Summary
Traditional environmental management programmes require extensive coverage of larval habitats to reduce drastically the emergence of adult mosquitoes. Recent studies have highlighted the impact of reduced availability of aquatic habitats on mosquito foraging for hosts and oviposition sites. In this study, we developed an agent-based model to track the status and movement of mosquitoes individually. Mosquito foraging was represented as a two-stage process: random flight when the resource was not within the mosquito’s perception range and directional flight to the resource when it was detected. Three scenarios of targeted source reduction were devised to eliminate all aquatic habitats within certain distances of human habitations. For comparison, three conventional source reductions randomly eliminated the same numbers of aquatic habitats as their corresponding targeted scenarios. Our results show that the elimination of habitats within 100 m, 200 m and 300 m of surrounding houses resulted in 13%, 91% and 94% reductions in malaria incidence, respectively; compared with −3%, 19% and 44%, respectively, for the corresponding conventional interventions. These findings indicate that source reduction might not require coverage of extensive areas, as previously thought, and that the distance to human habitations can be used for habitat targeting.
Keywords: Mosquito control, Habitats, Resource seeking, Gonotrophic cycle, Oviposition, Theoretical model
1. Introduction
Malaria campaigns in tropical Africa rely mainly on insecticide-treated bed nets (ITNs) and indoor residual spraying to suppress Anopheles gambiae Giles populations. The development of alternative tools is needed to reduce reliance on insecticide-based control. Environmental management (EM) of larval habitats played the lead role in the campaign against malaria before the 1950s.1 However, traditional EM is contentious because these programmes emphasized extensive coverage of aquatic habitats. In many cases, source reduction was implemented by indiscriminately treating all water bodies.2-4 Control areas were, furthermore, recommended to extend at least 2 km beyond the protected population.5,6 Coverage of such large areas is clearly expensive and unsustainable in resource-deprived African communities.
Advancements in mosquito ecology have cast EM in a new light. First, heterogeneity in mosquito productivity between aquatic habitats has been widely observed.7 If the treatment of each habitat is presumed to cost the same, in terms of resources, then targeted intervention in prolific habitats would be more cost-effective.8 Variations in the mechanisms underlying mosquito productivity, however, pose a challenge for the development of targeted interventions.4 In addition, various measures of mosquito productivity, for example the presence or density of anopheline larvae, are used and may bear little relevance to the abundance of emergent adult mosquitoes in a habitat.7 Therefore, targeted interventions need an understanding of mosquito productivity and operationally appropriate measures for habitat targeting in the field.
Second, theoretical work has revealed that reduced availability of oviposition sites prolongs the gonotrophic cycle, leading to unexpected impacts on malaria transmission.9,10 The lifecycle of female mosquitoes requires at least two resources:hosts for a blood meal and oviposition sites. Searching for hosts and oviposition sites, referred to as foraging in wildlife sciences, can have significant implications for malaria transmission. Due to the cyclic nature of blood feeding and egg laying, denying a female mosquito an opportunity to lay her eggs should reduce the frequency of blood feeding and increase mortality over the cycle.11 Understanding mosquito foraging assists in the development of intervention programmes, especially for demarcating the perimeter of environmental sanitation areas.12 Nevertheless, mosquito foraging has long been neglected in the examination of population dynamics, and the duration of the gonotrophic cycle is treated as a constant in malaria models.13
Control intervention measures, e.g. ITNs and source reduction of aquatic habitats, can reduce the availability of resources. The impact on the gonotrophic cycle has been the focus of several investigations in which the probability of encountering resources was modelled as a function of the number of resources in the study area with an assumption of a universal foraging mechanism.14-16 In these models, mosquitoes were presumed to have equal access to all resources in the landscape and thus the location of resources became irrelevant. Although mathematically convenient, this assumption is not compatible with empirical observations, which show limited flight ability and perceptual ranges of An. gambiae.17,18 Apparently, mosquito foraging is a local process consisting of non-directional, opportunistic flight that switches to directional, confirmatory flight when cues originating from a resource are perceived.19
Models of foraging behaviour are complicated because mosquito movements are status-dependent, e.g. only gravid females respond to cues of oviposition sites. Conventional population-based models become intractable when they try to take into account the complexities and idiosyncrasies associated with foraging. With the development of computer technology and software, agent-based modelling has increasingly become a useful tool for representing complex systems as it allows the construction of model frameworks to include substantial detail and reality.20
In this paper, we describe the development of an agent-based model to simulate the lifecycle of individual mosquitoes by explicitly tracking the movement of individual females in heterogeneous landscapes. The objective of our study was to examine the impact of various source reduction programmes and to formulate operational guidelines for EM by source reduction.
2. Materials and methods
2.1. Landscape
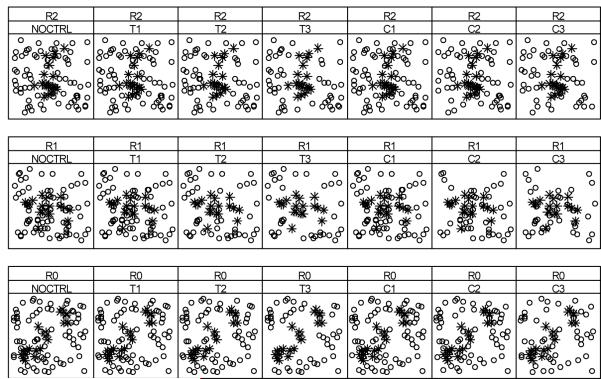
Hypothetical villages were created in grid-based landscapes (40 × 40). Twenty houses were arranged in the middle and 70 aquatic habitats were randomly distributed across the landscapes (Figure 1). These settings bore some resemblance to African rural communities in terms of the numbers of houses and habitats.21 Three landscapes (R0, R1, R2) were generated in which houses were aligned diagonally, vertically or horizontally, respectively (Figure 1). The number of residents in each house was assumed to be a normal variate with an average of five and a minimum of two. All the houses and aquatic habitats were assumed to be identical in their attractiveness to mosquitoes. Therefore, variations in the distributions of host-seeking mosquitoes and in larval abundance among habitats were solely dependent upon their location relative to the foraging paths of mosquitoes.
Figure 1.
Diagram of three landscapes (bottom to top) R0, R1, R2, with different arrangements of 20 houses (asterisk) and 70 habitats (open circle) and seven scenarios of source reduction (left to right) NOCTRL (no intervention as control), T1, T2, T3, C1, C2, C3 with T denoting targeted and C non-targeted interventions. T1, T2 and T3 covered all habitats within 100, 200 and 300 m of surrounding houses, accounting for 4, 17 and 28 of 70 habitats, respectively. C1, C2 and C3 randomly eliminated the same numbers of aquatic habitats as the corresponding targeted interventions.
2.2. Larval populations and density-dependent oviposition
The dynamics of the immature stages of the mosquito lifecycle were modelled using a matrix transition that assumed that populations of eggs, larvae and pupae had constant rates of development and survival over the period of simulation (Table 1). Several studies have shown that An. gambiae tends to avoid oviposition in habitats with crowded conspecific larval populations.22,23 To reflect the density-dependent effect on oviposition, we arbitrarily set a threshold of 5000 eggs/habitat so that gravid mosquitoes would skip those saturated habitats and continue to search for oviposition sites. Density-dependent effects have also been observed on larval development, survival and body size of emerged adults.24-26 The varying mechanisms of density-dependent regulation might influence mosquito abundance in similar ways, i.e. by stabilizing simulated mosquito populations. However, the density-dependent regulation adopted in our model also affected mosquito foraging. In our simulation, we included the density-dependent effect mainly for maintaining stable mosquito populations. Therefore, the adoption of mechanisms of density-dependent regulation would be unlikely to affect our results.
Table 1.
Parameter values used in the agent-based foraging model
| Parameter | Value | Reference |
|---|---|---|
| Larval demography | ||
| Daily development rate of egg | 0.3 | This study |
| Daily development rate of larva | 0.2 | This study |
| Daily development rate of pupa | 0.3 | This study |
| Daily mortality of immature stages | 0.2 | 37 |
| Female demography | ||
| Daily mortality | 0.2 | 38 |
| Fecundity | 80 eggs/ oviposition |
39 |
| Time before first blood meal | 2 days | 40 |
| Time for blood digestion and egg maturation |
1 day | 40 |
| Malaria transmission | ||
| Daily recovery rate | 0.01 | 41 |
| Extrinsic incubation | 10 days | 42 |
| Intrinsic incubation | 15 days | This study |
| Probability of mosquito infection after biting an infected person (rate c) |
0.15 | 43 |
| Probability of human infection after being bitten by an infectious mosquito (rate b) |
0.5 | 43 |
2.3. Adult populations and foraging behaviour
For each emergent female, we tracked the movement and the lifecycle individually. Females were categorized into three statuses, i.e. newly emerged, host-seeking and gravid mosquitoes. The transitions from newly emerged to host-seeking and host-seeking to gravid were accomplished by tracking the dates of emergence and last blood feeding (Table 1). Foraging was treated like a moving window sliding through the landscape. The size of the window was defined as the perceptual range and the movement of the window defined by the maximal flight length of the mosquito. We assumed that foraging for hosts and oviposition sites was identical and consisted of a two-phase process: (1) random flight in which the mosquito moved randomly into one of the adjacent grids when resources were not in the grid where the mosquito was located and the adjacent grids, and the flight did not stop until the maximal flight length was reached or the resource was located; and (2) directional flight proceeding to a resource located in any one of the grids adjacent to the central grid where the mosquito was located.19 The two parameters governing mosquito foraging were the maximal flight length and perception range. The former was defined as the number of grids the mosquito could fly through if there was no perception along the path and the latter was defined as the grid size, so that resources in any of the adjacent grids could be accessible by a direct flight. Based on a few studies showing the limited perceptual range of An. gambiae,6,27 we arbitrarily set the grid size to 50 m, equivalent to a detection range of 2.25 (150 × 150 m) ha. For the random flight search, we assumed that the probability moving to diagonally adjacent grids was half that of moving to horizontally or vertically adjacent grids. Three kinds of virtual mosquito were constructed with maximal flight lengths of 1, 3 and 5 grids/day. We assumed that mosquitoes flying out of the study area would be dead, i.e. an absorbing border effect.
2.4. Transmission dynamics of malaria
The dynamics of malaria transmission were simulated as a susceptible-infected model. No immunity was assumed and recovered persons became susceptible again. At the beginning of simulation, 50% of the resident population was assumed to be infected, with random dates of infection in the range −100 to −1 day. Each infection was presumed to last for 100 days before recovery. Once bitten by an infectious mosquito a person became infected at the rate b. After 15 days of incubation infected persons were able to transmit malaria parasites to uninfected mosquitoes, and uninfected mosquitoes became infected when biting infected persons at the rate c. After a 10 day extrinsic incubation period infected mosquitoes became infectious and remained so for the rest of their life (Table 1).
2.5. Environmental management by source reduction
We devised three scenarios for targeted interventions (T1, T2, T3) aimed at eliminating all habitats within 100 m 200 m and 300 m of the nearest houses, respectively. The control areas covered, on average, 4, 17 and 28 habitats for the three landscapes. For comparison, we constructed three scenarios for non-targeted interventions (C1, C2, C3) in which the same numbers of aquatic habitats were randomly eliminated as in the corresponding targeted interventions. Therefore, the difference in the impact of these two control strategies of source reduction could be attributed to disruption of foraging processes by the targeted interventions.
2.6. Simulations
Simulations were carried out daily on a total of 63 scenarios (three flights × seven EM scenarios × three landscapes) with an initial population of 20 000 gravid mosquitoes originating from randomly selected houses. Simulated populations reached plateaus after 80 days and intervention measures were introduced at 100 days. Each simulation lasted for 200 days. Averages of total mosquito abundance, human-biting rates (the number of host-seeking mosquitoes in a house divided by the number of persons), entomological inoculation rates (EIR; the number of infected mosquitoes divided by the number of persons), and the incidence and prevalence of malaria were recorded for the period of 100 to 200 days. The simulations were programmed using C++ and were run on a Dell dual-core CPU Xeon 5100 computer.
3. Results
3.1. Impact on mosquito abundance
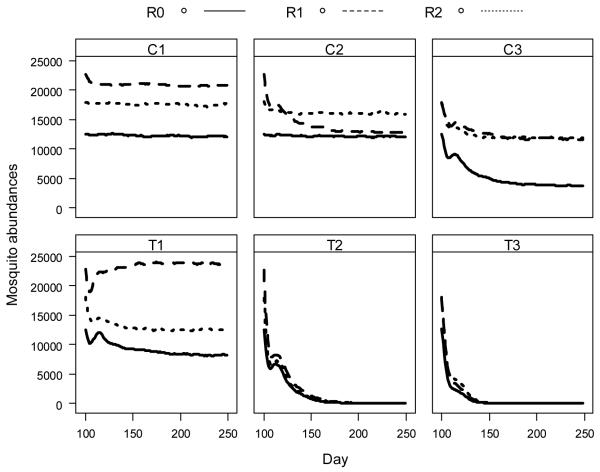
For the non-targeted control scenarios (C1, C2, C3) mosquito abundance declined with increased coverage, with reductions of 4, 20 and 43%, respectively (Figure 2). By contrast, the targeted source reduction scenarios T2 and T3 resulted in sharp declines in mosquito abundance of 90 and 95%, respectively, although T1 had only a marginal impact. Importantly, mosquito populations were not sustainable when aquatic habitats within 200 and 300 m of surrounding houses were eliminated.
Figure 2.
Simulated abundance of mosquitoes with a flight capability of 250 m under various intervention scenarios. Simulations of control scenarios started on day 100 with the initial population harvested from a simulation of mosquito dynamics without control interventions. (see Figure 1 legend for symbols).
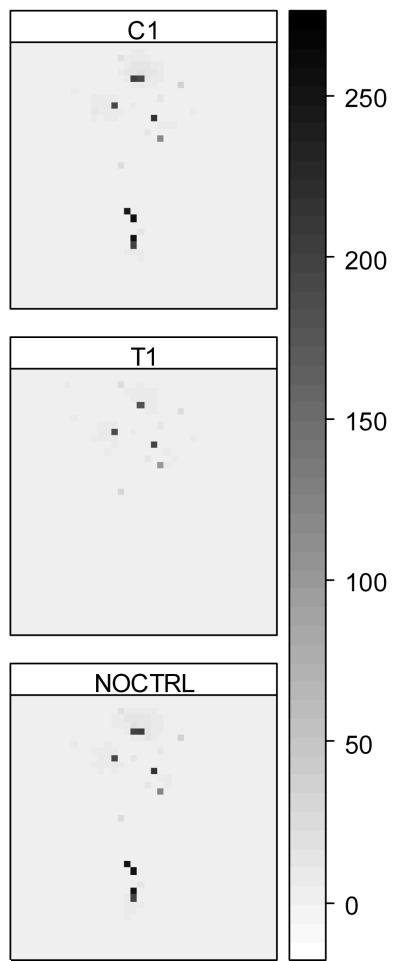
The spatial arrangement of houses and habitats alone yielded considerable variation in mosquito abundance and response to interventions. The greater abundance observed in landscape R1 might be attributable to the shorter average distance to the nearest houses, which was 454 m, 370 m and 455 m for landscapes R0, R1 and R2, respectively. The abundance of host-seeking mosquitoes reflected the spatial relationship between houses and oviposition sites. For example, host-seeking mosquitoes were abundant around houses (Figure 3). The distribution of host-seeking mosquitoes was substantially altered by the most restricted targeted intervention (T1 in Figure 3), although the majority of aquatic habitats were still present across the landscape.
Figure 3.
Predicted abundance of host-seeking mosquitoes in landscape 3 (see R2 in Figure 1). T1: targeted source-reduction scenario covering all habitats within 100 m of surrounding houses; C1: non-targeted scenario randomly eliminating the same number of aquatic habitats as its corresponding targeted intervention; NOCTRL: no intervention as control. The shaded bar and numbers are averages of host-seeking mosquitoes/grid.
3.2. Impact on malaria transmission
Similar to their impact on mosquito abundance, targeted interventions T2 and T3 yielded substantial reductions in EIR and incidence, while their impact on prevalence was less drastic because of the time required for parasites to clear from infected persons. By contrast, the non-targeted interventions yielded small reductions in EIR, incidence and prevalence, except for C3, which had a noticeable impact on EIR and incidence (Table 2).
Table 2.
Reductions (%) in mosquito abundance, entomological inoculation rates (EIR), incidence and prevalence of malaria, over the simulation period day 100 to day 250, for mosquitoes with a flight capability of 250 m
| Intervention | Remaining habitats (N = 70) |
EIR | Malaria incidence |
Malaria prevalence |
|---|---|---|---|---|
| T1 | 66 | 5.7 | 12.5 | 5 |
| T2 | 53 | 86.8 | 90.6 | 52.5 |
| T3 | 42 | 94.3 | 93.8 | 57.5 |
| C1 | 66 | 0 | −3.1 | −5 |
| C2 | 53 | 16.9 | 18.8 | 5 |
| C3 | 42 | 43.4 | 43.8 | 25 |
T1, T2, T3: targeted source-reduction scenarios covering areas of 100, 200 and 300 m from houses, respectively; C1, C2, C3: non-targeted interventions that randomly eliminated the same numbers of aquatic habitats as their corresponding targeted scenarios.
3.3. Impact on flight capability
Mosquitoes with a longer flight range were more abundant due to their better searching capability (Table 3). However, searching efficiency did not increase linearly with an increase in flight range; the increase in abundance was much larger between populations of mosquitoes flying one grid length and three grid lengths than between populations flying three grid lengths and five grid lengths. Thus, the efficiency of resource searching was not solely dependent on flight capability; although good flyers covered larger areas in search of resources than poor flyers, they might fly out of the area when resources were limited. The same effect, of a reduced gain in search efficiency with increased flight capability, was observed on the incidence and prevalence of malaria (Table 3).
Table 3.
Summary of mosquito populations and malaria transmission in relation to flight capability in the absence of interventions
| Flight capability (grid length) |
Total mosquito abundance |
Daily incidence |
Malaria prevalence (%) |
|---|---|---|---|
| 1 | 6712 | 0.12 | 0.17 |
| 3 | 14 813 | 0.25 | 0.33 |
| 5 | 17 583 | 0.32 | 0.40 |
4. Discussion
Two important findings emerged from our study. First, source reduction does not need to cover as extensive an area as previously conceived - 2 km around villages as the control perimeter. Our simulations of mosquito foraging indicate that coverage up to 300 m surrounding houses could lead to interruption of the gonotrophic cycle and significant reductions in malaria transmission. Therefore, mosquito foraging might be a promising target for malaria control using source reduction. Second, distance to the nearest houses can be the primary measure for habitat targeting. Traditionally, aquatic habitats are assessed based on measures of mosquito productivity, such as numbers of larvae or pupae, reflecting the emphasis on reducing mosquito emergence by anti-larval interventions. However, measures of mosquito productivity are controversial and difficult to estimate in the field.4,7,28 Here, we show that the distance to human habitation may serve as an operational indicator for habitat targeting in the field. Priority for source reduction should, at least partly, be placed to interfere with the spatial connection between human residences and oviposition sites.
In our model, the detection range played a major role in mosquitoes’ ability to locate resources. Unfortunately, the distance at which mosquitoes respond to a certain resource is poorly understood. Limited field data point to short ranges of perception. In Florida, mosquitoes including Aedes vexans and Psorophora columbiae visually responded to large, unpainted suction traps in less than 20 m.27 Gillies and Wilkes29 demonstrated that the distance over which mosquitoes detected and oriented to bait calves, although variable among mosquitoes, was 40 m. Building on these findings, our model shows that eliminating oviposition sites within 300 m of houses would be sufficient to suppress malaria transmission to extremely low levels. This finding suggests that mosquitoes’ poor perception of resources may limit their ability to explore oviposition sites far away from human habitations.
Interestingly, we found that the efficiency of searching for resources was not linearly related to the flight range. Good dispersers might simply drift out of the area, equivalent to deaths in our model, due to the random searching pattern in the absence of detectable cues. Field studies showed that An. gambiae travelled less than ¼ mile per day.6 Similarly, a recent mark-release-recapture study in Kenya indicated daily flight ranges of 200–400 m,30 corresponding to the mosquito with the maximal flight length of five grids in our model. Therefore, searching efficiency would increase with flight capability to a certain extent. Beyond that, long-range flyers would be disadvantaged because they might lose track of resources while in random flight. Additionally, long-range flight for resource seeking may be a disadvantage because it consumes a great deal of energy that could otherwise be used for egg maturation and oviposition and renders mosquitoes susceptible to predation.
Foraging movement is influenced by the arrangement of resources in the landscape.31-35 For example, aquatic sites might act as directional cues for dispersal between neighbouring villages31 or as the core feature for aggregation of host-seeking mosquitoes.36
With unprecedented action focused on combating malaria in Africa, especially scaling-up the application of ITNs, more research is needed to investigate the impact on mosquito foraging. It should be noted that our results do not apply to larval control by larviciding using chemical or microbial insecticides because these measures have little impact on oviposition. Killing larvae in water can reduce adult emergence but has little influence on egg-laying by gravid females. By contrast, source reduction can affect both adult emergence and the availability of oviposition sites, and the former may be an important mechanism for malaria control in situations where larval habitats are limited and scattered across large areas.9,16
Agent-based models allow the description of spatial networks of resources from the mosquito’s viewpoint. Our study highlights the gap in our understanding of the relationship between resource-seeking behaviours of vector mosquitoes and malaria transmission. As applies to all modelling work, it should be noted that uncertainties in the parameter values and underlying assumptions in our model affect predicted quantities and prohibit literal interpretations of the model prediction. However, the qualitative insight derived from our simulation is relatively robust because we focused on spatial patterns and comparisons of predicted quantities under various intervention scenarios, by which uncertainties of demographic parameters would be largely cancelled out.11 Nevertheless, our finding that coverage of 300 m surrounding human habitations provided adequate control needs to be vigorously tested in future studies because it is inconsistent with the conventional wisdom of source reduction by EM. Our simulation suggests that resource seeking by Anopheles mosquitoes might be a bottleneck in the mosquito lifecycle and, thus, a promising target for malaria control.
Acknowledgements
We thank the two anonymous reviewers for their critical and insightful comments and suggestions.
Funding: This research was supported by the US National Institutes of Health (UO1 A154889 to RJN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared.
Ethical approval: Not required.
References
- 1.Kitron U, Spielman A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Rev Infect Dis. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- 2.Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health. 2006;11:1629–42. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 3.Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. Am J Trop Med Hyg. 2008;79:19–27. [PubMed] [Google Scholar]
- 4.Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, et al. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. Am J Trop Med Hyg. 2006;74:517–8. [PubMed] [Google Scholar]
- 5.Service MW. The Anopheles vector. In: Bruce-Chwatt LJ, editor. Essential malariology. 3rd ed. Heinemann; London: 1993. pp. 96–123. [Google Scholar]
- 6.Gillies MT. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull Entomol Res. 1961;52:99–127. [Google Scholar]
- 7.Gu W, Utzinger J, Novak R. Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg. 2008;78:2–6. [PubMed] [Google Scholar]
- 8.Gu W, Novak RJ. Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546–52. [PubMed] [Google Scholar]
- 9.Gu W, Regens JL, Beier JC, Novak RJ. Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc Natl Acad Sci USA. 2006;103:17560–3. doi: 10.1073/pnas.0608452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killeen GF, McKenzie FE, Foy BD, Bogh C, Beier JC. The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by African mosquito populations. Trans R Soc Trop Med Hyg. 2001;95:469–76. doi: 10.1016/s0035-9203(01)90005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye C. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- 12.Service MW. Mosquito (Diptera: Culicidae) dispersal--the long and short of it. J Med Entomol. 1997;34:579–88. doi: 10.1093/jmedent/34.6.579. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald G. The epidemiology and control of malaria. Oxford University Press; Oxford, UK: 1957. [Google Scholar]
- 14.Kelly DW, Thompson CE. Epidemiology and optimal foraging: modelling the ideal free distribution of insect vectors. Parasitology. 2000;120:319–27. doi: 10.1017/s0031182099005442. [DOI] [PubMed] [Google Scholar]
- 15.Saul A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J. 2003;2:32. doi: 10.1186/1475-2875-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killeen GF, Seyoum A, Knols BG. Rationalizing historical successes of malaria control in Africa in terms of mosquito resource availability management. Am J Trop Med Hyg. 2004;71:87–93. [PubMed] [Google Scholar]
- 17.Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res. 1969;59:441–56. doi: 10.1017/s0007485300003412. [DOI] [PubMed] [Google Scholar]
- 18.Gillies MT, Wilkes TJ. The range of attraction of single baits for some West African mosquitoes. Bull Entomol Res. 1970;60:225–235. doi: 10.1017/S000748530004075X. [DOI] [PubMed] [Google Scholar]
- 19.Bidlingmayer WL. The measurement of adult mosquito population changes--some considerations. J Am Mosq Control Assoc. 1985;1:328–348. [PubMed] [Google Scholar]
- 20.Bian L. The representation of the environment in the context of individual-based modeling. Ecol. Model. 2003;159:279–296. [Google Scholar]
- 21.Takken W, Charlwood JD, Billingsley PF, Gort G. Dispersal and survival of Anopheles funestus and A. gambiae s.l. during the rainy season in southeast Tanzania. Bull Entomol Res. 1998;88:561–566. [Google Scholar]
- 22.Munga S, Minakawa N, Zhou G, Barrack OO, Githeko AK, Yan G. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J Med Entomol. 2006;43:221–4. doi: 10.1603/0022-2585(2006)043[0221:eolcap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Sumba LA, Ogbunugafor CB, Deng AL, Hassanali A. Regulation of oviposition in Anopheles gambiae s.s.: role of inter- and intra-specific signals. J Chem Ecol. 2008;34:1430–6. doi: 10.1007/s10886-008-9549-5. [DOI] [PubMed] [Google Scholar]
- 24.Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol. 2002;39:162–72. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- 25.Ng’habi KR, John B, Nkwengulila G, Knols BG, Killeen GF, Ferguson HM. Effect of larval crowding on mating competitiveness of Anopheles gambiae mosquitoes. Malar J. 2005;4:49. doi: 10.1186/1475-2875-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am J Trop Med Hyg. 2003;68:748–52. [PubMed] [Google Scholar]
- 27.Bidlingmayer WL, Hem DG. The range of visual attraction and the effect of competitive visual attractants upon mosquito flight. Bull Entomol Res. 1980;70:321–342. [Google Scholar]
- 28.Gu W, Novak R. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. Am. J. Trop. Med. Hyg. 2006;74:519–520. [PubMed] [Google Scholar]
- 29.Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res. 1969;59:441–56. doi: 10.1017/s0007485300003412. [DOI] [PubMed] [Google Scholar]
- 30.Midega JT, Mbogo CM, Mwnambi H, Wilson MD, Ojwang G, Mwangangi JM, et al. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan coast by using mark-release-recapture methods. J Med Entomol. 2007;44:923–9. doi: 10.1603/0022-2585(2007)44[923:edasoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costantini C, Li SG, Della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Med Vet Entomol. 1996;10:203–19. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinones ML, Lines JD, Thomson MC, Jawara M, Morris J, Greenwood BM. Anopheles gambiae gonotrophic cycle duration, biting and exiting behaviour unaffected by permethrin-impregnated bednets in The Gambia. Med Vet Entomol. 1997;11:71–8. doi: 10.1111/j.1365-2915.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomson MC, Connor SJ, Quinones ML, Jawara M, Todd J, Greenwood BM. Movement of Anopheles gambiae s.l. malaria vectors between villages in The Gambia. Med Vet Entomol. 1995;9:413–9. doi: 10.1111/j.1365-2915.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 35.Toure YT, Dolo G, Petrarca V, Traoré SF, Bouaré M, Dao A, et al. Mark-release-recapture experiments with Anopheles gambiae s.l. in Banambani Village, Mali, to determine population size and structure. Med Vet Entomol. 1998;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 36.Le Menach A, McKenzie FE, Flahault A, Smith DL. The unexpected importance of mosquito oviposition behaviour for malaria: non-productive larval habitats can be sources for malaria transmission. Malar J. 2005;4:23. doi: 10.1186/1475-2875-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munga S, Minakawa N, Zhou G, Githeko AK, Yan G. Survivorship of immature stages of Anopheles gambiae s.l. (Diptera: Culicidae) in natural habitats in western Kenya highlands. J Med Entomol. 2007;44:758–64. doi: 10.1603/0022-2585(2007)44[758:soisoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JHET. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull Entomol Res. 1997;87:445–53. [Google Scholar]
- 39.Roitberg BD, Gordon I. Does the Anopheles blood meal-fecundity curve, curve? J Vector Ecol. 2005;30:83–6. [PubMed] [Google Scholar]
- 40.Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, Rosario Vdo E. ‘A mate or a meal’--pre-gravid behaviour of female Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Malar J. 2003;2:9. doi: 10.1186/1475-2875-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar J. 2004;3:13. doi: 10.1186/1475-2875-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lines JD, Wilkes TJ, Lyimo EO. Human malaria infectiousness measured by age-specific sporozoite rates in Anopheles gambiae in Tanzania. Parasitology. 1991;102:167–77. doi: 10.1017/s0031182000062454. [DOI] [PubMed] [Google Scholar]
- 43.Gu W, Mbogo CM, Githure JI, Regens JL, Killeen GF, Swalm CM, et al. Low recovery rates stabilize malaria endemicity in areas of low transmission in coastal Kenya. Acta Trop. 2003;86:71–81. doi: 10.1016/s0001-706x(03)00020-2. [DOI] [PubMed] [Google Scholar]