages are depleted from infected mice there is an increase in viral titer which is associated with HSV-1 dissemination within the ganglion (Kodukula et al., 1999). This leads to an increase in the number of HSV-1 Ag-positive neurons in the TG of macrophage-depleted mice when compared to control mice. This effect is likely due to reduced production of macrophage derived cytokines, such as TNF-α, which are crucial for the termination of viral replication (Kodukula et al., 1999).
Neuroendocrine mediators can have a major regulatory influence on innate and adaptive immune responses. For example, glucocorticoid hormones secreted in response to stressor can suppress inflammatory and immune responses by upregulating the expression of IκB which prevents nuclear translocation of NFκB, a transcription factor that is essential for transcribing inflammatory genes (Vermeulen et al., 2006). However, not all stressors result in immunosuppression. Social disruption (SDR) is a stressor that is marked by the generation of glucocorticoid-resistant monocytes/macrophages, immune cell priming, and cellular activation (Avitsur et al., 2001, 2002, 2003; Stark et al., 2001; Bailey et al., 2007). SDR enhances the trafficking of glucocorticoid-resistant monocytes/macrophages from the bone marrow to the spleen and increases pro-inflammatory cytokine production both in vitro and in vivo (Sheridan et al., 2000; Avitsur et al., 2001, 2003; Stark et al., 2001; Quan et al., 2003; Engler et al., 2004). The impact of SDR on trafficking of these cells to loci of infection, however, has not been studied. Therefore, these studies were designed to determine the effect of SDR on a primary HSV-1 infection by examining the accumulation and activation of macrophages in the cornea and TG.
2. Materials and methods
2.1. Mice
HSV-1 antibody-negative Balb/c male mice at 4–6 weeks of age were obtained from Charles River Breeding Laboratories (San Diego, CA) and allowed to acclimate to their surroundings for 7–10 days before initiation of any experimental procedures. Balb/c mice were chosen because of their susceptibility to a primary HSV-1 infection and their ability to reactivate latent virus. All mice were housed 5 per cage and allowed free access to food and water. The animal facility, accredited by the American Association for the Accreditation of Laboratory Animal Care, maintains a 12-h light dark cycle with lights out at 1800 h.
2.2. Virus and cells
HSV-1 McKrae strain was used for ocular infections. Virus stock was grown and assayed on VERO cells in modified Eagle's medium containing 10% fetal bovine serum and 4× penicillin/streptomycin. Cells were cultured at 36 °C in a humidified incubator containing 5% CO2.
2.3. Ocular viral infection
Before experimentation, the eyes of all mice were examined for any abnormalities. Prior to infection, the mice were anesthetized with an intramuscular injection (0.1 ml) of 0.44 mg/ml xylazine (Phoenix Scientific, St. Joseph, MO) and 7.8 mg/ml ketamine (Phoenix Scientific, St. Joseph, MO). Both surfaces of the right and left cornea were lightly abraded in a 10 × 10 grid pattern with a 25-gauge needle (care was taken to avoid disruption of the stroma) (Nauss et al., 1985). A 5 μl drop of DMEM media containing 7.5 × 105 plaque-forming units of HSV-1 McKrae strain per ml was placed on the right eye cornea while a 5 μl drop of DMEM media was placed on the left cornea.
2.4. Social disruption stress paradigm (SDR)
This stress paradigm has been established in our laboratory (Sheridan et al., 2000; Avitsur et al., 2001, 2003; Stark et al., 2001; Quan et al., 2003; Engler et al., 2004). Cages of 5 mice were placed into either control or SDR groups. Control mice remained undisturbed in their home cage. During each SDR cycle, an aggressive intruder was introduced into the home cage. The aggressor attacked resident mice within 5–10 min of the beginning of the session and all residents exhibited passive responses to these attacks. Behavior was observed to ensure that the intruder remained aggressive and that the resident mice displayed signs of submissive behaviors. If the intruder did not attack, or was attacked by any of the resident mice, a new intruder replaced the initial intruder. In general, the attacks last for approximately 20–30 s, after which the intruder rested for 1–2 min before attacking again. All SDR cycles began at 4:30 PM and ended at 6:30 PM. The health status of each mouse was examined after each SDR cycle. Typically, animals underwent six cycles of SDR before being infected with virus. Different intruders were used on consecutive nights. In all the experiments, the subjects in the SDR group were defeated residents. All procedures were performed according to guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.5. Total RNA extraction
Animals were sacrificed and the ipsilateral TG was excised prior to and 1, 3, 5, and 7 days post-infection (p.i.). Tissue samples were submerged in TRIzol reagent (Life Technologies, Rockville, MD) and then stored in 5 ml polypropylene tubes at −80 °C. Samples were homogenized using a Tissue Tearor (Biospec Products Inc., Bartlesville, OK). Total RNA were extracted according to manufacturer's protocol for the TRIzol reagent.
2.6. Reverse transcription
A solution containing poly(A)-tailed RNA, oligo(dt) primer, dNTP mix, ribonuclease inhibitor Rnasin (Promega, Madison, WI) and 15 U of AMV reverse transcriptase (Promega, Madison, WI) in reaction buffer (250 mM Tris–HCl, pH 8.3, 250 mM KCl, 50 mM MgCl2, 50 mM DTT, and 2.5 spermidine) were incubated at 42 °C for 60 min. The solution was heated at 90 °C for 5 min to destroy AMV-RT and cooled to 4 °C.
2.7. Real-time PCR analysis
Reactions were performed in 25 μl volumes containing 2× Taqman Universal PCR Master Mix (Perkin-Elmer, Norwalk, Conn.) 2.5 μl of cDNA. Reactions also contained a 300 mM concentration of Taqman forward and reverse primers and 250 mM concentration of Taqman probe. Primer pairs and probes were designed using Primer Express software (Perkin-Elmer) and analyzed in 96-well optical plates. Probes were labeled at the 5′ end with the fluorescent reporter dye Fam and at the 3′ end with fluorescent quencher dye Tamra to allow direct detection of the PCR product. Primer and probe sequences (5′ → 3′) used in real-time RT-PCR reactions are shown in Table 1. Real-time detection and quantification were performed using ABI 7700 Sequence Detector (PE Biosystems). As a negative control, each plate contained a minimum of three wells lacking template. Each sample was analyzed in triplicate. The copy numbers for each individual gene transcript were normalized to 18S levels by dividing the copy number obtained from standard curves to that obtained for 18S.
Table 1.
Primer and probe sequences used in real-time RT-PCR reactions.
| Name | Type | Sequence (5′–3′) |
|---|---|---|
| IFN-α | F | TGCAACCCTCCTAGACTCATTCT |
| IFN-α | R | CCAGCAGGGCGTCTTCCT |
| IFN-α | Probe | CTGCATCAGACAGCCTTGCAGGTCATT |
| IFN-β | F | TGAATGGAAAGATCAACCTCACCTA |
| IFN-β | R | CTCTTCTGCATCTTCTCCGTCA |
| IFN-β | Probe | AGGGCGGACTTCAAGATCCCTATGGA |
| TNF-α | F | CTGTCTACTGAACTTCGGGGTGAT |
| TNF-α | R | GGTCTGGGCCATAGAACTGATG |
| TNF-α | Probe | ATGAGAAGTTCCCAAATGGCCTCCCTC |
| iNOS | F | CAGCTGGGCTGTACAAACCTT |
| iNOS | R | TGAATGTGATGTTTGCTTCGG |
| iNOS | Probe | CGGGCAGCCTGTGAGACCTTTGA |
| ICP0 | F | ATGTTTCCCGTCTGGTCCAC |
| ICP0 | R | CCCTGTCGCCTTACGTGAA |
| ICP0 | Probe | CCCCGTCTCCATGTCCAGGATGG |
| gB | F | GGTGTGTTTGTTTGGTACGCC |
| gB | R | GGAAAGAGGAAACAGGCCG |
| gB | Probe | TTGTGTGTGTGGGAAGAAAGAAAAGGGAAC |
| gH | F | CGACCACCAGAAAACCCTCTTT |
| gH | R | ACGCTCTCGTCTAGATCAAAGC |
| gH | Probe | TCCGGACCACTTTTC |
| LAT | F | CCCCTCCCACCCTTAGTCAG |
| LAT | R | CCAGCCGGCCCTTAGATAA |
| LAT | Probe | CGTCCGACCACCAACTGCCCC |
F = forward primer; R = reverse primer.
2.8. Flow cytometry
Mice were euthanized on days 0, 1, 2, 3, and 6 post-infection via CO2 asphyxiation and immediately decapitated. After carefully removing the brain to expose the TG on the base of the skull, the ipsilateral TG was excised and placed into 1 ml of sterile, ice cold Hanks' buffer. The tissues were homogenized, filtered, and washed to yield a single cell suspension. A total of 2.5 × 105 cells were then stained for flow cytometric analysis using established protocols as previously described (Bailey et al., 2007). Two sets of antibodies were used for staining. In the first set, optimal concentrations of FITC-conjugated anti-CD45/common leukocyte antigen, PerCP-conjugated anti-Gr-1/Ly-6G, and APC-conjugated anti-CD11b were added to stain for total leukocytes, monocyte/macrophages and neutrophils. Matched isotype controls were used to set negative staining criteria. After staining, 10,000 cells were analyzed on a FACSCalibur dual-laser flow cytometer (Becton–Dickinson Immunocytometry Systems, San Jose, CA). All antibodies were purchased from BD Pharmingen (San Jose, CA). Data were analyzed using BD CellQuest Pro software (Becton–Dickinson, San Jose, CA).
2.9. Statistics
To assess the influence of SDR on gene expression during infection, the quantity of specific RNA (i.e., TNF-α, IFN-α, IFN-β) obtained from a sample was compared to the quantity of the same RNA species present in uninfected healthy tissue samples using the comparative Ct method (i.e., ΔΔCt) as previously published (Livnat and Schmittgen, 2001; Schmitten and Livak, 2008). Briefly, real-time PCR generates a Ct value, which is the PCR cycle where amplification of the cDNA of interest begins exponential expansion. For analysis, the Ct value for the internal standard (i.e., 18S RNA) was first subtracted from the Ct value for the cDNA of interest (i.e., TNF-α, IFN-α, IFN-β). This subtraction controls for differences in reverse transcription and sample loading, and the value is denoted as the ΔCt. Next, the average ΔCt value generated from uninfected tissue samples was then subtracted from the ΔCt for each experimental sample. This equation sets the control sample to a reference value of 0 and generates a ΔΔCt for each unknown. And finally, these values were averaged for each treatment group; these mean values were used to generate the N-fold difference in RNA expression relative to the control. Expression of each anti-viral cytokine in uninfected tissue samples from the control group was assigned the value of 1 to represent the level that would be expected to be expressed in tissue not involved during infection. Therefore, for comparative analysis of the effects of SDR, expression of each cytokine in the experimental samples is expressed relative to this reference point. Although this data transformation accurately illustrates the logarithmic amplification following each PCR cycle and is used in each of the figures, we chose to perform statistical tests on the rew Ct values. We chose to use Ct values because the transformation involved in calculating the ΔΔCt positively skews the data, thus violating a primary assumption for the use of parametric statistics. Because the raw Ct values reflect normal biological activity, they can be assumed to be normally distributed and parametric statistics can be used. Two-way, repeated measure analysis of variance was performed using SPSS statistical software version 14.0 (Chicago, IL). p-values of less than 0.05 were considered statistically significant. Post hoc tests were performed using Bonferoni post hoc tests with p-values of less that 0.05 being considered significant.
3. Results
3.1. SDR increased gene expression of anti-viral cytokines in the cornea
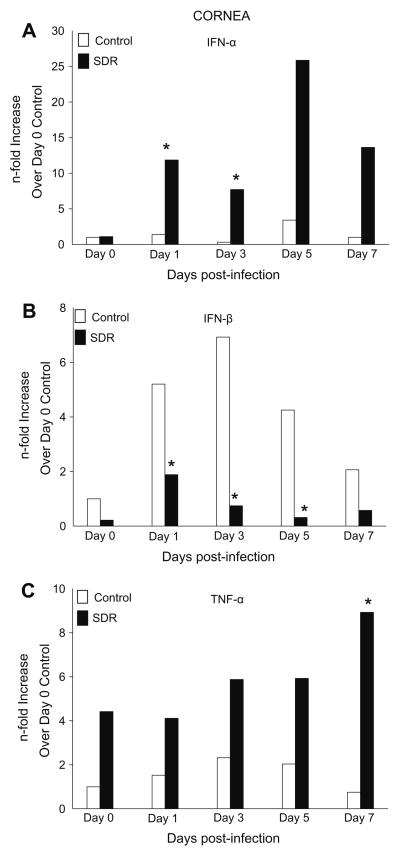
The corneas of infected mice were collected to determine whether SDR affected the expression of genes encoding anti-viral cytokines. Gene expression of IFN-α prior to infection was similar in both groups. On days 1 and 3 p.i., levels of IFN-α in control groups remained similar to values on day 0, but in SDR mice expression significantly increased by 12-fold and 8-fold, respectively. Expression of IFN-α peaked in both groups on day 5 p.i. at 3.4-fold (HCC) and 25.9-fold (SDR) however statistical analysis revealed no significant difference between the two groups. On day 7, levels of IFN-α in control animals decreased to levels prior to infection. SDR mice also had a reduction in the expression of IFN-α, although, it was 13-fold higher than the control group. In general, mice exposed to SDR had an increase in the production of IFN-α after infection with HSV-1 compared to the controls (F(1, 66) = 14.949, p < 0.001; Fig. 1A). Post hoc tests revealed a significant increase in IFN-α on days 1 and 3 p.i. in SDR mice compared to controls (p < 0.05).
Fig. 1.
Gene expression of pro-inflammatory cytokines in the cornea during a primary HSV-1 infection. (A) IFN-α expression was significantly increased on days 1 and 3 in SDR mice compared to controls. (B) IFN-β expression was significantly decreased in SDR mice on days 1, 3, and 5 post-infection. (C) TNF-α expression was significantly increased on day 7 between the two groups. n = 10 per treatment group per time point. *p < 0.05.
Gene expression of IFN-β was also examined using real-time PCR. In control mice, expression of IFN-β increased progressively, peaking on day 3 p.i. by 7-fold and then decreasing by day 7 to levels similar to day 0. The pattern of expression of IFN-β in SDR mice was also similar to control mice, however, IFN-β was 3-fold, 7-fold, and 4-fold lower on days 1, 3, and 5 p.i., respectively. There was no significant difference between both groups on days 0 and 7. Overall, gene expression of IFN-β was significantly lower in SDR mice compared to control mice (F(1, 56) = 10.310, p < 0.01, Fig. 1B) with post hoc testing indicating that this was due to a significant decrease on days 1, 3, and 5 post-infection (p < 0.05).
In the cornea of control mice, there was an increase in TNF-α which peaked 2.3-fold on day 3 p.i. Expression of TNF-α then decreased to levels similar to those on day 0. SDR mice had around a 4-fold increase in expression compared to controls on days 0, 1, 3, and 5 p.i. Its expression peaked on day 7 and was 9-fold greater than the controls. TNF-α expression was generally higher in SDR mice compared to controls (F(1, 68) = 34.166, p < 0.001, Fig. 1C). Post hoc tests indicated a significant difference on day 7 between the two groups (p < 0.05).
When iNOS expression was examined, there was no upregulation in its expression in either group, or on either day except for on day 5. There was a 4-fold increase in iNOS expression on day 5 in control mice compared to SDR but there was no significant difference between the two groups (data not shown).
3.2. SDR increased gene expression of anti-viral cytokines in the TG
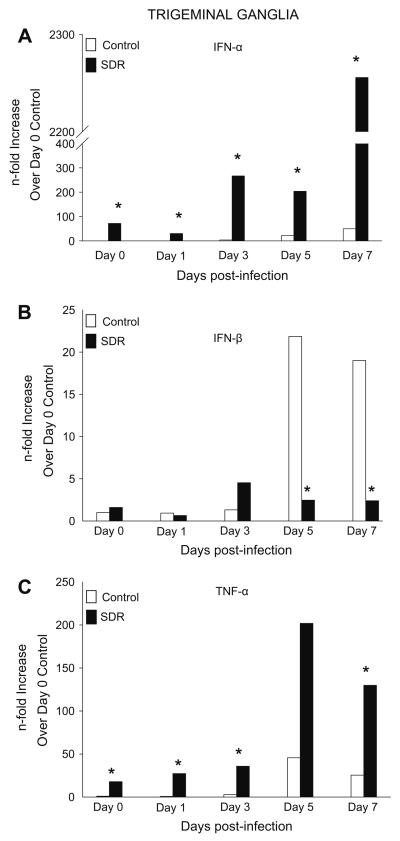
We next examined infected TGs to determine whether SDR affected expression of genes encoding for anti-viral cytokines. Prior to infection (day 0), gene expression of IFN-α was 71-fold higher compared to controls. Expression in control mice progressively increased each day peaking at 49-fold on day 7 p.i. SDR mice had a similar pattern of expression but had a significantly higher expression of IFN-α, with a 2200-fold difference by day 7 (F(1, 78) = 19.718, p < 0.001; Fig. 2A). Post hoc tests revealed significance on all days that were examined (p < 0.05).
Fig. 2.
Gene expression of pro-inflammatory cytokines in the TG during a primary HSV-1 infection. (A) IFN-α expression was significantly increased on all days in SDR mice compared to control. (B) IFN-β expression was significantly decreased on days 5 and 7 post-infection. (C) TNF-α expression was significantly increased on days 0, 1, 3 and 7 in SDR mice compared to controls. n = 10 per treatment group per time point. *p < 0.05.
Gene expression of IFN-β in the TG of control mice did not change from uninfected tissue until days 5 and 7 p.i., with a 22-fold and 19-fold increase, respectively (F(4, 75) = 3.753, p < 0.01, Fig. 2B). In SDR mice, gene expression remained similar to levels prior to infection on all days p.i. with levels being no higher than 4-fold.
In the TG, gene expression of TNF-α on days 0, 1, and 3 p.i. was on average 2-fold in control mice. Expression peaked on day 5 p.i. by 45-fold and then declined to 25-fold by day 7 p.i. In SDR mice, expression of TNF-α was 17-fold higher prior to infection compared to controls. The pattern of expression was similar to control mice but expression was significantly higher with levels peaking on day 5 p.i. by 150-fold (F(1, 64) = 52.928, p < 0.001; Fig. 2C). Post hoc tests revealed a significant difference between groups on days 0, 1, 3 and 7 (p < 0.05).
iNOS expression was also determined in these experiments. In both the control and SDR group, iNOS expression peaked on day 5 but there was no significant difference between the two groups on any of the days that were examined (data not shown).
3.3. SDR increased trafficking of CD11b+ macrophages to the TG
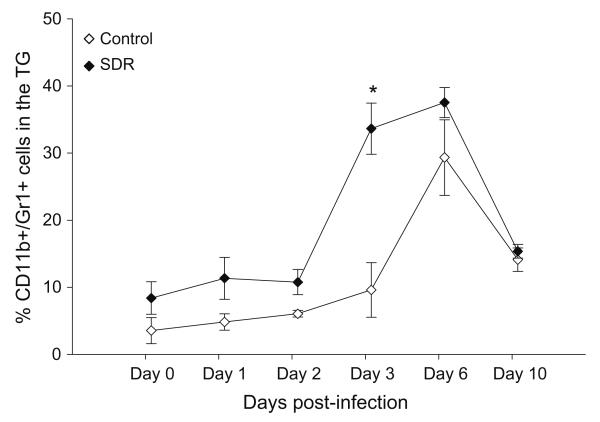
Since mice exposed to social stress had higher levels of gene expression of anti-viral cytokines, we investigated the effect of SDR on trafficking of macrophages to the TG during primary HSV-1 infection. Infiltration of immune cells was examined one day prior to infection and on days 1, 2, 3, 6, and 10 p.i. The presence of macrophages was determined as a percentage of total TG CD11b+ cells. On days 0, 1, and 2 the percentage of macrophages was 4%, 5%, and 6% in control TGs, respectively. In SDR TGs, the percentage of cells was approximately 5% higher than controls. On day 3 post-infection, the percentage of macrophages in the control TGs was 10% while the percentage in SDR TGs jumped to 34%. On day 6 p.i., infiltration peaked in both control and SDR TGs with percentages of 29% and 38%, respectively. By day 10 p.i., trafficking of macrophages decreased to 14.4% and 15.7% in control and SDR TGs, respectively. In general, mice exposed to SDR prior to infection had an increase in the percentage of CD11b+ macrophages compared to the controls (F(1, 32) = 14.953, p < 0.001; Fig. 3). Post hoc tests revealed a significant increase in macrophages on day 3 in SDR mice compared to controls (p < 0.05).
Fig. 3.
Effect of SDR on infiltration of CD11b+ macrophages in the TG during a primary HSV-1 infection. SDR increases the percentage of leukocytes in the TG that were CD11b+/Gr-1+ macrophages during a primary HSV-1 infection. BALB/c mice were exposed to six consecutive cycles of SDR. One day after the last cycle of SDR, the mice were ocularly infected with HSV-1 McKrae strain. On days 0, 1, 2, 3, and 6 post primary infection, the infected right TG was excised and separated into single cell suspensions. The cell population from the infected TG was counted and identified using flow cytometric analysis. n = 10 per treatment group per time point. Two-way, repeated measure analysis of variance was performed using SPSS statistical software version 14.0. p-values of less that 0.05 were considered significant. Statistical analysis revealed a significant SDR effect (F(1, 32) = 14.953, p < 0.001). Post hoc tests revealed a significant increase in macrophages on day 3 (p < 0.05).
3.4. SDR affected gene expression of viral proteins in the cornea
Exposure to SDR prior to an HSV-1 infection enhanced the innate immune response in the cornea as indicated by an increase in gene expression of anti-viral cytokines. In order to determine the effects of the enhanced immune response on viral replication, specific viral genes were chosen that are representative of a complete replication cycle.
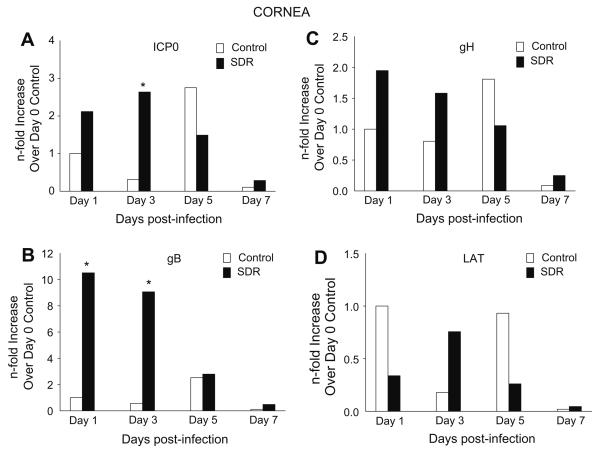
Gene expression of ICP0 in control mice was low with levels peaking on day 5 p.i. by only 2-fold. In SDR mice, expression also was low with levels peaking on day 3 p.i. by only 2-fold. However, post hoc tests revealed a significant difference between control and SDR mice on day 3 post-infection (p < 0.05, Fig. 4A). Expression of gB in the control mice did not go above 2-fold on any day p.i. However, in SDR mice expression of gB was 10-fold and 9-fold higher on days 1 and 3 p.i., respectively, compared to controls (F(3, 98) = 1.210, p < 0.05; Fig. 4B). There was no significant difference by days 5 and 7 p.i. Post hoc tests showed a significant difference on days 1 and 3 between the two groups (p < 0.05). In addition to gene expression for ICP0 and gB, gene expression of gH and LAT was also determined in these experiments. In both the control and SDR groups, gH and LAT levels remained below 2-fold and no significant differences were found between control and SDR groups on any of the days post-infection (Fig. 4C and D).
Fig. 4.
Gene expression of HSV-1 genes in the cornea during a primary HSV-1 infection. (A) ICP0 expression was significantly increased on day 3 post-infection in SDR mice. (B) gB expression was significantly increased on days 1 and 3 in SDR mice compared to controls. (C) Statistical analysis revealed no significant SDR effect on gH expression. (D) Statistical analysis revealed no significant SDR effect on LAT expression. n = 10 per treatment group per time point. p < 0.05.
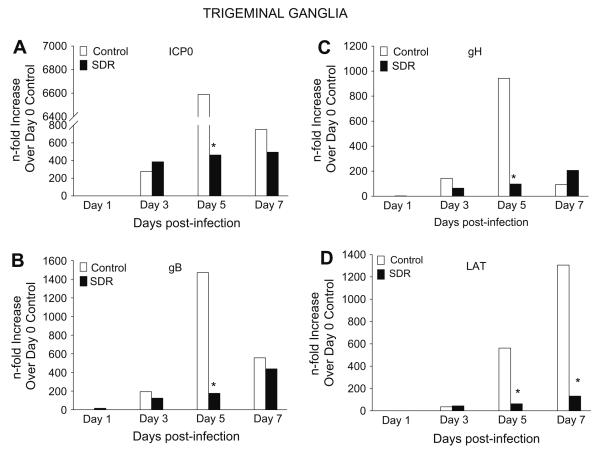
3.5. SDR reduced gene expression of viral proteins in the TG
In a fashion that was similar to the cornea, SDR enhanced innate immune responses in the TG that was evidenced by increased trafficking of CD11b+ macrophages and elevated gene expression of anti-viral cytokines. In order to determine the effects of this enhanced immune response on viral replication in the TG, gene expression of ICP0, gB, gH and LAT was examined on days 1, 3, 5, and 7 p.i. using real-time PCR.
In control mice, expression of ICP0 on day 3 p.i. was 275-fold higher than day 1 p.i. and then jumped to 6500-fold on day 5 p.i. It declined to 750-fold by day 7 p.i. SDR mice had similar expression on days 1, 3 and 7 p.i., but were significantly lower on day 5 p.i. with an expression of 450-fold (Fig. 5A). The pattern of expression in gB and gH was similar to that seen with ICP0. Although there was no difference between the two groups on days 1, 3, and 7 p.i., there was a significant difference on day 5 p.i. Glycoprotein B expression on day 5 p.i. showed control mice had peak expression of 1470-fold while SDR TGs peaked at only 175-fold (Fig. 5B). Also with gH, control mice peaked at 942-fold, while SDR mice had expression levels of only 93-fold (Fig. 5C). Post hoc tests revealed a significant difference on day 5 for ICP0, gB, and gH between SDR and control groups (p < 0.05). When the LAT gene was examined, it followed a different pattern than the previous viral genes. In the control TGs, expression of LAT was 35-fold on day 3 p.i., 560-fold on day 5 p.i., and 1300-fold on day 7 p.i. Expression in SDR TGs did not progressively increase like control TGs, but remained constant on days 3, 5 and 7 p.i. with expression being, on average, 70-fold. Post hoc test indicated a significant difference with SDR and control groups on day 5 and 7 p.i. (p < 0.05, Fig. 5D).
Fig. 5.
Gene expression of HSV-1 genes in the TG during a primary HSV-1 infection. (A) ICP0 expression was significantly decreased on day 5 in SDR mice compared to controls. (B) gB expression was significantly decreased on day 5 in SDR mice compared to controls. (C) Statistical analysis revealed a significant decrease in gH expression on day 5 in SDR mice compared to controls. (D) LAT expression was significantly decreased on days 5 and 7 in SDR mice compared to control mice. n = 10 per treatment group per time point. p < 0.05.
4. Discussion
In a mouse model of HSV-1 corneal infection, the type I IFNs, TNF-α, and iNOS are essential for cessation of viral replication. In this study, high levels of IFN-α and TNF-α were expressed in both the cornea and TG of SDR mice and expression of IFN-β was reduced. Upregulation of anti-viral cytokines in the cornea and TG results in the reduction of viral replication, however, differential outcomes can result as a consequence of infection in each tissue. Previous studies of corneal HSV-1 infections show immunopatho-logical complications resulting in tissue scarring of the cornea (Thomas and Rouse, 1997; Keadle et al., 2000; Mistry et al., 2001), while the TG infected neurons do not undergo apoptosis induced by secreted cytokines (Ozaki et al., 1997; Shimeld et al., 1999). Therefore, the reduction in IFN-β in the cornea may be due to destruction of epithelial cells and it may be an indication of fewer neurons being infected in the TG. The type I IFNs mediate the early innate immune response to viral infection by inducing the production of enzymes which interfere with the transcription of viral RNA or DNA. Type I INFs can also bind to protein kinase R (PKR) which leads to the loss of host transcriptional machinery and the induction of host cell apoptosis (Der and Lau, 1995). Although most cells have the transcriptional machinery to produce both IFN-α and IFN-β, during a viral infection, non-infected immune cells typically produce IFN-α, while virally-infected cells typically produce IFN-β (Noisakran et al., 1999; Smiley, 2004). The likely source of increased expression of IFN-α after SDR is the SDR-induced increase in infiltrating macrophages in the cornea and TG. In contrast, gene expression of IFN-β was reduced in SDR mice. This reduction of IFN-β is likely due to apoptosis of infected cells, or an overall decrease in viral infection.
TNF-α is an important anti-viral cytokine that is produced predominately by monocytes and macrophages. Studies have shown that it reduces HSV-1 viral replication, but the mechanisms by which this occurs are incompletely understood (Shubayev et al., 2006). Similar to the type I IFNs, TNF-α can induce apoptosis. If apoptosis were to occur in corneal epithelial cells and neurons, viral replication would likely cease in these tissues. It is possible, however, that increased TNF-α levels in the cornea would have more harmful rather than helpful effects as TNF-α induced apoptosis in corneal epithelial cells contributes to the pathogenesis of herpetic stromal keratitis (Keadle et al., 2000; Mistry et al., 2001). Unlike the cornea, studies have shown that TNF-α does not induce apoptosis in neurons; therefore, over-expression of TNF-α in the TG may be protective rather than detrimental to the host (Ozaki et al., 1997; Shimeld et al., 1999).
SDR-induced expression of IL-6 and IL-1β in the brain may be contributing to the elevation of TNF-α and IFN-α in the TG prior to infection. SDR has been shown to increase circulating levels of IL-6 and IL-1β (Quan et al., 2001; Stark et al., 2001) and tissue levels in the cortex and cerebellum (Sheridan et al., unpublished observations). In addition, expression of these cytokines has been shown to upregulate expression of other cytokines. It is unclear if SDR-induced enhancement of IFN-α and TNF-α in the TG was transient or prolonged since mice were infected one day after the last cycle of SDR. In this study, expression of viral genes jumped on average 300-fold by day 3 p.i. when compared to day 1 p.i.; this event may contribute to triggering high level production of IFN-α and TNF-α on day 3 p.i. It is possible that elevated levels of IFN-α and TNF- α are a result of both SDR and viral replication.
Previous studies have shown that SDR increased trafficking of glucocorticoid-resistant CD11b+ monocytes from the bone marrow to the spleen. Glucocorticoid resistance was due, in part, to the inability of the intracellular glucocorticoid receptor to translocate to the nucleus to perform its function (Quan et al., 2001; Avitsur et al., 2003). Further studies demonstrated that SDR increases macrophage pro-inflammatory cytokine production both in vitro and in vivo (Sheridan et al., 2000; Avitsur et al., 2001, 2003; Stark et al., 2001; Engler et al., 2004). In vitro studies have shown that the addition of the synthetic glucocorticoid hormone dexamethasone (DEX) to cells prior to being infected with HSV-1, increases the amount of virus produced in these cells because DEX acts on the glucocorticoid response element (GRE) within the oriL of the virus (Nasisse et al., 1989; Hardwicke and Schaffer, 1997; Mistry et al., 2001). Macrophages have been shown to be the predominant infiltrating cell in the TG 3–5 days after a primary infection and they are important for reducing viral replication (Liu et al., 1996). In this study, SDR significantly enhanced trafficking of macrophages in the TG and it is likely that these cells play a role in the elevated expression of anti-viral cytokines. Additionally, infiltrating lead to decreased glucocorticoid-resistant macrophages may viral replication.
In order to determine the effects of the SDR-enhanced immune response on viral replication, specific viral genes were chosen that are representative of the viral replication cycle. Examination of the cornea for viral genes in SDR mice showed increased gene expression of ICP0 and gB compared to controls on days 1 and 3 p.i. By days 5 and 7 p.i., levels of ICP0 and gB were reduced to levels similar to the controls. There was no difference in the expression of gH and LAT between groups. Similar to other stressors, SDR elevates glucocorticoid levels circulating in the host. The rise in glucocorticoids may play a role in the enhanced expression of ICP0 and gB early during infection. Corneal epithelial cells have been shown to have high levels of glucocorticoid receptors (Bourcier et al., 1999, 2000; Chen et al., 2006), possibly explaining why there is a higher expression of ICP0 and gB on days 1 and 3 p.i. in SDR mice. Increased levels of ICP0 and gB may stimulate more cytokine production from immune cells which would reduce viral replication in SDR mice to levels comparable to control mice by days 5 and 7 p.i.
Although SDR did not prevent neurons from being infected, expression of ICP0, gB, gH and LAT was reduced suggesting that SDR impeded viral replication. As previous studies indicated, most neurons do not undergo apoptosis when exposed to anti-viral cytokines. Thus, it is likely that the number of genome copies per neuron and/or the number of neurons that became latently infected was reduced. Because it has been suggested that the likelihood of HSV-1 reactivation is influenced by the number of genome copy numbers per neuron and/or the number of neurons that become latently infected after the primary infection, it is possible that stress prior to a primary infection will impact the development of latency.
Stress enhanced the innate immune response to a primary HSV-1 infection in both the cornea and TG as indicated by increased expression of anti-viral cytokines and infiltration of macrophages. While the SDR-induced enhanced immune response may have reduced viral replication in both the cornea and TG, it may lead to differential outcomes in each tissue. SDR may reduce the number of neurons that become infected in the TG, ultimately reducing the severity and frequency of future recurrences. Although this enhanced immune response may have a protective effect on the TG, such a pronounced immune response may come at a cost in other tissues. For example, in the cornea, immune cells lead to immuno-pathological complications by infiltrating the stroma and causing opacity and edema of the tissue. Therefore, in order to protect part of the CNS, namely the TG, the host must sacrifice the peripheral tissue. However, the effects of SDR may not be completely destructive to the cornea. Although the cornea may have been damaged after primary infection, studies indicate that the most destruction comes from repeated, recurrent infections (Thomas and Rouse, 1997). Therefore, SDR-enhanced immunity may increase the initial damage to the eye but the possibility of future recurrences and subsequent damage will be reduced.
For many years, it was thought that immune activation could only be driven by the presence of an antigen. However, these studies show that behavioral interactions alone can cause changes in the immune system. Furthermore, SDR-induced immune changes can have a major impact on the outcome of a viral infection. This study suggests that exposure to repeated social disruption prior to an HSV-1 infection primes macrophages, enhancing their capacity to respond to antigens, and reduces viral replication as a consequence.
5. Uncited references
Amir et al. (1999), MacMicking et al. (1997), Minagawa et al. (2004), Minami et al. (2002), Noisakran and Carr (2000), Pestka et al. (1987), Pozharskaya et al. (2004), Sawiris et al. (1994), and Wickham et al. (2004).
Acknowledgments
This work would not have been possible without the excellent technical assistance of Jacqueline Verity and Mark Hanke. This research was supported by NIH/NIDCR T32DE014320-07 and RO1MH046801-18 to Dr. John F. Sheridan.
References
- Amir J, Harel L, Smetana Z, Varsano I. The natural history of primary herpes simplex type 1 gingivostomatitis in children. Pediatr. Dermatol. 1999;16(4):259–263. doi: 10.1046/j.1525-1470.1999.00072.x. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J. Leukoc. Biol. 2003;74(4):507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J. Neuroimmunol. 2002;124(1–2):54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bourcier T, Borderie V, Forgez P, Lombet A, Rostène W, Laroche L. In vitro effects of dexamethasone on human corneal keratocytes. Invest. Ophthalmol. Vis. Sci. 1999;40(6):1061–1070. [PubMed] [Google Scholar]
- Bourcier T, Forgez P, Borderie V, Scheer S, Rostène W, Laroche L. Regulation of human corneal epithelial cell proliferation and apoptosis by dexamethasone. Invest. Ophthalmol. Vis. Sci. 2000;41(13):4133–4141. [PubMed] [Google Scholar]
- Chen WL, Lin CT, Yao CC, Huang YH, Chou YB, Yin HS, Hu FR. Invitro effects of dexamethasone on cellular proliferation, apoptosis, and Na+–K+ATPase activity of bovine corneal endothelial cells. Ocul. Immunol. Inflamm. 2006;14(4):215–223. doi: 10.1080/09273940600732380. [DOI] [PubMed] [Google Scholar]
- Cheng H, Tumpey TM, Staats HF, Van Rooijen N, Oakes JE, Lausch RN. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1402–1409. [PubMed] [Google Scholar]
- Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. USA. 1995;92(19):8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148(1–2):106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Gebhardt BM, Hill JM. Cellular neuroimmunologic responses to ocular herpes simplex virus infection. J. Neuroimmunol. 1990;28(3):227–236. doi: 10.1016/0165-5728(90)90016-g. [DOI] [PubMed] [Google Scholar]
- Goade DE, Nofchissey RA, Kusewitt DF, Hjelle B, Kreisel J, Moore J, Lyons CR. Ultraviolet light induces reactivation in a murine model of cutaneous herpes simplex virus-1 infection. Photochem. Photobiol. 2001;74(1):108–114. doi: 10.1562/0031-8655(2001)074<0108:uliria>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hardwicke MA, Schaffer PA. Differential effects of nerve growth factor and dexamethasone on herpes simplex virus type 1 oriL- and oriS-dependent DNA replication in PC12 cells. J. Virol. 1997;71(5):3580–3587. doi: 10.1128/jvi.71.5.3580-3587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Inoue Y, Kosaki R, Inoue Y, Nishida K, Shimomura Y, Tano Y, Hayashi K. Immunohistological study of infiltrated cells and cytokines in murine herpetic keratitis. Acta Ophthalmol. Scand. 2001;79(5):484–487. doi: 10.1034/j.1600-0420.2001.790511.x. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Miller JK, Pepose JS, Stuart PM. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci. 2000;41(1):96–102. [PubMed] [Google Scholar]
- Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J. Immunol. 1999;162(5):2895–2905. [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996;70(1):264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnat KJ, Schmittgen TD. Analysis of relative gene expression data using time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Minagawa H, Hashimoto K, Yanagi Y. Absence of tumour necrosis factor facilitates primary and recurrent herpes simplex virus-1 infections. J. Gen. Virol. 2004;85(Pt 2):343–347. doi: 10.1099/vir.0.19627-0. [DOI] [PubMed] [Google Scholar]
- Minami M, Kita M, Yan XQ, Yamamoto T, Iida T, Sekikawa K, Iwakura Y, Imanishi J. Role of IFN-gamma and tumor necrosis factor-alpha in herpes simplex virus type 1 infection. J. Interferon Cytokine Res. 2002;22(6):671–676. doi: 10.1089/10799900260100150. [DOI] [PubMed] [Google Scholar]
- Mistry SK, Zheng M, Rouse BT, Morris SM., Jr. Induction of arginases I and II in cornea during herpes simplex virus infection. Virus Res. 2001;73(2):177–182. doi: 10.1016/s0168-1702(00)00243-4. [DOI] [PubMed] [Google Scholar]
- Nasisse MP, Guy JS, Davidson MG, Sussman WA, Fairley NM. Experimental ocular herpesvirus infection in the cat. Sites of virus replication, clinical features and effects of corticosteroid administration. Invest. Ophthalmol. Vis. Sci. 1989;30(8):1758–1768. [PubMed] [Google Scholar]
- Noisakran S, Campbell IL, Carr DJ. Ectopic expression of DNA encoding IFN-alpha 1 in the cornea protects mice from herpes simplex virus type 1-induced encephalitis. J. Immunol. 1999;162(7):4184–4190. [PubMed] [Google Scholar]
- Noisakran SJ, Carr DJ. Therapeutic efficacy of DNA encoding IFN-alpha1 against corneal HSV-1 infection. Curr. Eye Res. 2000;20(5):405–412. [PubMed] [Google Scholar]
- Ozaki N, Sugiura Y, Yamamoto M, Yokoya S, Wanaka A, Nishiyama Y. Apoptosis induced in the spinal cord and dorsal root ganglion by infection of herpes simplex virus type 2 in the mouse. Neurosci. Lett. 1997;228(2):99–102. doi: 10.1016/s0304-3940(97)00364-9. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA. 1998;95(12):7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu. Rev. Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pozharskaya VP, Weakland LL, Offermann MK. Inhibition of infectious human herpesvirus 8 production by gamma interferon and alpha interferon in BCBL-1 cells. J. Gen. Virol. 2004;85(Pt 10):2779–2787. doi: 10.1099/vir.0.80214-0. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J. Neuroimmunol. 2003;137(1–2):51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J. Neuroimmunol. 2001;115(1–2):36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Raguin G, Malkin JE. Genital herpes: epidemiology and pathophysiology. Update and new perspectives. Ann. Med. Interne (Paris) 1997;148(8):530–533. [PubMed] [Google Scholar]
- Sawiris GP, Sydiskis RJ, Bashirelahi N. Hormonal modulation of herpes simplex virus replication in a mouse neuroblastoma cell line. J. Clin. Lab. Anal. 1994;8(3):135–139. doi: 10.1002/jcla.1860080304. [DOI] [PubMed] [Google Scholar]
- Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 1998;72(8):6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitten TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann. NY Acad. Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Easty DL, Hill TJ. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and cytokines. J. Virol. 1999;73(3):1767–1773. doi: 10.1128/jvi.73.3.1767-1773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell. Neurosci. 2006;31(3):407–415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MA. Diagnosis and management of recurrent herpes simplex infections. J. Am. Dent. Assoc. 2002;133(9):1245–1249. doi: 10.14219/jada.archive.2002.0366. [DOI] [PubMed] [Google Scholar]
- Smiley JR. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase. J. Virol. 2004;78(3):1063–1068. doi: 10.1128/JVI.78.3.1063-1068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(6):R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Stumpf TH, Case R, Shimeld C, Easty DL, Hill TJ. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. J. Gen. Virol. 2002;83(Pt 7):1579–1590. doi: 10.1099/0022-1317-83-7-1579. [DOI] [PubMed] [Google Scholar]
- Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 1997;16(4):375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Vanden Berghe W, Haegeman G. Regulation of NF-kappaB transcriptional activity. Cancer Treat. Res. 2006;130:89–102. [PubMed] [Google Scholar]
- Wickham S, Ash J, Lane TE, Carr DJ. Consequences of CXCL10 and IL-6 induction by the murine IFN-alpha1 transgene in ocular herpes simplex virus type 1 infection. Immunol. Res. 2004;30(2):191–200. doi: 10.1385/IR:30:2:191. [DOI] [PMC free article] [PubMed] [Google Scholar]