Abstract
A significant long-term side effect of radiation therapy for head and neck cancers is xerostomia, a dry mouth, due to salivary gland damage. Despite continuing efforts to eliminate this problem, many patients continue to suffer. This brief review describes our efforts to develop a gene transfer approach, employing the aquaporin-1 cDNA, to treat patients with existing radiation-induced salivary hypofunction. A Phase I/II clinical trial, using a recombinant adenoviral vector to mediate gene transfer, is currently underway.
Keywords: xerostomia, radiation, gene therapy, aquaporin-1, adenovirus
Introduction
Each year in the United States there are 35,000–40,000 new cases of oral cavity and pharyngeal (oral) cancers1. The five-year relative survival rate for these cancers has changed modestly, but significantly, over the last three decades, i.e., from 53% in 1975–77 to 60% for 1996–2004, the most recent period examined1. The treatment of most patients with oral cancers includes radiation therapy (RT). It has been long recognized that salivary glands in the radiation field can suffer irreversible damage leading to a marked reduction in salivary flow and, as a consequence, xerostomia, dysphagia and oral infections 2,3. This RT-induced salivary hypofunction results in a significant diminution in quality of life for a large number of surviving oral cancer patients4,5. As a result, there has been a substantial effort to minimize or eliminate this major side effect of RT for oral cancers. Among the strategies that have been employed are the following: use of hyperbaric oxygen6, chemoprevention with Amifostine7, surgical gland transfer8, and intensity-modulated RT9–11. Despite these efforts, there remain many patients who survive oral cancer but experience significant xerostomia, i.e., patients in RTOG categories 2 and 312. It is for these individuals that we have tried to develop a novel, gene transfer-based corrective treatment for RT-induced salivary hypofunction13.
Specifically, for the past ~15 years, our laboratory has studied the value of transferring the human aquaporin-114 (hAQP1) cDNA to restore salivary flow in patients with RT-damaged salivary glands13 (Table 1). hAQP1 encodes a water channel, a plasma membrane protein that facilitates rapid transmembrane water movement in response to an osmotic gradient. While the idea for these studies was conceived in late 1991, the actual progression of research from the bench into the clinic began in 1995 (Table 1). The following brief description (see also Figure 1) outlines the premise behind our studies13,15. RT damages most of the fluid secreting acinar cells in salivary glands, leaving patients with relatively water impermeable duct cells as the principle surviving epithelial cells in the glands. Normally, salivary ducts are an absorptive epithelia, i.e., they re-absorb almost all of the NaCl secreted by acinar cells in the isotonic primary salivary fluid16. However, in the absence of substantial numbers of acinar cells and a significant volume of primary fluid, such a role is absent. We hypothesized that under such conditions duct cells could generate an osmotic gradient (lumen > interstitium) that water could follow, however, they lacked a facilitated water permeability pathway in their luminal membrane13,15,17. Transfer of the hAQP1 cDNA into the surviving duct epithelial cells would provide a pathway for water to follow if an osmotic gradient was produced and, consequently, increased fluid secretion from the irradiated gland would result13,15,17.
Table 1.
Key steps in taking AdhAQP1 from the bench to the clinic
|
GLP and GMP are abbreviations, respectively, for the United States Food and Drug Administration's Good Laboratory Practice and Good Manufacturing Practice standards.
Figure 1.
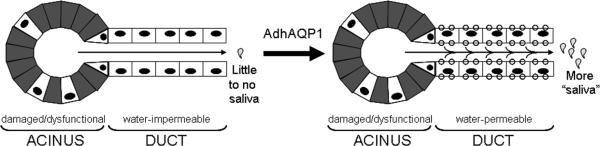
Schematic depiction of the general mechanism hypothesized to occur following AdhAQP1-mediated gene transfer to irradiation-induced hypofunctional parotid glands. Shown to the left is a parotid gland that has been damaged as a result of radiation therapy. The acinar cells shown in gray are either damaged or dysfunctional (acinar cells could also be lost), while some surviving and functional acinar cells remain (in white with black nuclei). Acinar cells are normally the only water-permeable epithelial cells in these glands and are considered a secretory epithelium16. Duct cells (in white with black nuclei), either not or minimally damaged by radiation therapy, are normally water-impermeable and are an absorptive epithelium. The overall result is little to no saliva is secreted from this gland. Following administration of AdhAQP1 (large arrow) the acinar cells are unchanged, but the duct cells now are water permeable, i.e., capable of secreting fluid in an exocrine direction, and thus more “saliva” is secreted into the mouth. The presence of the aquaporin-1 transgenic protein is indicated as small circles in the luminal and basal plasma membranes of the duct cells. The secreted “saliva” is placed in quotation marks because it would not be of exactly the same composition as the normal saliva secreted by parotid glands. While it would contain exocrine proteins and various electrolytes, its specific composition would be different from that produced by a parotid gland before radiation therapy. This hypothesized mechanistic scheme is based on experimental results obtained in pre-clinical studies with miniature pigs23. See that study, the study with irradiated rats by Delporte et al 15 and the text herein, for additional details.
In vivo animal model studies
We originally tested this hypothesis in irradiated rats, using a recombinant serotype 5, adenoviral vector to transfer the hAQP1 cDNA15 (AdhAQP1, Table 2). Rats have long been used as a model of RT-induced salivary hypofunction (e.g., see references18, 19). Our initial studies were cross-sectional in design, i.e., comparing different groups of rats subjected to a single relatively high radiation dose (data for 21 Gy shown) and a relatively high AdhAQP1 dose (5×109 plaque forming units; pfu), given their size. Controls employed included sham-irradiation and treatment with a control adenoviral vector15. As shown in Table 1, AdhAQP1 was effective in restoring salivary flow to near-normal levels (~84%) in this rat model.
Table 2.
Comparison of the efficacy of AdhAQP1 in two animal models of salivary gland irradiation damage *
| Species and gland used | Animal size (Kg) | Study design | Radiation dose (Gy) | AdhAQP1 dose (pfu) | Change>control | Percent of “normal flow” |
|---|---|---|---|---|---|---|
| Rat submandibular | ~0.3 | Cross-sectional | 1×21 | 5×109 | 2.3-fold | ~84 |
| Miniature pig parotid | ~30 | Longitudinal | 1×20 | 1×109 | 2.7-fold | ~81 |
These results are based on data presented in Delporte et al15 (rat) and Shan et al23 (miniature pig). The data for changes>control and percent of normal salivary flow are based on a summary presented in Table 1 of Baum et al37. Key data from the miniature pig study23 are shown in Figure 1. Kg-kilogram; Gy-Gray; pfu-plaque forming units. See text for additional details.
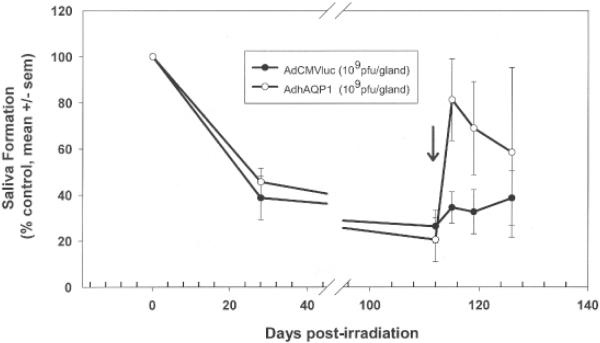
A critical step in the development of a clinically useful gene transfer treatment is the extension of results, with reasonable dose scaling, to a large animal model20. We initially conducted a small longitudinal study in five-rhesus macaques21. The results, however, were equivocal, in part we suspect because of the small number of animals studied (one control; two animals in each of two dosage groups). We needed to utilize a large animal that was more affordable in order to increase the number of animals studied. Miniature pigs provide an excellent and affordable large animal model of salivary gland irradiation damage22 and we next tested the AdhAQP1 vector in irradiated parotid glands in this species23 (Tables 1, 2; Figure 2). The data shown in the figure are representative of results from three animal cohorts studied longitudinally. The radiation dose used was 20Gy and the AdhAQP1 vector dose in the experiments shown in Figure 1 was 1×109 pfu, i.e., both doses lower than used in the rat experiments. As can be clearly seen from Figure 1 and Table 1, the gene transfer maneuver in miniature pigs was also able to restore salivary flow to near-normal levels in the targeted parotid gland, albeit transiently.
Figure 2.
Pattern of parotid salivary flow in miniature pigs following irradiation (20 Gy) and adenoviral vector administration (1×109 pfu). Parotid saliva was collected twice prior to irradiation and normalized to 100%. All other results shown are relative to that initial normalized salivary output (mean ±SEM). Animals were administered (arrow) either a control vector (AdCMVluc; encodes luciferase; filled circles) or the experimental vector (AdhAQP1; open circles) 17 weeks following irradiation. This figure is reproduced from Shan et al23.
Given the strongly positive results of these efficacy studies, we next conducted a detailed vector biodistribution and toxicology study, under the US Food and Drug Administration's (FDA's) Good Laboratory Practice (GLP) standards, to assess the safety of AdhAQP124. This large study (100 adult rats of each gender) supported the earlier non-GLP assessments made in macaques21 and miniature pigs23, i.e., that administration of AdhAQP1 to a single salivary gland was safe.
The clinical trial
Based on those safety findings, plus the pre-clinical efficacy studies conducted in rats and miniature pigs, we submitted a clinical protocol for testing this vector in patients (Tables 1,3). The protocol, “Open-label, dose-escalation study evaluating the safety of a single administration of an adenoviral vector encoding human aquaporin-1 to one parotid salivary gland in individuals with irradiation-induced parotid salivary hypofunction”, received all required US approvals (NIDCR Institutional Review Board, NIH Institutional Biosafety Committee, Recombinant DNA Advisory Committee, and the FDA), and is overseen by an independent Data Safety and Monitoring Board. The NIH protocol number for this trial is 06-D-0206 and the FDA's Investigational New Drug number for AdhAQP1 is 13102. Production of the clinical grade (FDA's Good Manufacturing Practice standards) AdhAQP1 vector occurred at the Belfer Gene Therapy Core Facility at Cornell University Medical College (New York, NY).
Table 3.
Summary of key characteristics of NIH protocol 06-D-0206
|
This trial is currently recruiting subjects and is registered at the clinical trials.gov website (http://www.clinicaltrials.gov/ct/show/NCT00372320?order). There is also a patient oriented website (http://www.drymouthstudy.com/) that provides general information about the study and study personnel, as well as a contact link for more information. Key eligibility criteria for the study are listed in Table 4, but the detailed inclusion and exclusion criteria can be found at the clinicaltrials.gov website. The trial is designed as a single site, open label, dose escalation study (Table 3), i.e. Phase I/II. Safety is being evaluated using conventional clinical and immunological parameters. The primary outcome measures for biological efficacy are parotid gland salivary output and subjective responses to a standardized questionnaire and visual analogue scale25,26. As a Phase I/II trial, the major purpose of this study is to determine vector safety in humans. However, as noted above in the miniature pig pre-clinical study, the effects of AdhAQP1 treatment are transient (~2–4 weeks). Such a result is expected with any first generation adenoviral vector27, given the typical immune response to their administration in salivary glands28. There is no intent by us to treat patients with additional doses of AdhAQP1.
Table 4.
Key enrollment criteria for NIH protocol 06-D-0206
|
Thus, this study also is testing an important proof of concept: does hAQP1 gene transfer increase salivary flow and improve dry mouth in humans with RT-induced parotid hypofunction? If the treatment increases salivary flow, even transiently, we have developed a serotype 2 adenoassociated viral (AAV2) vector (rAAVhAQP1)29 and AAV2 vectors are capable of mediating long lived transgene expression in the salivary glands of rodents and non-human primates30,31. In the non-human primate study31, AAV2-mediated reporter transgene (rhesus erythropoietin) expression was stable for ~6 months, the longest time studied. Also, biodistribution assays at animal sacrifice demonstrated that the vast majority of the AAV2 vector was found in the targeted parotid gland (>99%), with little vector detected elsewhere31. Furthermore, we found no consistent adverse changes in serum chemistry or hematology parameters in the AAV2-vector treated animals. Thus, using an AAV2 vector to mediate extended hAQP1 expression should be beneficial for the long-term correction of salivary gland radiation damage. Accordingly, we have begun extensive testing of the rAAVhAQP1 vector in radiation damaged miniature pig parotid glands. Patients enrolled in the current AdhAQP1 clinical trial would be eligible to enroll in future studies with rAAVhAQP1.
The current study takes one year to complete and requires 10–12 visits to the NIH Clinical Center in Bethesda, MD. There is 1 required inpatient visit that lasts 3–4 days, and the remaining outpatient visits typically last 2 days. There is no expense to a patient for their participation. The first two visits involve multiple clinical and imaging evaluations and are designed to ensure an individual's eligibility. The AdhAQP1 vector is administered on the third visit, and all remaining visits are designed to learn more about the safety and efficacy of the hAQP1 gene transfer. After establishing all necessary infrastructures for the study, we began treating patients with AdhAQP1 in 2008 and have completed the first dose cohort (4.8×107 vector genomes, vg, to a single parotid gland; see Table 5). We are currently treating subjects enrolled in the second dose cohort.
Table 5.
Approved AdhAQP1 doses for NIH protocol 06-D-0206*
| Dose Cohort | Dose in vector genomes |
|---|---|
| 1 | 4.8 × 107 |
| 2 | 2.9 × 108 |
| 3 | 1.3 × 109 |
| 4 | 5.8 × 109 |
| 5 | 3.5 × 1010 |
Three subjects are planned for each dose cohort. The first cohort has been completed. Dose level 4 corresponds approximately to that shown to be effective in miniature pigs when normalized for infusate volume. See text for additional details.
It is important to recognize that the dose units (vg) employed in the clinical trial are different from those used in our pre-clinical studies (pfu). This is because of a change recommended by the FDA. The pfu designation indicates the number of infectious units administered, while vg defines the number of vector particles delivered (both infectious and non-infectious, i.e., including “defective” ones). The FDA reasoned that the latter is more reproducible to assess in different laboratories, as it is measured by quantitative PCR. The former is the result of a functional assay and shows more inter-laboratory variability. Thus, the dosage units used in clinical studies are vg, with an FDA-specified maximum number of vg tolerated per measurable infectious unit. Comparing the vg/pfu ratio of the laboratory grade AdhAQP1 vector used in the miniature pig studies with similar values from the clinical vector preparation, administration of 5.8 × 109 vg/gland (the fourth dose cohort; Table 5) provides a similar multiplicity of infection (MOI; vector dose; defined as infectious units/mL infusate; assuming a 500 μL infusate volume) to that successfully used pre-clinically (Figure 1). It also is important to recognize that comparable (scaled) transgene expression in murine submandibular glands and miniature pig parotid glands is seen with this same effective MOI32.
The highest proposed dose of 3.5 × 1010 vg/gland for this clinical study has not been associated with any reported major adenoviral vector-adverse events when administered to other tissues and is one well tolerated by patients (e.g., see references33,34). Furthermore, the highest dose is about 1000-times less than the adenoviral vector dose that was associated with a death in a liver gene therapy study at the University of Pennsylvania35. However, it is essential to recognize that a clinical trial employing a novel biological reagent such as AdhAQP1 poses several theoretical major risks for participants (Table 6). The parotid glands, however, have several distinct advantages as a gene transfer target site36, including: (i) being well-encapsulated to limit undesirable vector spread, (ii) ductal access uses a limited fluid volume, not diluted on administration and allowing use of low vector doses, and (iii) not being critical for life in case of a local severe adverse event. Thus, while the theoretical risks listed in Table 6 have been recognized during the review process of this protocol, the anticipated likelihood of the occurrence of one is considered to be extremely low.
Table 6.
Theoretical major risks associated with salivary gland gene therapy
|
Concluding remarks
This brief review has described the development of a gene transfer-based treatment for RT-induced salivary hypofunction. The ongoing clinical trial represents the first use of gene therapy in human salivary glands. Pre-clinical animal model results with the AdhAQP1 vector were quite encouraging, although relatively short-lived because a first-generation adenoviral vector was used. If the AdhAQP1 clinical trial shows positive results, an alternative strategy using an AAV2 vector, which should lead to extended transgene expression in humans, has also been developed.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None declared
References
- 1.Jamal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Burlage FR, Spijkervet FKL, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–225. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 4.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Ho KF, Farnell DJJ, Routledge JA, Burns MP, Sykes AJ, Slevin NJ, et al. Developing a CTCAEs patient questionnaire for late toxicity after head and neck radiotherapy. Eur J Cancer. 2009;45:1992–1998. doi: 10.1016/j.ejca.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Teguh DN, Levendag PC, Noever I, Voet P, van der Est H, van Roou P, et al. Early hyperbaric oxygen therapy for reducing radiotherapy side effects: early results of a randomized trial in oropharyngeal and nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2008.11.056. doi:10.1016/j.irobp.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 7.Brizel DM, Overgaard J. Does amifostine have a role in chemoradiation treatment? Lancet Oncol. 2003;4:378–381. doi: 10.1016/s1470-2045(03)01132-x. [DOI] [PubMed] [Google Scholar]
- 8.Jha N, Seikaly H, Harris J, Williamd D, Sultanem K, Hier M, et al. Phase III randomized study: oral pilocarpine versus submandibular salivary gland transfer protocol for the management of radiation-induced xerostomia. Head Neck. 2009;31:234–243. doi: 10.1002/hed.20961. [DOI] [PubMed] [Google Scholar]
- 9.Chen W-C, Hwang T-Z, Wang W-H, Lu C-H, Chen C-C, Chen C-M, et al. Comparison between conventional and intensity-modulated post-operative radiotherapy for stage III and IV oral cavity cancer in terms of treatment results and toxicity. Oral Oncol. 2009;45:505–510. doi: 10.1016/j.oraloncology.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Vergeer MR, Doornaert P, Reitvelt DHF, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a non-randomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation Therapy Oncology Group phase III trial 0225. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.9109. doi:10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox JD, Steitz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for research and treatment of cancer. Int J Radiation Oncology Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Baum BJ, Zheng C, Cotrim AP, McCullagh L, Goldsmith CM, Brahim JS, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. In: Beitz E, editor. Handbook of Experimental Pharmacology. Vol. 190. Springer-Verlag; Berlin Heidelberg: 2009. pp. 403–418. Aquaporins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum BJ. Principles of saliva secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- 17.Vitolo JM, Baum BJ. The use of gene transfer for the protection and repair of salivary glands. Oral Dis. 2002;8:183–191. doi: 10.1034/j.1601-0825.2002.02865.x. [DOI] [PubMed] [Google Scholar]
- 18.Cherry CP, Glucksman A. Injury and repair following irradiation of salivary glands in male rats. Brit J Radiol. 1959;32:596–608. doi: 10.1259/0007-1285-32-381-596. [DOI] [PubMed] [Google Scholar]
- 19.Abok K, Brunck U, Jung B, Ericsson J. Morphologic and histochemical studies of the differing radiosensitivity of ductular and acinar cells of the rat submandibular gland. Virchows Arch B Cell Pathol. 1984;45:443–460. doi: 10.1007/BF02889885. [DOI] [PubMed] [Google Scholar]
- 20.Casals M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 21.O'Connell AC, Baccaglini L, Fox PC, O'Connell BC, Kenshalo D, Oweisy H, et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999;6:505–513. doi: 10.1038/sj.cgt.7700078. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Shan Z, Ou G, Liu X, Zhang C, Baum BJ, et al. Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys. 2005;62:1510–1516. doi: 10.1016/j.ijrobp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Zheng C, Goldsmith CM, Mineshiba F, Chiorini JA, Kerr A, Wenk ML, et al. Toxicity and biodistribution of a first-generation recombinant adenoviral vector, encoding aquaporin-1, after retroductal delivery to a single rat submandibular gland. Hum Gene Ther. 2006;17:1122–1133. doi: 10.1089/hum.2006.17.1122. [DOI] [PubMed] [Google Scholar]
- 25.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of gland performance. J Amer Dent Assoc. 1987;115:581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 26.Pai S, Ghezzi EM, Ship JA. Development of a visual analogue scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg. 2001;91:311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 27.Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 28.Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ, et al. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998;9:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- 29.Braddon VR, Chiorini JA, Wang S, Kotin RM, Baum BJ. Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther. 1998;9:2777–2785. doi: 10.1089/hum.1998.9.18-2777. [DOI] [PubMed] [Google Scholar]
- 30.Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voutetakis A, Zheng C, Mineshiba F, Cotrim AP, Goldsmith CM, Schmidt M, et al. Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates. Hum Gene Ther. 2007;18:142–150. doi: 10.1089/hum.2006.154. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Zheng C, Zhang X, Liu X, Zhang C, Goldsmith CM, et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo. J Gene Med. 2004;6:55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- 33.Harvey BG, Maroni J, O'Donoghue KA, Chu KW, Muscat JC, Pippo AL, et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- 34.Crystal RG, Harvey BG, Wisnivesky JP, O'Donoghue KA, Chu KW, Maroni J, et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13:65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- 35.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10:585–590. doi: 10.1016/j.molmed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758:1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Delporte CD, Hoque ATM, Kulakusky JA, Braddon VR, Goldsmith CM, Wellner RB, et al. Relationship between adenovirus-mediated aquaporin-1 expression and fluid movement across epithelial cells. Biochem Biophys Res Commun. 1998;246:584–588. doi: 10.1006/bbrc.1998.8668. [DOI] [PubMed] [Google Scholar]