Abstract
Background:
Midregional proadrenomedullin (MR-proADM) is a potential prognostic biomarker in patients with community-acquired pneumonia (CAP). Previous work has been hampered by sample size and illness spectrum limits. We sought to describe the pattern of MR-proADM in a broad CAP cohort, confirm its prognostic role, and compare its performance to procalcitonin, a novel biomarker of infection.
Methods:
We conducted a multicenter prospective cohort study in 28 community and teaching EDs. Patients with a clinical and radiographic diagnosis of CAP were enrolled. We stratified MR-proADM levels a priori into quartiles and quantified severity of illness using the pneumonia severity index (PSI); and confusion (abbreviated mental test score of ≤ 8), urea ≥ 7 mmol/L, respiratory rate ≥ 30 breaths/min, BP < 90 mm Hg systolic or < 60 mm Hg diastolic, age ≥ 65 years (CURB-65). The primary outcome was 30-day mortality.
Results:
A total of 1,653 patients formed the study cohort. MR-proADM levels consistently rose with PSI class and 30-day mortality (p < 0.001). MR-proADM had a higher area under the curve for 30-day mortality than procalcitonin (0.76 vs 0.65, respectively; p < 0.001), but adding MR-proADM to the PSI in all subjects minimally improved performance. Among low-risk subjects (PSI classes I to III), mortality was low and did not differ by MR-proADM quartile. However, among high-risk subjects (PSI class IV/V; n = 546), subjects in the highest MR-proADM quartile (n = 232; 42%) had higher 30-day mortality than those in MR-proADM quartiles 1 to 3 (23% vs 9%, respectively; p < 0.0001). Similar results were seen with CURB-65. MR-proADM and procalcitonin levels were generally concordant; only 6% of PSI class IV/V subjects in the highest MR-proADM quartile had very low procalcitonin levels (< 0.1 ng/mL).
Conclusions:
In our multicenter CAP cohort, MR-proADM levels correlate with increasing severity of illness and death. High MR-proADM levels offer additional risk stratification in high-risk CAP patients, but otherwise MR-proADM levels do not alter PSI-based risk assessment in most CAP patients.
The search for biomarkers that alter prognostic ability in pneumonia and sepsis has not yet produced a reliable tool. One biomarker of interest is midregional proadrenomedullin (MR-proADM), a more stable, easier to measure, stoichiometrically equivalent, midregion fragment of the parent precursor of adrenomedullin.1 Adrenomedullin is a peptide produced by multiple tissue types during physiologic stress, and has pluripotent function, including vasodilatory, antimicrobial, and antiinflammatory activity.2 In animal models of sepsis,3–5 exogenous adrenomedullin reduces acute lung injury, vascular permeability, and death; endogenous overexpression similarly ameliorates the septic insult. Human data are sparse, but early studies6,7 reported high adrenomedullin levels associated with increased vasodilation and severity of illness in systemic inflammation and septic shock.
In 2006, Christ-Crain et al8 reported in a single-center cohort of Swiss ED patients with community-acquired pneumonia (CAP) that MR-proADM slightly altered the prognostic accuracy of an established CAP clinical prediction rule (pneumonia severity index [PSI]9) and seemed to be a useful risk stratification tool. However, small sample size and high frequency of severe CAP (PSI classes IV/V) limited these findings, leaving the opportunity for clarity in a larger, more diverse CAP population.
We therefore examined MR-proADM levels in a multicenter cohort of ED patients with CAP. We sought to (1) describe the pattern of initial MR-proADM in CAP and (2) confirm the potential prognostic role in CAP. We also compared the prognostic performance of MR-proADM, the PSI, and procalcitonin, a novel biomarker of infection. We have previously examined the PSI and procalcitonin prognostic abilities.10
Materials and Methods
Study Design and Setting
We conducted a multicenter, prospective cohort study of patients presenting to the EDs of 28 teaching and nonteaching hospitals in southwestern Pennsylvania, Connecticut, southern Michigan, and western Tennessee between November 2001 and November 2003 (Genetic and Inflammatory Markers of Sepsis [GenIMS]). GenIMS investigated the relationship between inflammatory molecule expression, clinical course, and outcome in patients with CAP and sepsis. As part of this aim, we sought to determine the pattern and potential prognostic role of initial MR-proADM in CAP.
Selection of Participants
Eligible subjects were ≥ 18 years old with both a clinical and radiologic ED diagnosis of pneumonia as per Fine et al.9 We excluded those subjects who had transferred from another hospital, been discharged from a hospital within the prior 10 days, had experienced a previous episode of pneumonia within the past 30 days, were receiving long-term mechanical ventilation, had cystic fibrosis, had active pulmonary tuberculosis, had a known positive HIV antibody titer, had alcoholism with evidence of end-organ damage, had been admitted to the hospital for palliative care, had been enrolled previously in GenIMS, were incarcerated, or were pregnant. We obtained informed consent from the subjects or their legally authorized representative. The institutional review boards of the University of Pittsburgh and all participating sites approved the study.
Data Collection and Processing
We gathered baseline and sequential clinical information by structured patient or proxy interviews, bedside assessment by study nurses, and standardized medical record reviews. We did not obtain presenting day blood samples from patients presenting after 11:00 pm or on weekends and holidays for logistical reasons; all other patients had blood sampled as soon as possible after consent. Study personnel anticoagulated blood samples with heparin and separated plasma by centrifugation within 1 h of collection. The immediately frozen plasma was shipped on dry ice to a central laboratory where it was stored at −80°C. We tracked clinical data and blood samples using unique anonymized identification numbers, merging data only after assay completion. We observed strict data confidentiality and audited clinical data and assays for accuracy, including random chart audits, repeat blood assays, and computer flags for inconsistencies.
Methods of Measurement
We measured MR-proADM using a new sandwich immunoassay (MR-proADM; BRAHMS; Hennigsdorf, Germany).11 The assay has a functional assay sensitivity of 0.12 nmol/L. Study nurses ascertained deaths in the hospital. Post-hospital discharge deaths were ascertained by telephone and a National Death Index search. We enrolled subjects between November 2001 and November 2003, locked clinical data in 2004, completed assays in 2005, and petitioned complete National Death Index data when it became available in 2006. We prospectively assessed severity of illness using PSI, and a priori stratified MR-proADM into quartiles.12
Outcome Measures
Our primary outcome was 30-day mortality, the traditional end point used for clinical prediction rules in CAP, including the PSI. Secondary outcomes included 90-day and 48-day mortality, the latter to facilitate comparison with the primary mortality outcome in the study by Christ-Crain et al.8
Statistical Analysis
We used the Jonckheere-Terpstra test to determine the trend among levels of MR-proADM, temperature, and leukocyte count across PSI class. We also generated descriptive data, comparing initial presentation and outcome measures across MR-proADM quartiles. To test for differences across MR-proADM quartiles for continuous variables, we used the Kruskal-Wallis test. To test for trends across MR-proADM quartiles for ordinal variables, we used the Jonckheere-Terpstra trend test. We considered a p value < 0.05 as statistically significant.
We generated Kaplan-Meier plots stratified by MR-proADM quartile and used log-rank testing to test for difference. We generated receiver operating characteristic curves for 30-day mortality, for MR-proADM and PSI alone, and then together in a combined model. We also evaluated the test characteristics of the Christ-Crain et al8 proposed an optimal cutoff MR-proADM value of 1.8 nmol/L to predict mortality. Last, to understand potential MR-proADM independent prognostic values beyond PSI, we generated Kaplan-Meier plots, stratified by MR-proADM quartile within each PSI class. We defined low risk as PSI classes I to III, and high risk as PSI classes IV and V.8 We used the same approach to determine the prognostic value of adding MR-proADM to confusion (abbreviated mental test score of ≤ 8), urea ≥ 7 mmol/L, respiratory rate ≥ 30 breaths/min, BP < 90 mm Hg systolic or < 60 mm Hg diastolic, age ≥ 65 years (CURB-65) score, and defined low risk as CURB-65 group 1 and high risk as CURB-65 groups 2 and 3.13
To compare the prognostic performance of MR-proADM and procalcitonin, we used the Wald statistic to test for differences in the area under the curve (AUC) for each biomarker for 30-day mortality prediction. We also compared MR-proADM quartile vs procalcitonin tier (I, < 0.1; II, ≥ 0.1, < 0.25; III, ≥ 0.25, < 0.5; IV, ≥ 0.5 ng/mL),10 for all subjects and for the high-risk subjects in particular, to understand the similarities and differences between the two biomarkers.
Results
Baseline Characteristics
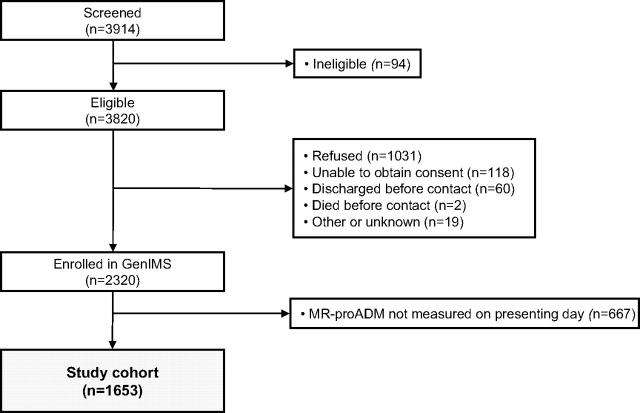
Of the 2,320 subjects enrolled in GenIMS, 1,653 (71.3%) had an MR-proADM level measured on the presenting day and formed the study cohort (Fig 1). Median time from ED admission to first blood sample collection was 1.3 h. Table 1 lists the baseline characteristics of the study cohort. We had complete ED PSI data in 1,385 of the 1,653 cohort subjects (84%). Subjects were predominantly white, and 39% were identified as being at high risk by PSI, with most subjects admitted to the hospital. The mean age was 65 years (SD, 18.5) and one-half were men. Median MR-proADM and interquartile range (IQR) at presentation were 1.0 nmol/L (IQR, 0.7 to 1.5 nmol/L).
Figure 1.
Flow diagram of study.
Table 1.
Study Cohort Baseline Characteristics
| Variables | Value (n = 1,653) |
|---|---|
| Age, yr* | 65.0 (18.5) |
| Male sex | 862 (52) |
| Race | |
| White | 1,336 (81) |
| Black | 229 (14) |
| Other | 88 (5) |
| Prior antibiotics | 271 (16) |
| Charlson comorbidity index score < 0 | 1,095 (66) |
| Laboratory findings | |
| MR-proADM, nmol/L | 1.0 (0.7–1.5) |
| Procalcitonin, μg/L | 0.2 (0.08–1.3) |
| Leukocyte count, cells ×109* | 11.9 (12.0) |
| PSI | |
| Mean (SD) | 82.0 (34.2)† |
| Class | |
| I, II | 542 (39) |
| III | 297 (21) |
| IV | 419 (30) |
| V | 127 (9) |
| Received appropriate antibiotics‡ | 1,280 (77) |
| ED discharges | 266 (16) |
| Mortality, % (end point) | 6.4 (30 d), 7.2 (48 d), 9.8 (90 d) |
Values are given as No. (%) or median (IQR), unless otherwise indicated.
*Values are given as the mean (SD).
†PSI was measured in the ED in 1,385 subjects (84%). There were no significant differences between subjects who did and did not have a PSI measured.
‡Appropriate antibiotics were administered within the first 24 h of presentation, as per the 2001 American Thoracic Society guidelines.18
MR-proADM and CAP Severity
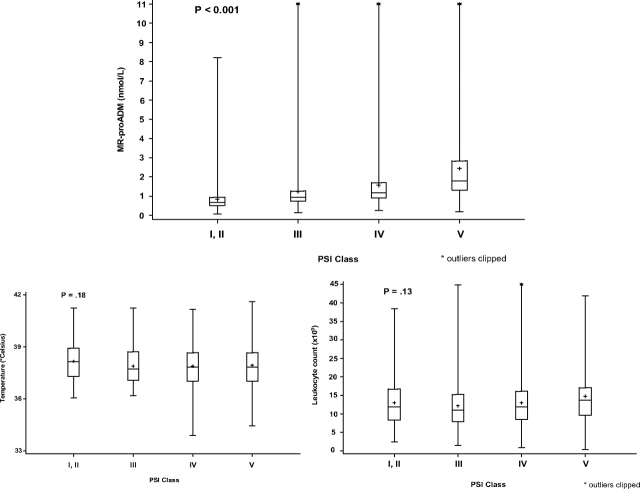
MR-proADM levels consistently rose as PSI class advanced from I/II to V (p < 0.001) [Fig 2]. Differences across PSI class were not significant for temperature (p = 0.18) and leukocyte count (p = 0.13). In MR-proADM quartile 1, 73% of subjects were in PSI class I/II; in MR-proADM quartile 4, 70% were in PSI class IV/V (Table 2). As MR-proADM quartile rose, length of stay and the proportions of subjects who were admitted to the ICU, received vasopressors, and had a clinically significant positive culture finding also rose.
Figure 2.
Distribution of MR-proADM levels and other markers by PSI class. Lower and upper limits of boxes indicate the 25th and 75th percentiles; horizontal lines indicate the 50th percentiles, and crosses (+) within boxes indicate the mean values. The lines extending from the boxes indicate the entire range of values, except for four outlier values in the MR-proADM box plots (14.7, 15.4, 16.9, and 20.9 nmol/L) and two outlier values in the leukocyte count box plots (63/mL, 359/mL). The extreme leukocyte count outlier (359/mL) was excluded from box-plot analyses.
Table 2.
Initial Presentation and Outcomes, by MR-proADM Quartile
| MR-proADM Quartile |
||||||
|---|---|---|---|---|---|---|
| Initial presentation | p Value* | All1,653 (100%) | I (≤ 0.669 nmol/L) | II (> 0.669,≤ 0.965 nmol/L) | III (> 0.965,≤ 1.45 nmol/L) | IV (> 1.45 nmol/L) |
| PSI† | ||||||
| Mean (SD) | < 0.0001 | 82.0 (34.2) | 56.8 (27.1) | 76.1 (27.4) | 89.5 (27.5) | 107.9 (32.6) |
| Class | ||||||
| I, II | 542 | 268 (49) | 144 (27) | 85 (16) | 45 (8) | |
| III | 297 | 54 (18) | 97 (33) | 91 (31) | 55 (19) | |
| IV | 419 | 38 (9) | 90 (22) | 147 (35) | 144 (34) | |
| V | 127 | 6 (5) | 10 (8) | 23 (18) | 88 (69) | |
| CURB-65 | ||||||
| Mean (SD) | < 0.0001 | 1.6 (1.2) | 0.7 (0.9) | 1.3 (1.0) | 1.8 (1.1) | 2.5 (1.0) |
| Group | ||||||
| 1 | 827 | 344 (42) | 261 (32) | 152 (18) | 70 (8) | |
| 2 | 422 | 50 (12) | 102 (24) | 145 (34) | 135 (30) | |
| 3 | 404 | 20 (5) | 50 (12) | 116 (29) | 218 (54) | |
| Prior antibiotics | 0.70 | 271 (16) | 63 (15) | 78 (19) | 69 (17) | 61 (15) |
| Preenrollment duration of symptoms, d‡ | < 0.0001 | 4.7 (14.5) | 5.2 (9.9) | 4.9 (7.5) | 4.3 (18.5) | 4.4 (18.5) |
| Procalcitonin, ng/mL‡ | < 0.0001 | 3.4 (16.5) | 1.2 (8.8) | 1.2 (5.5) | 2.4 (6.1) | 8.7 (30.1) |
| Outcomes | ||||||
| 30-d mortality | < 0.0001 | 106 (6) | 4 (1) | 14 (3) | 24 (6) | 64 (15) |
| 48-d mortality | < 0.0001 | 119 (7) | 4 (1) | 16 (4) | 28 (7) | 71 (17) |
| 90-d mortality | < 0.0001 | 161 (10) | 6 (1) | 24 (6) | 39 (9) | 92 (22) |
| Hospital LOS§ | < 0.0001 | 6.2 (5.3)/5.0 | 3.9 (3.7)/3.0 | 5.6 (4.9)/5.0 | 6.8 (4.7)/6.0 | 8.7 (6.4)/7.0 |
| ICU admission | < 0.0001 | 215 (13) | 20 (5) | 27 (7) | 57 (14) | 111 (27) |
| Vasopressor use | < 0.0001 | 22 (1) | 2 (0.5) | 1 (0.2) | 3 (1) | 16 (4) |
| Clinically significant culture‖ | < 0.0001 | 215 (13) | 31 (7) | 45 (11) | 50 (12) | 89 (22) |
| Bacteremia | < 0.0001 | 107 (6) | 7 (2) | 19 (5) | 26 (6) | 55 (13) |
Values are given as No. (%), unless otherwise indicated.
*Test for trend across quartiles, using the Jonckheere-Terpstra test for trend for ordinal variables, and test for differences across quartiles using the Kruskal-Wallis test for continuous variables. LOS = length of stay (admitted patients).
†PSI was measured in the ED in 1,385 subjects (84%). There were no significant differences between subjects who did, and did not, have a PSI measured.
‡Values are given as the mean (SD).
§Values are given as the mean (SD)/median.
‖We defined a clinically significant culture result according to published guidelines and previous literature.18–25 For example, single blood cultures that yielded coagulase-negative staphylococci sputum culture findings that yielded normal oral flora were not counted as being clinically significant.
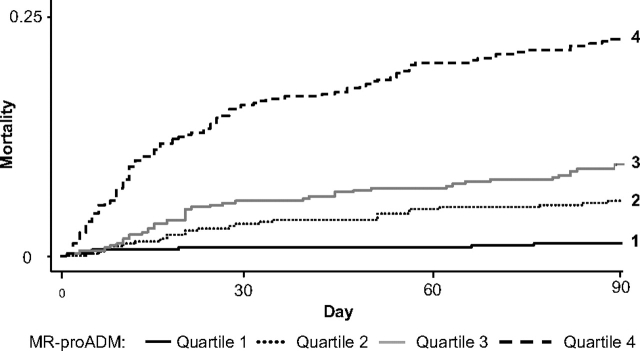
MR-proADM as a Prognostic Marker for Mortality
MR-proADM quartile strongly correlated with mortality, showing consistent progression across quartiles (Fig 3, Table 2). Median MR-proADM levels were higher (p < 0.0001) in 30-day nonsurvivors (1.6 nmol/L; IQR, 1.1 to 2.8 nmol/L) vs survivors (0.9 nmol/L; IQR, 0.7 to 1.4 nmol/L).
Figure 3.
Kaplan-Meier survival curves by MR-proADM quartile.
Receiver Operating Characteristic Curve Analyses
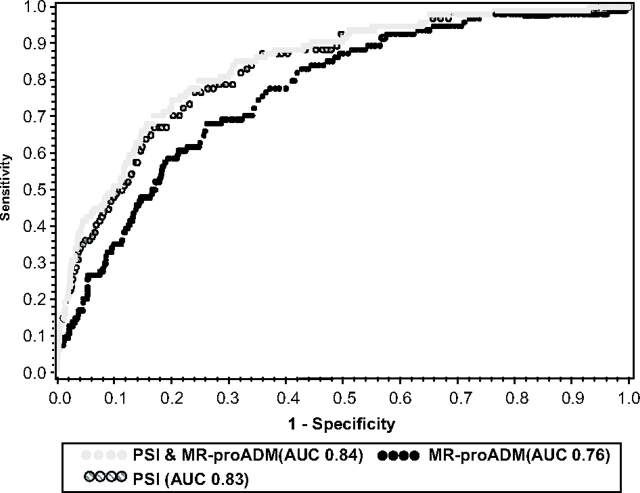
For our primary outcome of 30-day mortality, the AUC for MR-proADM was 0.76. Testing the 1.8 nmol/L cutoff proposed by Christ-Crain et al,8 we found a sensitivity of 0.46, specificity of 0.86, positive likelihood ratio of 3.3, and negative likelihood ratio of 0.6. Our optimal prognostic cutoff (maximum combined sensitivity and specificity) for MR-proADM was 1.3 nmol/L, with a sensitivity of 0.68, specificity of 0.73, positive likelihood ratio of 2.5, and negative likelihood ratio of 0.44.
The AUC of a combined model using MR-proADM and PSI together was 0.84 and did not differ from the AUC of PSI alone (0.83) [Fig 4]. Similar AUC results were seen for a combined model using MR-proADM and CURB-65 (0.80), and CURB-65 alone (0.78). Similar test characteristics were seen for 48-day and 90-day mortality.
Figure 4.
Receiver operating characteristic curve analysis of MR-proADM, PSI, and a combined model of MR-proADM and PSI. Outcome is 30-day mortality. Adding MR-proADM to PSI did not significantly increase the AUC.
MR-proADM Stratification Within PSI Class
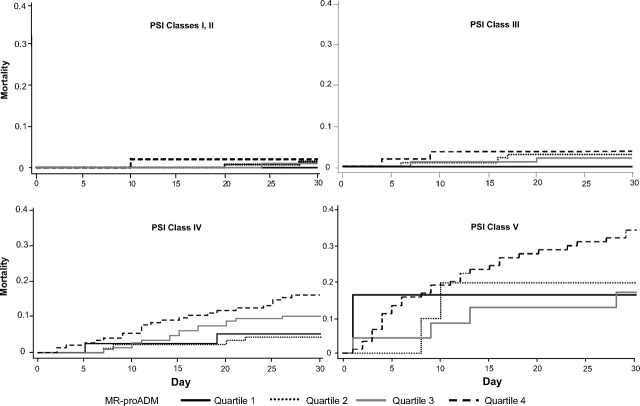
In PSI classes I to III, mortality rates were low and did not significantly differ across MR-proADM quartiles (Fig 5, Table 2). However, within PSI classes IV/V (n = 546), subjects in the highest MR-proADM quartile (n = 232; 42%) had a higher 30-day mortality rate vs those in MR-proADM quartiles 1 to 3 (23% vs 9%, respectively; p < 0.0001). Differences were also significant at 48 days (26% vs 10%, respectively; p < 0.0001) and 90 days (33% vs 14%, respectively; p < 0.0001). Similar results were seen with CURB-65. In CURB-65 group 1, mortality rates were low for all MR-ProADM quartiles; within CURB-65 groups 2 and 3 (n = 826), subjects in the highest MR-proADM quartile (n = 353; 43%) had a higher 30-day mortality rate vs those in MR-proADM quartiles 1 to 3 (18% vs 6%, respectively; p < 0.0001).
Figure 5.
Kaplan-Meier survival curves by PSI class and MR-proADM quartile. In PSI classes I to III, mortality was low, and stratification by MR-proADM quartile did not provide additional information. In PSI classes IV/V, patients in the highest proADM quartile had the highest mortality.
Low MR-proADM values (quartile 1) had a limited negative predictive value among subjects in PSI classes IV/V. This occurred rarely (8%; 44 of 546 subjects), and the 30-day mortality rate in these subjects, although below that predicted by PSI, was still clinically important (7%; 3 of 44 subjects).
MR-proADM vs Procalcitonin
The AUC for 30-day mortality for MR-proADM was higher than that for procalcitonin (0.76 vs 0.65, respectively; p < 0.001). The two biomarkers were generally concordant (Table 3). Among subjects in MR-proADM quartile 1, 61% were in procalcitonin tier 1; among subjects in MR-proADM quartile 4, 66% were in procalcitonin tier 4. Similar patterns were seen when restricted only to the high-risk PSI class IV/V subjects. Notably, only 6% of the PSI IV/V subjects in the highest MR-proADM quartile were in the lowest procalcitonin tier.
Table 3.
MR-proADM Quartile vs Procalcitonin Tier
| MR-proADM Quartile |
||||
|---|---|---|---|---|
| Variables | I (≤ 0.669 nmol/L) | II (< 0.669, ≤ 0.965 nmol/L) | III (> 0.965, ≤ 1.45 nmol/L) | IV (> 1.45 nmol/L) |
| All patients (n = 1,653) | ||||
| Procalcitonin tier, ng/mL | ||||
| I (< 0.1) | 251 (61) | 162 (39) | 107 (26) | 24 (6) |
| II (≥ 0.1, < 0.25) | 90 (22) | 103 (25) | 99 (24) | 64 (16) |
| III (> 0.25, < 0.5) | 26 (6) | 53 (13) | 38 (9) | 52 (13) |
| IV (> 0.5) | 47 (11) | 95 (23) | 169 (41) | 273 (66) |
| PSI IV/V patients (n = 546) | ||||
| Procalcitonin tier, ng/mL | ||||
| I (> 0.1) | 23 (52) | 39 (39) | 50 (29) | 14 (6) |
| II (≥ 0.1, > 0.25) | 13 (30) | 23 (23) | 48 (28) | 36 (16) |
| III (> 0.25, < 0.5) | 4 (9) | 11 (11) | 17 (10) | 36 (16) |
| IV (> 0.5) | 4 (9) | 27 (27) | 55 (32) | 146 (63) |
Values are given as No. (%).
Discussion
Our findings confirm the prognostic value of MR-proADM in patients with CAP, but we also note important differences from the original Swiss study. Although we also found that MR-proADM strongly correlated with PSI and mortality, we identified a lower optimal cutoff value in our study cohort. Furthermore, we observed that the prognostic utility of MR-proADM beyond PSI was limited to high-risk subjects.
Differences in study cohort and methodology may explain the differing findings. Our study cohort had a more broadly spread level of CAP severity as measured by PSI class distribution than the Swiss cohort, especially more class I to III patients who may better represent ED CAP populations. Christ-Crain et al8 also used “failure at follow-up” as a primary outcome, defining failure as a combination of death, recurrence, or persistence of CAP. Using a combined end point created a higher event rate and may have improved test characteristic results, although the combined events clearly vary widely in impact despite being analyzed together. For mortality alone, although our end points differed from theirs (30-day vs at follow-up, respectively), we found identical AUC results for MR-proADM.
Although the previous authors found a very small prognostic benefit of MR-proADM when added to the PSI based on AUC evaluation, we believe the absence of that same enhancement in our study is not a meaningful conflict. Specifically, their incremental AUC increase (0.73 to 0.77) does not largely differ from our observation (0.83 to 0.84). Last, our larger, multicenter cohort improved generalizability and allowed us to perform within-PSI class analyses.
Although adrenomedullin appears protective in animal models of infection, we found that subjects in the highest MR-proADM quartile had the highest mortality, most notably subjects already judged to be high risk. Previous laboratory work14 examining adrenomedullin in cardiovascular disease also found that adrenomedullin has beneficial properties, whereas clinical cardiovascular studies12,15,16 showed that high levels of adrenomedullin and MR-proADM were associated with worse patient outcomes. Similar to our results, Khan et al12 reported that in acute myocardial infarction, subjects in the highest MR-proADM quartile had the highest mortality, especially in those subjects with an elevated pro-B-type natriuretic peptide level. Elevated MR-proADM levels have also been associated with increased mortality in patients experiencing acute exacerbations of COPD.17 This seeming dichotomy likely represents a “fire vs firefighter” epiphenomenon, where the proportional presence of firefighters at a fire reflects their protective, rather than causative, role. The high MR-proADM levels seen with increased illness severity and mortality may represent an endogenous, protective role for adrenomedullin. In particular, adrenomedullin may decrease infection-induced organ damage,5 inflammation, and apoptosis.3 Our observational design cannot unravel this protective role, yet we confirm previous observations that high MR-proADM levels add a prognostic marker in patients clinically deemed to be high risk for death.
We sought to examine MR-proADM prognostic ability, alone and in conjunction with the PSI score. Although MR-proADM performed similarly to PSI in receiver operating characteristic curve analyses, it is unknown whether MR-proADM could serve as a substitute for PSI. Clearly, PSI use in daily practice can be limited by clinician recall, lack of implementation tool availability, and appropriate application. Biomarkers may provide objective, individualized, prognostic information not subject to physician recall or bias, yet limited data support this notion.
Both MR-proADM and procalcitonin correlate with CAP severity and mortality, and although MR-proADM performs better as a stand-alone test in receiver operating characteristic curve analyses, when added to established clinical prediction rules, neither substantially improves prediction rule performance when applied to all patients.10 However, Kaplan-Meier curves stratified by PSI and biomarker levels yield intriguing observations. Neither biomarker could differentiate mortality among low-risk patients, whereas in high-risk patients both biomarkers showed significant, yet unique differentiation, with the highest levels of MR-proADM associated with the highest mortality, and the lowest levels of procalcitonin associated with the lowest mortality. However, because the two biomarkers are rarely discordant at the extremes, their incremental value to each other appears low. Future work should examine models incorporating MR-proADM, procalcitonin, other potential biomarkers of infection, and the individual elements of established clinical prediction rules, and attempt to create a de novo risk stratification tool with greater prognostic power and ease of use.
Our study has limitations. First, we did not track the recurrence or persistence of CAP and could not examine the combined end point of failure of the study by Christ-Crain et al.8 Second, the identification of additional risk in a patient already deemed to be at high risk limits the overall clinical value. Last, we did not study MR-proADM serially, and we cannot comment on the value of that strategy in detecting subclinical deterioration, guiding care, or enhancing prognosis.
Conclusions
In our multicenter CAP cohort, MR-proADM level correlated with severity of illness and death. High MR-proADM levels offer additional risk stratification in high-risk CAP patients, but otherwise MR-proADM levels do not alter PSI-based risk assessment in most CAP patients.
Acknowledgments
Author contributions: Drs. Huang, Angus, Weissfeld, Kellum, and Yealy conceived and designed the study, analyzed and interpreted the data, and provided important critical revisions of the article. Drs. Huang, Angus, and Yealy drafted the article. Drs. Huang, Angus, Kellum, and Yealy provided final approval. Dr. Weissfeld and Mr. Pugh provided statistical expertise, and they contributed to the analysis and interpretation of the data and the drafting of the article. Dr. Struck provided technical expertise regarding the MR-proADM assay and contributed to the drafting of the article. Drs. Angus and Kellum provided administrative, technical, and logistic support. Dr. Delude provided laboratory expertise and contributed to the drafting of the article. Dr. Rosengart contributed to the drafting of the article. All authors read and approved the final manuscript.
Financial/nonfinancial disclosures: Drs. Huang, Weissfeld, Kellum, and Angus have consulted for BRAHMS Diagnostica, the manufacturer of the MR-proADM assay used in this study. Dr. Huang has received one speaking honorarium and travel expenses for a presentation at a satellite symposium of the September 2006 European Respiratory Society Meeting in Munich, Germany. Dr. Struck is an employee of BRAHMS Diagnostica. BRAHMS Diagnostica covered sample shipping and MR-proADM assay costs; no direct monetary grant support was provided. Mr. Pugh and Drs. Struck, Delude, and Rosengart have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank the patients for their participation in this study. We are also indebted to the investigators, coordinators, clinical, laboratory, and research personnel at each of the participating hospitals for their efforts. A complete list of GenIMS investigators is available at http://www.ccm.upmc.edu/genims_investigators.
Abbreviations:
- AUC
area under the curve
- CAP
community-acquired pneumonia
- CURB-65
confusion (abbreviated mental test score of ≤ 8), urea ≥ 7 mmol/L, respiratory rate ≥ 30 breaths/min, BP < 90 mm Hg systolic or < 60 mm Hg diastolic, age ≥ 65 years
- GenIMS
Genetic and Inflammatory Markers of Sepsis
- IQR
interquartile range
- MR-proADM
midregional proadrenomedullin
- PSI
pneumonia severity index
Footnotes
Funding/Support: Funding for this research was received from National Institute of General Medical Sciences grant No. R01 GM061992.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.xhtml).
References
- 1.Struck J, Tao C, Morgenthaler NG, et al. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25:1369–1372. doi: 10.1016/j.peptides.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Beltowski J, Jamroz A. Adrenomedullin: what do we know 10 years since its discovery? Pol J Pharmacol. 2004;56:5–27. [PubMed] [Google Scholar]
- 3.Itoh T, Obata H, Mmurakami S, et al. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;293:L446–L452. doi: 10.1152/ajplung.00412.2005. [DOI] [PubMed] [Google Scholar]
- 4.Temmesfeld-Wollbruck B, Brell B, David I, et al. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. 2007;33:703–710. doi: 10.1007/s00134-007-0561-y. [DOI] [PubMed] [Google Scholar]
- 5.Shindo T, Kurihara H, Maemura K, et al. Hypotension and resistance to lipopolysaccharide-induced shock in transgenic mice overexpressing adrenomedullin in their vasculature. Circulation. 2000;101:2309–2316. doi: 10.1161/01.cir.101.19.2309. [DOI] [PubMed] [Google Scholar]
- 6.Nishio K, Akai Y, Murao Y, et al. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit Care Med. 1997;25:953–957. doi: 10.1097/00003246-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Ueda S, Nishio K, Minamino N, et al. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160:132–136. doi: 10.1164/ajrccm.160.1.9810006. [DOI] [PubMed] [Google Scholar]
- 8.Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTNO4176397] Crit Care. 2006;10:R96. doi: 10.1186/cc4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 10.Huang DT, Weissfeld LA, Kellum JA, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–58. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenthaler NG, Struck J, Alonso C, et al. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 12.Khan SQ, O'Brien RJ, Struck J, et al. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2007;49:1525–1532. doi: 10.1016/j.jacc.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eto T, Kitamura K, Kato J. Biological and clinical roles of adrenomedullin in circulation control and cardiovascular diseases. Clin Exp Pharmacol Physiol. 1999;26:371–380. doi: 10.1046/j.1440-1681.1999.03047.x. [DOI] [PubMed] [Google Scholar]
- 15.Gegenhuber A, Struck J, Dieplinger B, et al. Comparative evaluation of B-type natriuretic peptide, mid-regional pro-A-type natriuretic peptide, mid-regional pro-adrenomedullin, and Copeptin to predict 1-year mortality in patients with acute destabilized heart failure. J Card Fail. 2007;13:42–49. doi: 10.1016/j.cardfail.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Nishida H, Horio T, Suzuki Y, et al. Plasma adrenomedullin as an independent predictor of future cardiovascular events in high-risk patients: comparison with C-reactive protein and adiponectin. Peptides. 2008;29:599–605. doi: 10.1016/j.peptides.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest. 2008;134:263–272. doi: 10.1378/chest.08-0047. [DOI] [PubMed] [Google Scholar]
- 18.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 19.Richter SS, Beekmann SE, Croco JL, et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol. 2002;40:2437–2444. doi: 10.1128/JCM.40.7.2437-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein MP, Murphy JR, Reller LB, et al. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults; II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5:54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- 21.Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 22.Graham JC, Galloway A. ACP Best Practice No 167: the laboratory diagnosis of urinary tract infection. J Clin Pathol. 2001;54:911–919. doi: 10.1136/jcp.54.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults: Infectious Diseases Society of America. Clin Infect Dis. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayhall CG. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. Hospital epidemiology and infection control; pp. 1659–1702. [Google Scholar]
- 25.Granato PA. Pathogenic and indigenous microorganisms of humans. In: Murray PR, editor. Manual of clinical microbiology. Washington, DC: American Society for Microbiology Press; 2003. pp. 44–54. [Google Scholar]