Abstract
Background:
The National Emphysema Treatment Trial studied lung volume reduction surgery (LVRS) for its effects on gas exchange, breathing pattern, and dyspnea during exercise in severe emphysema.
Methods:
Exercise testing was performed at baseline, and 6, 12, and 24 months. Minute ventilation (V̇e), tidal volume (Vt), carbon dioxide output (V̇co2), dyspnea rating, and workload were recorded at rest, 3 min of unloaded pedaling, and maximum exercise. Pao2, Paco2, pH, fraction of expired carbon dioxide, and bicarbonate were also collected in some subjects at these time points and each minute of testing. There were 1,218 patients enrolled in the study (mean [± SD] age, 66.6 ± 6.1 years; mean, 61%; mean FEV1, 0.77 ± 0.24 L), with 238 patients participating in this substudy (mean age, 66.1 ± 6.8 years; mean, 67%; mean FEV1, 0.78 ± 0.25 L).
Results:
At 6 months, LVRS patients had higher maximum V̇e (32.8 vs 29.6 L/min, respectively; p = 0.001), V̇co2, (0.923 vs 0.820 L/min, respectively; p = 0.0003), Vt (1.18 vs 1.07 L, respectively; p = 0.001), heart rate (124 vs 121 beats/min, respectively; p = 0.02), and workload (49.3 vs 45.1 W, respectively; p = 0.04), but less breathlessness (as measured by Borg dyspnea scale score) [4.4 vs 5.2, respectively; p = 0.0001] and exercise ventilatory limitation (49.5% vs 71.9%, respectively; p = 0.001) than medical patients. LVRS patients with upper-lobe emphysema showed a downward shift in Paco2 vs V̇co2 (p = 0.001). During exercise, LVRS patients breathed slower and deeper at 6 months (p = 0.01) and 12 months (p = 0.006), with reduced dead space at 6 months (p = 0.007) and 24 months (p = 0.006). Twelve months after patients underwent LVRS, dyspnea was less in patients with upper-lobe emphysema (p = 0.001) and non–upper-lobe emphysema (p = 0.007).
Conclusion:
During exercise following LVRS, patients with severe emphysema improve carbon dioxide elimination and dead space, breathe slower and deeper, and report less dyspnea.
Keywords: cardiopulmonary exercise, COPD, emphysema
COPD markedly impairs exercise performance, especially in those with predominantly emphysema. Emphysema causes decreased lung elastic recoil, which increases expiratory airflow resistance and leads to dynamic hyperinflation.1–3 During exercise, dynamic hyperinflation progresses rapidly, decreasing chest wall compliance and impairing respiratory muscle function.3–9 Dynamic hyperinflation and an elevated work of breathing precipitate breathlessness, thereby decreasing exercise tolerance and quality of life.1,10,11
Lung volume reduction surgery (LVRS) increases lung elastic recoil12 and decreases end-expiratory lung volume,3,6,13,14 thereby improving lung15–18 and respiratory muscle mechanics5,7 and overall exercise tolerance.19–24 However, most of the published reports are uncontrolled, unicenter trials involving small numbers of patients with short-term follow-up.3,5,17–19,22,23,25–27
The National Emphysema Treatment Trial (NETT) represents the most extensively characterized patient cohort with severe emphysema undergoing repeated exercise testing. Here we report the effects of optimal medical therapy plus LVRS vs optimal medical therapy alone on maximum exercise after outpatient rehabilitation and through 2 years postrandomization to treatment. Specifically, we assessed the effects of LVRS vs medical treatment on gas exchange, breathing pattern, presence of exercise limitation, and sensation of dyspnea during exercise.
Materials and Methods
The design and methods of NETT have been previously detailed.20 All patients provided written informed consent, and the study was approved by the institutional review board at each center. All 17 NETT centers performed maximum exercise testing at baseline, 6 and 12 months after randomization, and yearly thereafter. Baseline measurements were completed after pulmonary rehabilitation and before randomization, except for Dlco, which was obtained before pulmonary rehabilitation. Five of the 17 centers additionally participated in the exercise substudy. Exercise substudy patients had additional measures collected during maximum exercise testing. There were 1,218 patients randomized in NETT, 608 to LVRS and 610 to medical treatment. Of these patients, 238 also participated in the exercise substudy; 122 were randomized to medical treatment and 116 to LVRS.
Patient Selection
Enrollment criteria for NETT have been previously reported.15 Exercise substudy participants satisfied NETT main study criteria and had no contraindications to exercise testing. Contraindications to exercise testing included unstable angina; lower extremity or back problems that prohibited pedaling; history of syncope, cardiac dysrhythmia, hypoxemia, arterial oxygen saturation (Sao2) < 80% within 2 min of unloaded cycling despite supplemental oxygen; uncontrolled systemic hypertension; bradycardia (< 50 beats/min), multifocal premature ventricular contractions; inability to coordinate a cycle cadence of > 40 revolutions per minute (rpm); fever; or exacerbation of COPD at test time.
Clinical Assessment
Demographic data and medical history were collected using standardized instruments.20 Pulmonary function testing was performed using American Thoracic Society guidelines.28–30 Lung volumes were measured via body plethysmography. Diffusing capacity of the lung for carbon monoxide (Dlco) was measured by the single-breath technique. All pulmonary function measures are postbronchodilator values (except Dlco) and are reported in absolute numbers or as a percentage of normal predicted.31–33 The craniocaudal distribution of emphysema on chest CT scan was classified by radiologists as upper lobe predominant, lower lobe predominant, diffuse, or superior segments of lower lobes predominantly involved; the latter three choices were grouped as non–upper-lobe-predominant.
Cardiopulmonary Exercise Test Setup
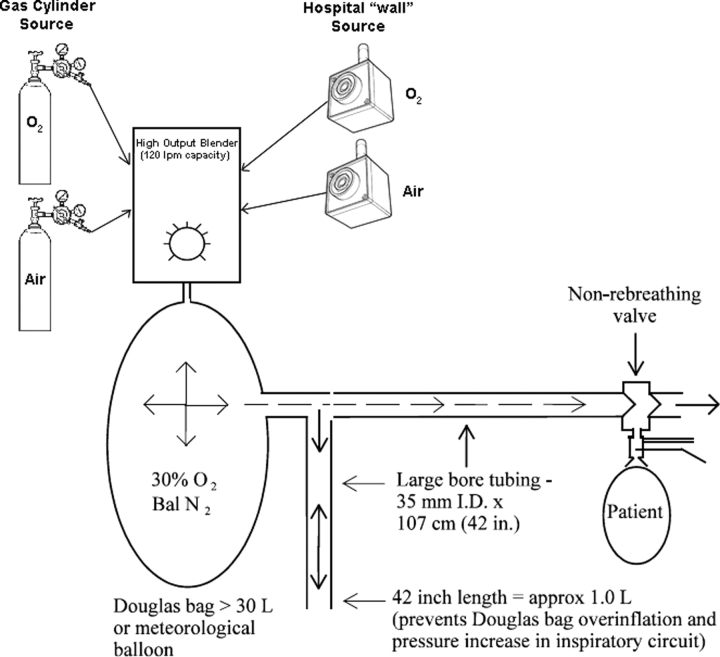
All clinics used an electromagnetically braked lower extremity cycle ergometer that had the capacity to provide ramped workloads at 5 W/min, metabolic cart systems capable of analyzing data in 20-s intervals using breath-by-breath analysis or mixing chamber systems, and continuous ECG and pulse oximetry monitoring. Supplemental oxygen (30%) was provided by a high-flow oxygen blender capable of delivering 100 L/min using a flow-by circuit to the inspiratory port of a unidirectional valve (Fig 1).
Figure 1.
Schematic showing delivery of high-flow 30% inspired oxygen to avoid fluctuations in inspired oxygen concentration at all levels of ventilation encountered during exercise.
Measures Collected During Exercise Testing
Measures were collected after 5 min at rest, after 3 min of unloaded pedaling, and at maximum exercise. Measures included Sao2, minute ventilation (V̇e), tidal volume (Vt), carbon dioxide output (V̇co2), heart rate, respiratory rate, systolic BP, diastolic BP, and modified Borg scale ratings (scale, 0 to 10)34,35 for breathlessness and leg muscle fatigue. Load was reported at maximum exertion. Under the substudy protocol, Paco2, Pao2, pH, fraction of expired carbon dioxide, and Sao2 were also collected after 5 min at rest, after 3 min of unloaded pedaling, after each minute of exertion, and at maximal exercise. Arterial blood samples were timed precisely to expired gas collections and used for dead space calculation.
Exercise Testing Protocol
At least 15 min and no more than 4 h prior to testing, patients received a short-acting inhaled bronchodilator. Before performing any exercise, patients sat for 10 min with a Venturi mask inspiring 30 to 31% oxygen. Patients were then transferred to the cycle ergometer and rested for 5 min prior to beginning pedaling. Patients were then instructed to begin 3 min of unloaded pedaling. Work increments were ramped at 5 W/min in patients with a maximum voluntary ventilation ≤ 40 L/min; 10 W/min work increments were used in patients with maximum voluntary ventilation > 40 L/min. During exercise the patient was instructed to maintain a cadence of 40 to 70 rpm. The test ended when the cadence fell below 40 rpm and did not return with exhortation, the patient requested termination, or the technician terminated the test for safety. Maximum exercise values included maximum watts on the cycle recorded when workload was terminated or cadence dropped below 40 rpm and did not return. All maximum data were recorded from the same 20-s interval. Borg Scale ratings were recorded at maximum exertion. Expired gas measurements were reported from the last 20-s interval of collection before test termination.
Dead Space Calculation
The dead space fraction was calculated using the Enghoff modification of the Borg equation.36 Arterial blood gas determinations and expired carbon dioxide concentrations were obtained at identical time points.
Definitions of Exercise Limitation
Ventilatory limitation was defined as either the presence of a maximum Ve (V̇emax)/maximal ventilatory volume (MVV) ratio ≥ 85, or MVV − V̇emax < 8 L. Cardiovascular limitation was defined as 100 × heart rate/(220 − age) ≥ 90 for men, and as 100 × heart rate/(226 − age) ≥ 90 for women. Patients were then classified as to the presence or absence of ventilatory or cardiac limitations to perform maximum exercise.
Statistical Analysis
Patients who could pedal with the cycle ergometer set at 0 W only were considered to have a maximum workload of 0 W, and values measured at unloaded pedaling were considered to be measures at maximum effort. Mean values for continuous variables were compared using two-sample t tests; distributions of categorical variables were compared using χ2 tests. Differences between the two treatment groups in the relationship between paired continuous measures from the three sampling times (after 5 min of rest on the mouthpiece, after 3 min of unloaded pedaling, and at maximum effort) were assessed for each follow-up time using generalized estimating equations with robust variance estimation37 to account for the correlation between observations from the same patient. The linear model included the dependent continuous measure as the outcome and terms for treatment group, sampling phase (rest, unloaded, or maximum), and the interactions between these two variables; the p value was derived under the assumption that the treatment and treatment times phase interaction terms were equal to zero. Analyses were limited to patients completing testing at each time point included in the analysis so a patient who missed an assessment was excluded.
Results
Baseline Patient Demographics and Lung Function
In comparison to the total population, patients enrolled in the exercise substudy were more likely to be men, were slightly more hypoxemic at rest, with lower Pao2 and lower Paco2 values, and were less likely to have upper-lobe-predominant emphysema (Table 1).
Table 1.
Baseline Demographics, Respiratory Function, and Emphysema Distribution*
| All Patients |
Substudy Patients |
|||
|---|---|---|---|---|
| Characteristics | LVRS(n = 608) | Medical(n = 610) | LVRS(n = 116) | Medical(n = 122) |
| Age at randomization, yr | 66.5 ± 6.3 | 66.7 ± 5.9 | 66.2 ± 7.2 | 66.0 ± 6.4 |
| Male gender, % | 58.4 | 64.1 | 62.1 | 71.3 |
| BMI, kg/m2 | 24.5 ± 3.7 | 24.7 ± 3.5 | 24.4 ± 3.7 | 24.7 ± 3.6 |
| Pao2, mm Hg | 64.5 ± 10.5 | 64.2 ± 10.1 | 62.9 ± 11.8 | 62.6 ± 10.2 |
| Paco2, mm Hg | 43.3 ± 5.9 | 43.0 ± 5.8 | 41.8 ± 5.2 | 42.4 ± 5.7 |
| PH | 7.42 ± 0.03 | 7.42 ± 0.03 | 7.43 ± 0.03 | 7.42 ± 0.03 |
| FEV1, L | 0.76 ± 0.24 | 0.78 ± 0.24 | 0.76 ± 0.24 | 0.80 ± 0.26 |
| FEV1, % predicted | 26.8 ± 7.4 | 26.7 ± 7.0 | 26.7 ± 7.6 | 27.1 ± 7.1 |
| RV, % predicted | 220.5 ± 49.9 | 223.3 ± 48.9 | 218.3 ± 52.2 | 219.8 ± 49.2 |
| TLC, % predicted | 128.0 ± 15.3 | 128.5 ± 15.0 | 128.3 ± 15.1 | 127.7 ± 14.2 |
| Dlco, % predicted | 28.3 ± 9.7 | 28.4 ± 9.7 | 27.7 ± 10.3 | 29.1 ± 8.9 |
| Hgb, g/dL | 14.4 ± 1.3 | 14.3 ± 1.3 | 14.4 ± 1.5 | 14.2 ± 1.3 |
| Emphysema location,† % | ||||
| Upper lobe | 67.7 ± 21.9 | 69.9 ± 20.8 | 69.1 ± 23.1 | 67.7 ± 22.4 |
| Lower lobe | 49.8 ± 18.4 | 51.5 ± 17.8 | 54.1 ± 18.4 | 52.3 ± 19.0 |
| Total | 55.8 ± 15.9 | 57.6 ± 15.3 | 59.1 ± 15.8 | 57.4 ± 16.8 |
| Upper lobe-predominant,‡ % | 63.3 | 66.5 | 62.1 | 55.4 |
*Values are given as the mean ± SD, unless otherwise indicated. BMI = body mass index; Hgb = hemoglobin; pH = arterial pH; RV = residual volume; TLC = total lung capacity.
†Determined from radiologist scores (0 to 4) for the percentage of emphysema seen on chest CT scans, in each of the upper, middle, and lower zones of each lung. The upper lobe percentage is the mean of the midpoints of the ranges represented by the right and left upper lobe scores. The lower-lobe percentage is the mean of the midpoints of the ranges represented by the right and left middle and lower lobe scores. The total percentage is the mean of the midpoints of the ranges represented by the upper, middle, and lower lobe scores of both lungs.
‡Determined from the radiologist's assessment of the craniocaudal distribution of emphysema seen on chest CT scans. The choices were upper lobe-predominant, lower lobe-predominant, diffuse, or superior segments of lower lobes predominantly involved. The latter three choices were grouped as non–upper lobe-predominant.
Maximum Exercise Outcomes at Baseline and LVRS Effects Postrandomization
At baseline, the medical group achieved higher V̇e and workload during maximum exercise than those randomized to LVRS (p < 0.05 for both measures; Table 2). Six months after randomization the LVRS patients achieved higher V̇e, V̇co2, Vt, heart rate, and workload (p < 0.05 for each measure), and lower Borg score for dyspnea (p = 0.0001) during maximum exercise than those patients assigned to medical therapy. LVRS and medical patients had similar systolic BP, diastolic BP, respiratory rate, and Borg score for leg fatigue.
Table 2.
Measurements During Maximum Exercise at Baseline and 6, 12, and 24 Months Postrandomization to Treatment, for Patients Completing Testing at All Time Points*
| Baseline |
6 mo |
12 mo |
24 mo |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | LVRS(n = 287) | Medical(n = 216) | p Value | LVRS(n = 287) | Medical(n = 216) | p Value | LVRS(n = 287) | Medical(n = 216) | p Value | LVRS(N = 287) | Medical(n = 216) | p Value |
| V̇e, L/min | 28.3 | 30.2 | 0.03 | 32.8 | 29.6 | 0.001 | 31.5 | 29.1 | 0.01 | 29.7 | 27.7 | 0.03 |
| V̇co2, L/m | 0.804 | 0.852 | 0.07 | 0.923 | 0.820 | 0.0003 | 0.890 | 0.807 | 0.005 | 0.831 | 0.759 | 0.01 |
| Vt, L | 1.01 | 1.06 | 0.08 | 1.18 | 1.07 | 0.001 | 1.15 | 1.05 | 0.002 | 1.09 | 1.01 | 0.03 |
| HR, beats/min | 121.5 | 121.0 | 0.75 | 124.4 | 120.7 | 0.02 | 123.9 | 120.7 | 0.03 | 121.6 | 118.8 | 0.06 |
| RR, breaths/min | 29.0 | 29.2 | 0.69 | 28.4 | 28.6 | 0.74 | 28.1 | 28.8 | 0.24 | 28.1 | 28.3 | 0.73 |
| SBP, mm Hg | 189.7 | 189.8 | 0.97 | 187.2 | 184.5 | 0.31 | 188.1 | 184.6 | 0.21 | 185.5 | 183.9 | 0.54 |
| DBP, mm Hg | 94.2 | 93.0 | 0.26 | 91.1 | 92.6 | 0.21 | 91.0 | 92.2 | 0.28 | 90.4 | 90.9 | 0.68 |
| Borg score for dyspnea | 5.0 | 5.0 | 0.97 | 4.4 | 5.2 | 0.0001 | 4.6 | 5.4 | < 0.0001 | 4.9 | 5.3 | 0.05 |
| Borg score for muscle fatigue | 4.1 | 4.4 | 0.20 | 4.5 | 4.7 | 0.31 | 4.5 | 4.7 | 0.46 | 4.6 | 4.8 | 0.31 |
| Load, W | 42.1 | 48.1 | 0.01 | 49.3 | 45.1 | 0.04 | 49.0 | 43.3 | 0.01 | 44.9 | 40.7 | 0.05 |
*The number of individual measures ranged from 277 to 287 for the LVRS group, and from 205 to 216 for the medical group. DBP = diastolic BP; HR = heart rate; RR = respiratory rate; SBP = systolic BP.
Effect of LVRS on Cardiac or Ventilatory Limitations to Perform Maximum Exercise
At baseline, approximately two thirds of patients randomized to the LVRS and medical groups manifested only ventilatory limitation during exercise (Table 3). Approximately 11 to 12% of patients had both ventilatory and cardiac limitation; few patients had only cardiac limitation. Distributions in the treatment groups were similar (p = 0.48). Six months after randomization, the distributions in the treatment groups were different (p = 0.001). In the LVRS group, there was a marked reduction in the percentage of patients who developed ventilatory limitation only. In contrast, the percentage of medical group patients with only ventilatory limitation had increased. These patterns of change were observed in patients with both upper-lobe and non–upper-lobe-predominant emphysema.
Table 3.
Ventilatory and Cardiovascular Limitation During Maximum Exercise at Baseline and 6, 12, and 24 Months Postrandomization to Treatment, by Chest CT Scan Pattern of Emphysema, for Patients with Measures at All Time Points*
| Baseline |
6 mo |
12 mo |
24 mo |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Limitation | LVRS, % | Medical, % | p Value | LVRS, % | Medical, % | p Value | LVRS, % | Medical, % | p Value | LVRS, % | Medical, % | p Value |
| All patients | ||||||||||||
| Ventilatory and cardiovascular | 11.2 | 12.1 | 8.3 | 9.6 | 11.9 | 8.5 | 10.5 | 12.1 | ||||
| Ventilatory only | 67.9 | 62.3 | 49.5 | 71.9 | 54.2 | 67.8 | 60.3 | 64.8 | ||||
| Cardiovascular only | 2.2 | 1.5 | 7.9 | 3.0 | 6.5 | 3.5 | 5.4 | 1.0 | ||||
| Neither | 18.8 | 24.1 | 34.3 | 15.6 | 27.4 | 20.1 | 23.8 | 22.1 | ||||
| Total | 100 | 100 | 0.48 | 100 | 100 | 0.001 | 100 | 100 | 0.03 | 100 | 100 | 0.07 |
| Patients, No. | 277 | 199 | 277 | 199 | 277 | 199 | 277 | 199 | ||||
| MVV, L/min BTPS | ||||||||||||
| Mean | 32.0 | 34.5 | 0.01 | 40.4 | 33.9 | < 0.001 | 38.0 | 33.7 | 0.001 | 35.1 | 33.1 | 0.12 |
| SD | 10.2 | 11.2 | 14.1 | 11.5 | 13.6 | 12.8 | 13.0 | 13.9 | ||||
| Patients with upper lobe-predominant emphysema | ||||||||||||
| Ventilatory and cardiovascular | 12.0 | 10.4 | 6.8 | 8.8 | 11.5 | 8.8 | 10.5 | 13.6 | ||||
| Ventilatory only | 67.0 | 67.2 | 48.2 | 73.6 | 50.8 | 69.6 | 57.1 | 65.6 | ||||
| Cardiovascular only | 1.6 | 0.8 | 7.9 | 2.4 | 8.4 | 1.6 | 7.9 | 0.8 | ||||
| Neither | 19.4 | 21.6 | 37.2 | 15.2 | 29.3 | 20.0 | 24.6 | 20.0 | ||||
| Total | 100 | 100 | 0.87 | 100 | 100 | 0.001 | 100 | 100 | 0.03 | 100 | 100 | 0.02 |
| Patients, No. | 191 | 125 | 191 | 125 | 191 | 125 | 191 | 125 | ||||
| MVV, L/min BTPS | ||||||||||||
| Mean | 31.8 | 34.0 | 0.08 | 41.4 | 33.6 | < 0.001 | 39.2 | 33.6 | 0.001 | 36.5 | 33.3 | 0.06 |
| SD | 10.2 | 11.3 | 14.8 | 12.1 | 14.2 | 13.6 | 13.6 | 15.3 | ||||
| Patients with non—upper-lobe-predominant emphysema | ||||||||||||
| Ventilatory and cardiovascular | 9.3 | 14.9 | 11.6 | 10.8 | 12.7 | 8.1 | 10.5 | 9.5 | ||||
| Ventilatory only | 69.8 | 54.1 | 52.3 | 68.9 | 61.6 | 64.9 | 67.4 | 63.5 | ||||
| Cardiovascular only | 3.5 | 2.7 | 8.1 | 4.1 | 2.3 | 6.8 | 0 | 1.4 | ||||
| Neither | 17.4 | 28.4 | 27.9 | 16.2 | 23.3 | 20.3 | 22.1 | 25.7 | ||||
| Total | 100 | 100 | 0.19 | 100 | 100 | 0.15 | 100 | 100 | 0.42 | 100 | 100 | 0.68 |
| Patients, No. | 86 | 74 | 86 | 74 | 86 | 74 | 86 | 74 | ||||
| MVV, L/min BTPS | ||||||||||||
| Mean | 32.4 | 35.3 | 0.09 | 38.2 | 34.4 | 0.03 | 35.4 | 34.0 | 0.44 | 31.8 | 32.7 | 0.61 |
| SD | 10.2 | 11.2 | 12.0 | 10.5 | 11.9 | 11.6 | 11.0 | 11.3 | ||||
*One patient in the medical group did not have a chest CT scan for classification of the pattern of emphysema. The p value for LVRS and medical groups in the distribution of the type of limitation was determined by χ2 test. The p values for the LVRS and medical groups in mean MVV was determined by t test. BTPS = body temperature and pressure saturated.
Effects of LVRS on Gas Exchange at Rest and During Maximum Exercise in Substudy Patients
The relationship of Paco2 to V̇co2 at rest, during unloaded cycling, and during maximum exercise is shown in Figure 2 for patients with measurements made at baseline and at 6, 12, and 24 months after randomization, by emphysema distribution. At baseline, regardless of emphysema distribution, patients were eucapnic at rest, and they developed mild hypercapnia during unloaded cycling, with sharp and steep increases in Paco2 during maximum exercise. Patients with upper-lobe-predominant emphysema randomized to LVRS had a downward shift in the relationship of Paco2 vs V̇co2 at rest, during unloaded cycling, and during maximum exercise that was evident at 6 months (p = 0.001), and was borderline significant at 12 months (p = 0.07) and 24 months (p = 0.06) compared to medical patients. These differences between treatment groups were not seen during follow-up in the non–upper-lobe-predominant group.
Figure 2.

Impact of upper-lobe vs non-upper-lobe emphysema on the relationship of Paco2 to V̇co2 during restful breathing, unloaded cycling, and maximum exercise at baseline and 6, 12, and 24 months postrandomization to treatment, for substudy patients completing testing at all time points. Error bars show the Paco2 SEM in each phase of testing.
Table 4 shows Pao2 at rest, during unloaded cycling, and at maximum exercise at baseline and 6, 12, and 24 months after randomization for patients tested at all four testing times. There was no difference between treatment groups in level of oxygenation during unloaded pedaling, and maximum exercise at any of the follow-up times.
Table 4.
Pao2 During Restful Breathing, Unloaded Pedaling, and Maximum Exercise at Baseline and at 6, 12, and 24 Months Postrandomization to Treatment, for Patients Completing Testing at All Time Points*
| Baseline |
6 mo |
12 mo |
24 mo |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | LVRS(n = 39) | Medical(n = 32) | p Value | LVRS(n = 39) | Medical(n = 32) | p Value | LVRS(n = 39) | Medical(n = 32) | p Value | LVRS(n = 39) | Medical(n = 32) | p Value |
| Resting | 107.2 | 106.9 | 0.96 | 117.0 | 110.2 | 0.24 | 121.6 | 110.4 | 0.06 | 122.4 | 110.7 | 0.08 |
| Unloaded | 97.6 | 101.6 | 0.49 | 107.8 | 103.9 | 0.51 | 112.0 | 104.6 | 0.25 | 113.0 | 102.3 | 0.10 |
| Maximum | 88.3 | 98.7 | 0.10 | 93.7 | 97.7 | 0.52 | 94.4 | 98.6 | 0.54 | 93.8 | 93.9 | 0.99 |
*Patients inspired 30% or 31% oxygen during testing.
Effects of LVRS on Breathing Pattern at Rest and During Exercise
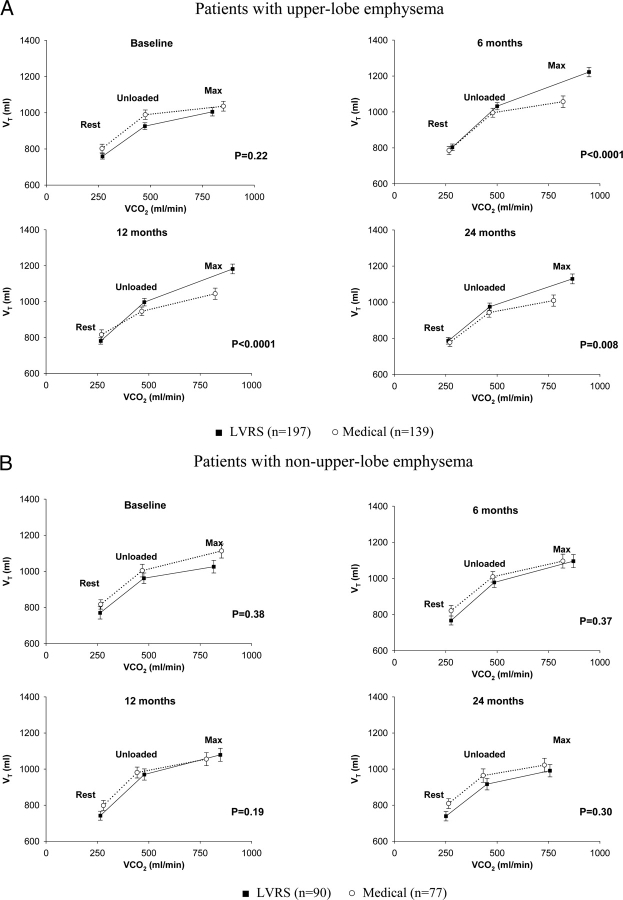
At baseline, LVRS and medical group patients with upper-lobe-predominant emphysema generated similar Vt during unloaded pedaling and at maximum exercise (Fig 3). Six, 12, and 24 months after randomization, LVRS patients with upper-lobe-predominant emphysema generated higher Vt values during unloaded cycling and maximum exercise than similar medical group patients (p < 0.01 for each time point). LVRS and medical group patients with non–upper-lobe-predominant emphysema had similar Vt generation during unloaded pedaling and at maximum exercise at each time point.
Figure 3.
Vt generation vs V̇co2 at rest, unloaded cycling, and maximum exercise at baseline and 6, 12, and 24 months postrandomization to treatment, by chest CT scan pattern of emphysema, for substudy patients completing testing at all time points. Error bars show the Vt SEM in each phase of testing.
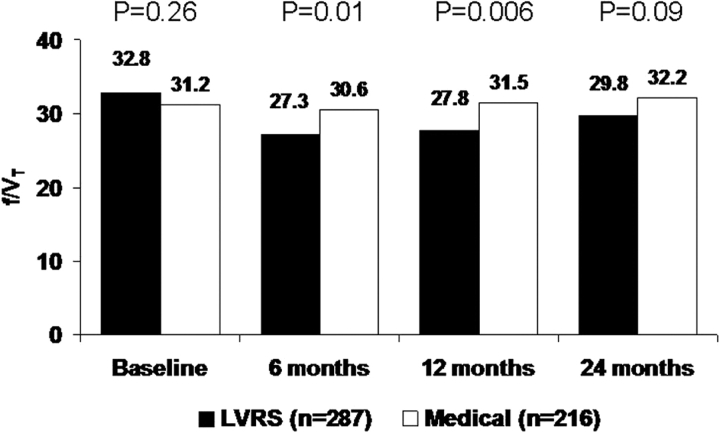
Figure 4 shows the effect of LVRS vs medical therapy on rapid shallow breathing during maximum exercise. Following LVRS, patients breathed deeper and slower at 6 months (p = 0.01) and 12 months (p = 0.006).
Figure 4.
Rapid shallow breathing index (f/Vt) at maximum exercise at baseline and 6, 12, and 24 months postrandomization to treatment, for patients completing testing at all time points.
Effects of LVRS on Ventilatory Dead Space During Maximum Exercise
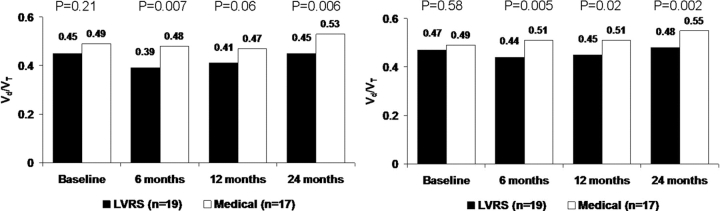
LVRS and medical patients had similar ventilatory dead space levels at baseline (p = 0.21), but LVRS patients had significantly lower levels than medical patients at 6 months (p = 0.007) and 24 months (p = 0.006) [Fig 5, left, A]. When ventilatory dead space was measured at an iso-workload time point (ie, unloaded pedaling) [Fig 5, right, B], LVRS and medical patients had similar ventilatory dead space levels at baseline (p = 0.58), but LVRS patients had significantly lower levels than medical patients at 6 months (p = 0.005), 12 months (p = 0.02), and 24 months (p = 0.002).
Figure 5.
Physiologic dead space ventilation (Vd/Vt) at maximum exercise (left, A) and iso-workload (unloaded cycling) [right, B] at baseline and 6, 12, and 24 months postrandomization to treatment, for patients completing testing at all time points.
Effects of LVRS on Dyspnea at Rest and During Maximum Exercise
At 12 months after randomization, regardless of the CT scan pattern of emphysema, LVRS patients had a significant reduction in breathlessness during unloaded cycling and maximum exercise compared to medical patients (Fig 6).
Figure 6.
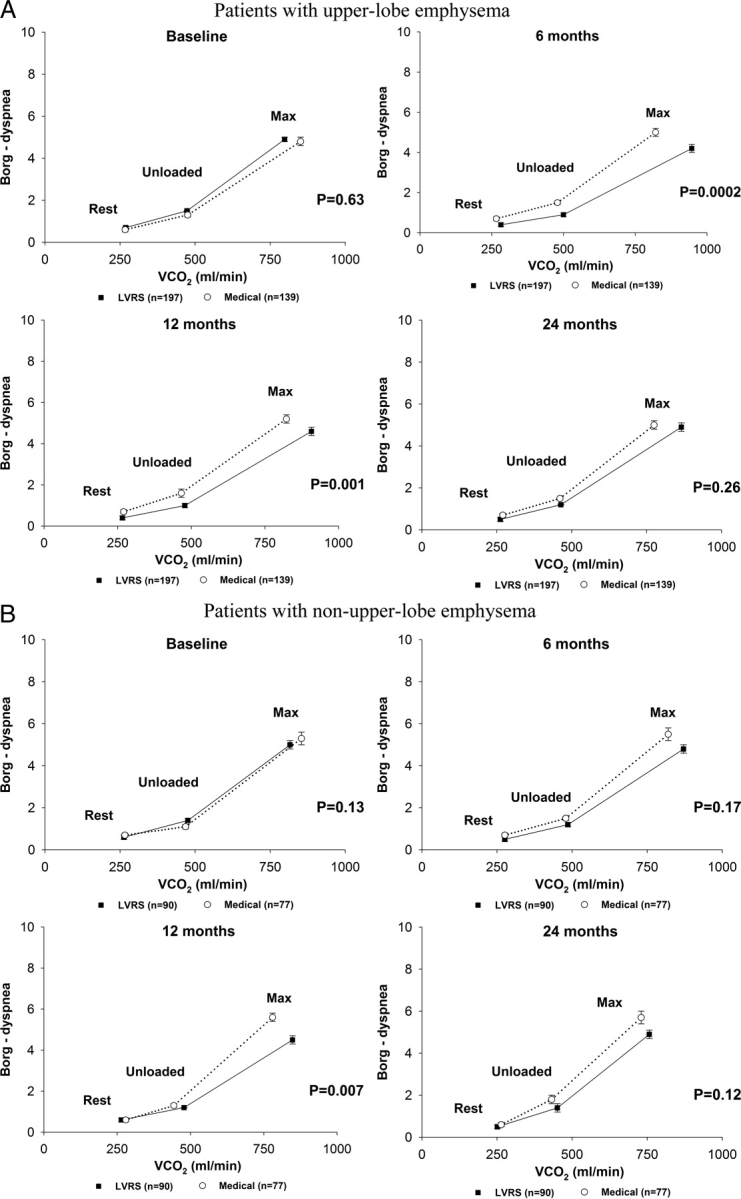
Relationship of Borg score rating of dyspnea to V̇co2 during restful breathing, unloaded pedaling, and maximum exercise at baseline and 6, 12, and 24 months postrandomization to treatment, by chest CT scan pattern of emphysema, for patients completing testing at all time points. Error bars show the SEM Borg score rating of dyspnea in each phase of testing.
Discussion
We previously reported that exercise capacity improved by > 10 W in 15% of LVRS patients compared with 3% of the medical therapy patients (p < 0.01).26 Our present study extends these findings by showing that improved exercise following LVRS is due to the following improvements in ventilatory mechanics: less rapid and shallow breathing, reductions in ventilatory dead space, and enhanced carbon dioxide elimination. Approximately two thirds of our patients had ventilatory limitation as the primary etiology of early exercise termination. Approximately 11 to 12% had ventilatory and cardiac limitation, and rare patients had cardiac limitation alone. LVRS resulted in an approximately 20% reduction in the percentage of patients exhibiting ventilatory limitation as a primary cause for exercise termination. These data support improvements in lung,14,15,18,26,27 chest wall,9,16 and respiratory muscle mechanics,5,7 as responsible for enhancing exercise performance following LVRS.
Our data show that ventilatory improvements occur following LVRS throughout the continuum of exercise, from submaximal (unloaded cycling) through maximum symptom-limited exercise workloads. Although a submaximal study protocol was not used in this study, we measured all physiologic, ventilatory, and patient symptom scores during unloaded cycling, as well as at maximum exercise. This allowed us directly to observe physiologic improvements across the spectrum of exercise.
The improvement in ventilatory function during maximum exercise in patients treated with LVRS is most likely related to an increase in ventilatory capacity and decreased dead space. Post LVRS, patients exhibited higher V̇emax and maximum Vt during maximum exercise, as well as a reduction in the rapid shallow breathing index. These data suggest that ventilatory capacity was enhanced simultaneously with a reduction in an end-expiratory lung volume, suggesting a higher inspiratory capacity. Our data support others who showed a decrease in end-expiratory esophageal pressure13,27 or an increase in inspiratory capacity6 during exercise following LVRS.
Our data show that a decrease in ventilatory dead space occurs during unloaded cycling, as well as at maximum exercise following LVRS. When coupled with simultaneous increases in V̇emax, the reductions in ventilatory dead space suggest an improvement in alveolar ventilation throughout the range of exercise, which resulted in improved carbon dioxide elimination.
The physiologic improvements we observed during unloaded cycling and maximum exercise were mirrored by reductions in their sensation of dyspnea. These data suggest that an improvement in ventilatory function is the major benefit of LVRS in terms of both exercise performance and symptoms.
The effect of the chest CT scan pattern of emphysema on influencing the changes in physiologic variables that we measured during maximum exercise is intriguing, but its mechanism is unclear. Patients with upper-lobe predominant emphysema had significant improvements in carbon dioxide elimination and their ability to generate Vt across the spectrum of exercise, whereas those with non–upper-lobe predominant disease did not. Whether the CT scan pattern of emphysema is only a marker of patients with more severe airflow obstruction and gas trapping or truly influences the surgical consequences of LVRS is uncertain at present and requires further study.
Our study may be limited because the exercise substudy population was more likely to be male, slightly more hypoxemic at rest, had lower Paco2 values, and had more upper-lobe-predominant emphysema than the nonexercise substudy NETT cohort. However, we do not think these factors would negate any of the observed physiologic effects of LVRS on ventilatory mechanics during exercise for the general NETT emphysema patient population. Additionally, despite statistical significance, some of the mean changes in physiologic function post LVRS are small in magnitude (eg, V̇co2, Borg scores for dyspnea and leg fatigue, and dead space) compared to the medically treated group. However, these mean changes in physiologic variables appear to be clinically meaningful because 20% of the LVRS patients group no longer had ventilatory limitation as a cause of exercise termination posttreatment.
Our data show that, following LVRS, patients with severe emphysema demonstrate an increase in exercise capacity, slower and deeper breathing, decreased ventilatory dead space, improved carbon dioxide elimination, a reduction in dyspnea, and less ventilatory limitation to perform maximum exercise. Additionally, the pattern of emphysema determined by CT scan inspection is associated with a change in breathing pattern and gas exchange during maximum exercise following LVRS.
Abbreviations:
- Dlco
diffusing capacity of the lung for carbon monoxide
- LVRS
lung volume reduction surgery
- MVV
maximal ventilatory volume
- NETT
National Emphysema Treatment Trial
- rpm
revolutions per minute
- Sao2
arterial oxygen saturation
- V̇co2
carbon dioxide output
- V̇e
minute ventilation
- V̇emax
maximum minute ventilation
- Vt
tidal volume
Appendix: Members of the NETT Research Group
Office of the Chair of the Steering Committee, University of Pennsylvania (Philadelphia, PA)
A.P. Fishman, B.A. Bozzarello, and A. Al-Amin.
Clinical Centers
Baylor College of Medicine (Houston, TX):
M. Katz, C. Wheeler, E. Baker, P. Barnard, J. Carter, S. Chatziioannou, K. Conejo-Gonzales, J. Haddad, D. Hicks, N. Kleiman, M. Milburn-Barnes, C. Nguyen, M. Reardon, J. Reeves-Viets, S. Sax, A. Sharafkhaneh, C. Young, R. Espada, R. Butanda, K. Dubose, M. Ellisor, P. Fox, K. Hale, E. Hood, A. Jahn, S. Jhingran, K. King, C. Miller, I. Nizami, T. Officer, J. Ricketts, J. Rodarte, R. Teague, and K. Williams.
Brigham and Women's Hospital (Boston, MA):
J. Reilly, D. Sugarbaker, C. Fanning, S. Body, S. Duffy, V. Formanek, A. Fuhlbrigge, P. Hartigan, S. Hooper, A. Hunsaker, F. Jacobson, M. Moy, S. Peterson, R. Russell, D. Saunders, and S. Swanson.
Cedars–Sinai Medical Center (Los Angeles, CA):
R. McKenna, Z. Mohsenifar, C. Geaga, M. Biring, S. Clark, R. Frantz, P. Julien, M. Lewis, J. Minkoff-Rau, V. Yegyan, and M. Joyner.
Cleveland Clinic Foundation (Cleveland, OH):
M. DeCamp, J. Stoller, Y. Meli, J. Apostolakis, D. Atwell, J. Chapman, P. DeVilliers, R. Dweik, E. Kraenzler, R. Lann, N. Kurokawa, S. Marlow, K. McCarthy, P. McCreight, A. Mehta, M. Meziane, O. Minai, P. O'Donovan, M. Steiger, K. White, J. Maurer, C. Hearn, S. Lubell, R. Schilz, and T. Durr.
Columbia University (New York, NY) and Long Island Jewish Medical Center (New Hyde Park, NY):
M. Ginsburg, B. Thomashow, P. Jellen, J. Austin, M. Bartels, Y. Berkman, P. Berkoski, F. Brogan, A. Chong, G. DeMercado, A. DiMango, B. Kachulis, A. Khan, B. Mets, M. O'Shea, G. Pearson, J. Pfeffer, L. Rossoff, S. Scharf, M. Shiau, P. Simonelli, K. Stavrolakes, D. Tsang, D. Vilotijevic, C. Yip, M. Mantinaos, and M. McKeon.
Duke University Medical Center (Durham, NC):
N. MacIntyre, R.D. Davis, J. Howe, R.E. Coleman, R. Crouch, D. Greene, K. Grichnik, D. Harpole, A. Krichman, B. Lawlor, H. McAdams, J. Plankeel, S. Rinaldo-Gallo, J. Smith, M. Stafford-Smith, V. Tapson, M. Steele, and J. Norten.
Mayo Foundation (Rochester, MN):
J. Utz, C. Deschamps, K. Mieras, M. Abel, M. Allen, D. Andrist, G. Aughenbaugh, S. Bendel, E. Edell, M. Edgar, B. Edwards, B. Elliot, J. Garrett, D. Gillespie, J. Gurney, B. Hammel, K. Hanson, L. Hanson, G. Harms, J. Hart, T. Hartman, R. Hyatt, E. Jensen, N. Jenson, S. Kalra, P. Karsell, D. Midthun, C. Mottram, S. Swensen, A.-M. Sykes, K. Taylor, N. Torres, R. Hubmayr, D. Miller, S. Bartling, and K. Bradt.
National Jewish Medical and Research Center (Denver, CO):
B. Make, M. Pomerantz, M. Gilmartin, J. Canterbury, M. Carlos, P. Dibbern, E. Fernandez, L. Geyman, C. Hudson, D. Lynch, J. Newell, R. Quaife, J. Propst, C. Raymond, J. Whalen-Price, K. Winner, M. Zamora, and R. Cherniack.
Ohio State University (Columbus, OH):
P. Diaz, P. Ross, T. Bees, H. Awad, J. Drake, C. Emery, M. Gerhardt, M. Kelsey, M. King, D. Rittinger, M. Rittinger.
Saint Louis University (St. Louis, MO):
K. Naunheim, F. Alvarez, J. Osterloh, S. Borosh, W. Chamberlain, S. Frese, A. Hibbit, ME Kleinhenz, G. Ruppel, C. Stolar, J. Willey, and C. Keller.
Temple University (Philadelphia, PA):
G. Criner, S. Furukawa, A.M. Kuzma, R. Barnette, N. Brister, K. Carney, W. Chatila, F. Cordova, G. D'Alonzo, M. Keresztury, K. Kirsch, C. Kwak, K. Lautensack, M. Lorenzon, U. Martin, P. Rising, S. Schartel, J. Travaline, G. Vance, P. Boiselle, and G. O'Brien.
University of California (San Diego, CA):
A. Ries, R. Kaplan, C. Ramirez, D. Frankville, P. Friedman, J. Harrell, J. Johnson, D. Kapelanski, D. Kupferberg, C. Larsen, T. Limberg, M. Magliocca, F.J. Papatheofanis, D. Sassi-Dambron, and M. Weeks.
University of Maryland at Baltimore, Baltimore, and Johns Hopkins Hospital (Baltimore, MD):
M. Krasna, H. Fessler, I. Moskowitz, T. Gilbert, J. Orens, S. Scharf, D. Shade, S. Siegelman, K. Silver, C. Weir, and C. White.
University of Michigan (Ann Arbor, MI):
F. Martinez, M. Iannettoni, C. Meldrum, W. Bria, K. Campbell, P. Christensen, K. Flaherty, S. Gay, P. Gill, P. Kazanjian, E. Kazerooni, V. Knieper, T. Ojo, L. Poole, L. Quint, P. Rysso, T. Sisson, M. True, B. Woodcock, and L. Zaremba.
University of Pennsylvania (Philadelphia, PA):
L. Kaiser, J. Hansen-Flaschen, M.L. Geraghty, A. Alavi, T. Alcorn, J. Aronchick, S. Aukberg, B. Benedict, S. Craemer, R. Daniele, J. Edelman, W. Gefter, L. Kotler-Klein, R. Kotloff, D. Lipson, W. Miller, Jr., R. O'Connell, S. Opelman, W. Russell, H. Sheaffer, R. Simcox, S. Snedeker, J. Stone-Wynne, G. Tino, P. Wahl, J. Walter, P. Ward, D. Zisman, J. Mendez, and A. Wurster.
University of Pittsburgh (Pittsburgh, PA):
F. Sciurba, J. Luketich, C. Witt, G. Ayres, M. Donahoe, C. Fuhrman, R. Hoffman, J. Lacomis, J. Sexton, W. Slivka, D. Strollo, E. Sullivan, T. Simon, C. Wrona, G. Bauldoff, M. Brown, E. George, R. Keenan, T. Kopp, and L. Silfies.
University of Washington (Seattle, WA):
J. Benditt, D. Wood, M. Snyder, K. Anable, N. Battaglia, L. Boitano, A. Bowdle, L. Chan, C. Chwalik, B. Culver, T. Gillespy, D. Godwin, J. Hoffman, A. Ibrahim, D. Lockhart, S. Marglin, K. Martay, P. McDowell, D. Oxorn, L. Roessler, M. Toshima, and S. Golden.
Other Participants
Agency for Healthcare Research and Quality (Rockville, MD):
L. Bosco, Y.-P. Chiang, C. Clancy, and H. Handelsman.
Centers for Medicare and Medicaid Services (Baltimore, MD):
S. Sheingold, T. Carino, J. Chin, J. Farrell, K. McVearry, A. Norris, S. Shirey, and C. Sikora.
Coordinating Center, Johns Hopkins University (Baltimore, MD):
S. Piantadosi, J. Tonascia, P. Belt, K. Collins, B. Collison, J. Dodge, M. Donithan, V. Edmonds, J. Fuller, J. Harle, R. Jackson, H. Koppelman, S. Lee, C. Levine, H. Livingston, J. Meinert, J. Meyers, D. Nowakowski, K. Owens, S. Qi, M. Smith, B. Simon, P. Smith, A. Sternberg, M. Van Natta, L. Wilson, and R. Wise.
Cost-Effectiveness Subcommittee:
R.M. Kaplan, J.S. Schwartz, Y-P. Chiang, M.C. Fahs, A.M. Fendrick, A.J. Moskowitz, D. Pathak, S. Ramsey, S. Sheingold, AL Shroyer, J. Wagner, and R. Yusen.
Cost-Effectiveness Data Center, Fred Hutchinson Cancer Research Center (Seattle, WA):
S. Ramsey, R. Etzioni, S. Sullivan, D. Wood, T. Schroeder, R. Smith, K. Berry, and N. Myers.
CT Scan Image Storage and Analysis Center (University of Iowa, Iowa City, IA):
E. Hoffman, J. Cook-Granroth, A. Delsing, J. Guo, G. McLennan, B. Mullan, C. Piker, J. Reinhardt, J. Sieren, and W. Stanford.
Data and Safety Monitoring Board:
J.A. Waldhausen, G. Bernard, D. DeMets, M. Ferguson, E. Hoover, R. Levine, D. Mahler, A.J. McSweeny, J. Wiener-Kronish, O.D. Williams, and M. Younes.
Marketing Center, Temple University (Philadelphia, PA):
G. Criner and C. Soltoff.
Project Office, National Heart, Lung, and Blood Institute (Bethesda, MD):
G. Weinmann, J. Deshler, D. Follmann, J. Kiley, and M. Wu.
Footnotes
Funding/Support: The NETT was supported by contracts with the National Heart, Lung, and Blood Institute (NO1HR76101, NO1HR76102, NO1HR76103, NO1HR76104, NO1HR76105, NO1HR76106, NO1HR76107, NO1HR76108, NO1HR76109, NO1HR761010, NO1HR76111, NO1HR76112, NO1HR76113, NO1HR76114, NO1HR76115, NO1HR76116, NO1HR76118, and NO1HR76119), the Centers for Medicare and Medicaid Services, and the Agency for Healthcare Research and Quality.
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.O'Donnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(suppl):S647–S655. doi: 10.1097/00005768-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bauerle O, Chrusch CA, Younes M. Mechanisms by which COPD affects exercise tolerance. Am J Respir Crit Care Med. 1998;157:57–68. doi: 10.1164/ajrccm.157.1.9609126. [DOI] [PubMed] [Google Scholar]
- 3.Homan S, Porter S, Peacock M, et al. Increased effective lung volume following lung volume reduction surgery in emphysema. Chest. 2001;120:1157–1162. doi: 10.1378/chest.120.4.1157. [DOI] [PubMed] [Google Scholar]
- 4.Celli B, ZuWallack R, Wang S, et al. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–1748. doi: 10.1378/chest.124.5.1743. [DOI] [PubMed] [Google Scholar]
- 5.Laghi F, Jubran A, Topeli A, et al. Effect of lung volume reduction surgery on neuromechanical coupling of the diaphragm. Am J Respir Crit Care Med. 1998;157:475–483. doi: 10.1164/ajrccm.157.2.9705082. [DOI] [PubMed] [Google Scholar]
- 6.Dolmage TE, Waddell TK, Maltais F, et al. The influence of lung volume reduction surgery on exercise in patients with COPD. Eur Respir J. 2004;23:269–274. doi: 10.1183/09031936.03.00068503. [DOI] [PubMed] [Google Scholar]
- 7.Criner G, Cordova FC, Leyenson V, et al. Effect of lung volume reduction surgery on diaphragm strength. Am J Respir Crit Care Med. 1998;157:1578–1585. doi: 10.1164/ajrccm.157.5.9607081. [DOI] [PubMed] [Google Scholar]
- 8.Aliverti A, Stevenson N, Dellaca RL, et al. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59:210–216. doi: 10.1136/thorax.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz O, Villafranca C, Ghezzo H, et al. Breathing pattern and gas exchange at peak exercise in COPD patients with and without tidal flow limitation at rest. Eur Respir J. 2001;17:1120–1127. doi: 10.1183/09031936.01.00057801. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell DE, Webb KA. Breathlessness in patients with severe chronic airflow limitation: physiologic correlations. Chest. 1992;102:824–831. doi: 10.1378/chest.102.3.824. [DOI] [PubMed] [Google Scholar]
- 11.Leyenson V, Furukawa S, Kuzma AM, et al. Correlation of changes in quality of life after lung volume reduction surgery with changes in lung function, exercise, and gas exchange. Chest. 2000;118:728–735. doi: 10.1378/chest.118.3.728. [DOI] [PubMed] [Google Scholar]
- 12.Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med. 1996;334:1095–1099. doi: 10.1056/NEJM199604253341704. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FJ, de Oca MM, Whyte RI, et al. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med. 1997;155:1984–1990. doi: 10.1164/ajrccm.155.6.9196106. [DOI] [PubMed] [Google Scholar]
- 14.Tschernko EM, Gruber EM, Jaksch P, et al. Ventilatory mechanics and gas exchange during exercise before and after lung volume reduction surgery. Am J Respir Crit Care Med. 1998;158:1424–1431. doi: 10.1164/ajrccm.158.5.9702086. [DOI] [PubMed] [Google Scholar]
- 15.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung volume reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 16.Jubran A, Laghi F, Mazur M, et al. Partitioning of lung and chest-wall mechanics before and after lung-volume-reduction surgery. Am J Respir Crit Care Med. 1998;158:306–310. doi: 10.1164/ajrccm.158.1.9706082. [DOI] [PubMed] [Google Scholar]
- 17.Geddes D, Davies M, Koyama H, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med. 2000;343:239–245. doi: 10.1056/NEJM200007273430402. [DOI] [PubMed] [Google Scholar]
- 18.Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:2018–2027. doi: 10.1164/ajrccm.160.6.9902117. [DOI] [PubMed] [Google Scholar]
- 19.Oswald-Mammosser M, Kessler R, Massard G, et al. Effect of lung volume reduction surgery on gas exchange and pulmonary hemodynamics at rest and during exercise. Am J Respir Crit Care Med. 1998;158:1020–1025. doi: 10.1164/ajrccm.158.4.9710057. [DOI] [PubMed] [Google Scholar]
- 20.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest. 1999;116:1750–1761. doi: 10.1378/chest.116.6.1750. [DOI] [PubMed] [Google Scholar]
- 21.Shade D, Jr, Cordova F, Lando Y, et al. Relationship between resting hypercapnia and physiologic parameters before and after lung volume reduction surgery in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1405–1411. doi: 10.1164/ajrccm.159.5.9810054. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson GT, Fernandez E, Zamora MR, et al. Improved exercise performance following lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 1998;157:1195–1203. doi: 10.1164/ajrccm.157.4.9705008. [DOI] [PubMed] [Google Scholar]
- 23.Benditt JO, Lewis S, Wood DE, et al. Lung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometry. Am J Respir Crit Care Med. 1997;156:561–566. doi: 10.1164/ajrccm.156.2.9611032. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell DE, Webb KA, Bertley JC, et al. Mechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in emphysema. Chest. 1996;110:18–27. doi: 10.1378/chest.110.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Keller CA, Ruppel G, Hibbett A, et al. Thoracoscopic lung volume reduction surgery reduces dyspnea and improves exercise capacity in patients with emphysema. Am J Respir Crit Care Med. 1997;156:60–67. doi: 10.1164/ajrccm.156.1.9609101. [DOI] [PubMed] [Google Scholar]
- 26.Gelb AF, McKenna RJ, Jr, Brenner M, et al. Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2001;163:1562–1566. doi: 10.1164/ajrccm.163.7.2009048. [DOI] [PubMed] [Google Scholar]
- 27.Benditt JO, Wood DE, McCool FD, et al. Changes in breathing and ventilatory muscle recruitment patterns induced by lung volume reduction surgery. Am J Respir Crit Care Med. 1997;155:279–284. doi: 10.1164/ajrccm.155.1.9001325. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society. Standardization of spirometry. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society. Lung function testing selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique; 1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 31.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 32.Crapo RO, Morris AH, Clayton PD, et al. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 33.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 34.Borg G. Psychophysical basis of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 35.Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16:55–58. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- 36.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 37.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]