Abstract
Though TGF-β inhibition enhances anti-tumor immunity mediated by CD8+ T cells in several tumor models, it is not always sufficient for rejection of tumors. In the present study, to maximize the anti-tumor effect of TGF-β blockade, we tested the effect of anti-TGF-β combined with an irradiated tumor vaccine in a subcutaneous CT26 colon carcinoma tumor model. The irradiated tumor cell vaccine alone in prophylactic setting significant delayed tumor growth, whereas anti-TGF-β antibodies alone did not show any anti-tumor effect. However, tumor growth was inhibited significantly more in vaccinated mice treated with anti-TGF-β antibodies compared to vaccinated mice without anti-TGF-β suggesting that anti-TGF-β synergistically enhanced irradiated tumor vaccine efficacy. CD8+ T cell-depletion completely abrogated the vaccine efficacy, so protection required CD8+ T cells. Depletion of CD25+ T regulatory cells led to the almost complete rejection of tumors without the vaccine, whereas anti-TGF-β did not change the number of CD25+ T regulatory cells in un-vaccinated and vaccinated mice. Though the abrogation of CD1d-restricted NKT cells, which have been reported to induce TGF-β production by MDSC through an IL-13-IL-4R-STAT6 pathway, partially enhanced anti-tumor immunity regardless of vaccination, abrogation of the NKT cell-IL-13-IL-4R-STAT-6 immunoregulatory pathway did not enhance vaccine efficacy. Taken together, these data indicated that anti-TGF-β enhances efficacy of a prophylactic vaccine in normal individuals despite their not having the elevated TGF-β levels found in cancer patients and that the effect is not dependent on TGF-β solely from CD4+CD25+ T regulatory cells or the NKT cell-IL-13-IL-4R-STAT-6 immunoregulatory pathway.
Introduction
The success of cancer immunotherapy depends on overcoming immune suppression in patients. There are multiple mechanisms suggested to suppress anti-tumor immunity. TGF-β plays important roles in several of such mechanisms of immune suppression.
TGF-β is a highly pleiotropic cytokine and can be produced by many lymphoid and non-lymphoid cells 1. TGF-β can directly enhance growth, metastasis, and angiogenesis of some tumors 2-7. In anti-tumor immunity, tumor antigen specific cytotoxic T lymphocytes (CTLs) play crucial roles in eradicating tumors. However, TGF-β inhibits the anti-tumor immune response at several levels including the production of perforin, granzyme A, granzyme B, FAS ligand, and IFN-γ by CTLs in vitro and in vivo 8. In human patients with melanoma, antigen-specific CD8+ T-cell effector function in vitro is inhibited by the addition of TGF-β 9.
TGF-β also influences dendritic cells (DCs), which are critical in priming protective CD4+ Th 1 and CD8+ CTL – mediated anti-tumor responses. TGF-β can inhibit DC migration and antigen transport to draining lymph nodes (LNs) within murine skin tumors, effectively obstructing T-cell activation 10. In addition to such an immobilization of DCs, TGF-β may also decrease DC numbers by escalating apoptosis 11 and limit their function by inhibiting maturation and expression of major histocompatibility complex (MHC) class II and costimulatory molecules 1. Moreover, TGF-β plays an important role in the development and / or function of several classes of regulatory T cells including T regulatory 1 cells (Tr1), T helper 3 cells (Th3), Th17 and CD4+CD25+Foxp3+ T regulatory cells 12-14. Some regulatory T cells suppress tumor-specific CD8+ T cell cytotoxicity through TGF-β signals in vivo 15. Since TGF-β maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells 16, TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells 17. T regulatory cells have been shown to suppress immunosurveillance in the CT26 subcutaneous tumor model 18, so blockade of TGF-β may suppress CD4+CD25+T regulatory cells, and lead to the enhancement of anti-tumor immunity.
Recently, we have identified another new immunosuppressive mechanism involving TGF-β in tumor immunity. Specifically, in a fibrosarcoma model, CD1d-restricted NKT cells activate a negative immunoregulatory pathway, in which IL-4R-STAT-6 signaling activated by IL-13 induces TGF-β production and as a result, this TGF-β is the final effector to suppress CD8+ CTL function 19, 20. In this tumor model, blockade of TGF-β leads not only to the complete prevention of tumor recurrence, but also to the enhancement of the cytotoxic activity of CTL in vitro. Moreover, in another tumor model, we have shown that the blockade of TGF-β, IL-13, or the abrogation of NKT cells leads to a significant reduction of lung metastases after iv injection of CT26 tumors 20, 21. Other studies have also shown improvement of anti-tumor immunity by TGF-β blockade 22-26. These data suggested that the blockade of TGF-β may enhance the CTL response against tumors and lead to the inhibition of tumor growth. However, in other tumor models, blockade of TGF-β did not always protect against tumor growth 27. In such cases in which TGF-β blockade was not sufficient to unmask spontaneous tumor immunosurveillance, we hypothesized that a prophylactic anti-tumor vaccine (whole cell vaccine or tumor-antigen specific peptide vaccine) may complement the effect of TGF-β blockade to enhance anti-tumor immunity. Such a complementary effect was seen in at least one tumor model 28.
In the present study, we showed that the blockade of TGF-β synergistically enhanced whole cell vaccine efficacy in the s.c. CT26 tumor model, and the protection was mediated by CD8+ T cells. Distinct from other tumor models, the immunological mechanism of the anti-tumor effect of TGF-β blockade in this s.c. CT26 model was independent of both the NKT-cell, IL-13, and IL-4R-STAT-6 pathway and CD4+CD25+ T regulatory cells. Our data indicated that the blockade of TGF-β may prevent the suppression of CD8+ T cell function in a prophylactic vaccine tumor-free setting in which TGF-β cannot be from the tumor but must have an immunologic origin, and leads to the enhancement of tumor vaccine efficacy.
Materials and Methods
Mice
Inbred BALB/c mice were purchased from the Frederick Cancer Research Facility. BALB/c CD1d knockout mice (CD1d KO mice; provided by M. Grusby, Harvard University, Boston, MA or purchased from Jackson Laboratory, Bar Harbor, ME), BALB/c IL-13 knockout mice, and BALB/c IL-4-IL-13 double knockout mice (provided from A.N.J. McKenzie) were bred at the National Cancer Institute. All gene-targeted mice were backcrossed onto the BALB/c background for at least 10 generations. IL-4 knockout mice and STAT6 knockout mice with the BALB/c background were obtained from the Jackson Laboratory. IL-4 receptor alpha knockout mice on the BALB/c background were obtained from TACONIC. All mice were maintained in a pathogen-free animal facility. Female mice > 6 wk old were used in all experiments. All animal experiments were approved by the Animal Care and Use Committee of the National Cancer Institute.
Antibodies
Purified mAbs reactive with mouse CD8 (2.43; American Type Culture Collection) and CD25 (PC61; American Type Culture Collection) were purified from ascites by Harlan Bioproducts for Science, Inc. Monoclonal anti-TGF-β (1D11.16 specific for TGF-β1, TGF-β2, and TGF-β3) and isotype-matched control antibody (13C4) were made and provided by Genzyme. FITC-conjugated anti-CD4 (RM4-5), APC-conjugated anti-CD25 (PC61.5), and PE-conjugated anti-Foxp3 (FJK-16s) were purchased from eBioscience.
Tumor cell lines
The CT26 cell line (a N-nitro-N-methylurethane–induced BALB/c murine colon carcinoma) was provided by Dr. N. Restifo (NCI, NIH, Bethesda, MD, USA) and maintained in RPMI-1640 complete medium supplemented with 10% FCS, penicillin/streptomycin, L-glutamine, sodium pyruvate, nonessential amino acids, and 2 mercaptoethanol (5 × 10 −5 M).
Vaccination and tumor inoculation
1×105 irradiated (25,000 rad) CT26 cells were injected subcutaneously (sc.) into various groups of mice. Some vaccinated or unvaccinated mice were treated with 200 μg (at the time of vaccination and CT26 challenge) or 100 μg (other time points) anti-TGF-β mAb or control mAb intraperitoneally (ip) three times a week from the time of vaccination to 2 weeks after CT26 challenge. 3 weeks after vaccination, 1×106 live CT26 cells were injected sc. into these mice. 2 and 1 day before, and 4, 7, 10, and 14 days after CT26 challenge, some vaccinated mice treated with anti-TGF-β mAb were also treated with 0.5mg anti-CD8 mAb.
Flow Cytometric Analysis (intracellular Foxp3 staining)
For the detection of CD4+ CD25+Foxp3+ T cells, spleen cells were obtained from vaccinated or unvaccinated mice treated with or without anti-TGF-β. Tumor infiltrating lymphocytes were prepared from pooled tumors. Briefly, the tumors were minced well with scissors in RPMI1640 and mashed on a nylon membrane (pore size 100 μm). After a wash of the cell suspension, lymphocytes were isolated by using Lymphoid M (Cederlane). The cells were stained with anti-CD3, anti-CD4 and anti-CD25 mAb for 30 min after blocking CD16/CD32 (2,4G2; BD Biosciences) for 15 min before permeabilization. These cells were then incubated with anti-Foxp3 for 30 min, and after washing, evaluated by flow cytometry on a FACSCaliber™ or LSRII (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc).
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA), log-rank test, or Student's t test. Data were considered significant at p < 0.05.
Results
1D11 synergistically enhances whole cell vaccine efficacy, and the tumor rejection is mediated by CD8+ T cells
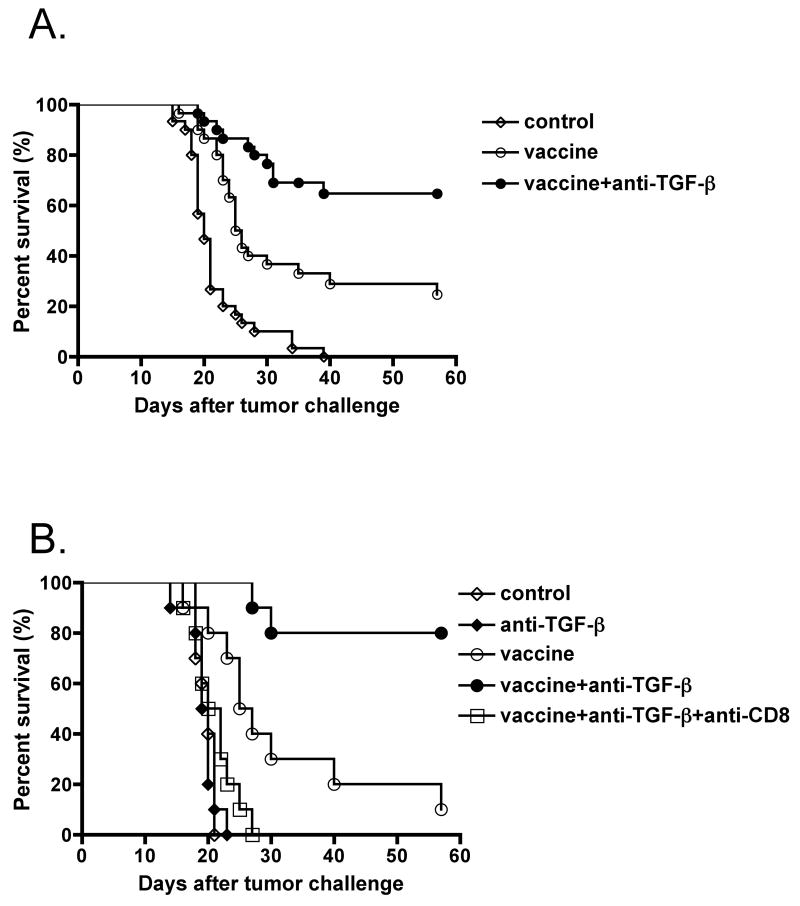
To test the hypothesis that anti-TGF-β antibody may synergize with an anti-cancer vaccine, we examined the anti-tumor effect of 1D11 (anti-TGF-β) on a whole cell vaccine in a prophylactic setting against s.c. injected CT26 tumors in syngeneic BALB/c mice. We immunized mice with 1×105 irradiated CT26 tumor cells. Some mice were also treated with 1D11 three times a week for 2 weeks. Three weeks after vaccination, these mice were challenged with 1×106 live CT26 tumor cells. As shown in Fig.1A & B, the whole tumor cell vaccine with control IgG (13C4) induced a significant delay of tumor growth compared to control or 1D11-treated mice without vaccination. However, vaccinated mice treated with 1D11 showed significantly better protection from tumor growth, even if palpable tumors temporarily occurred after the tumor challenge. The survival rate in vaccinated mice treated with 1D11 was significantly higher than that in vaccinated mice without 1D11 (67% vs. 23%, p< 0.002) (Fig.1A). To see whether this protection was mediated by CD8+ T cells, some vaccinated mice treated with 1D11 were also treated with anti-CD8 Ab for depletion of CD8+ T cells in vivo. As shown in Fig.1B, the anti-tumor effect in these mice was abrogated by the depletion of CD8+ T cells in vivo two days before tumor challenge. These data suggested that the blockade of TGF-β synergistically enhances anti-tumor immunity in conjunction with a prophylactic whole cell vaccine, and the protection is mediated by CD8+ T cells.
Fig.1. Blockade of TGF-β synergistically enhances whole cell vaccine efficacy in mice and the protection is mediated by CD8+ T cells.
1×105 irradiated (25,000 rad) CT26 cells were injected subcutaneously (sc.) into BALB/c mice. Some vaccinated or unvaccinated mice were treated with anti-TGF-β monoclonal antibody (1D11.16) or control antibody (13C4) intraperitoneally (ip) first with 200 μg at the time of vaccination and of the CT26 challenge, and then with 100 μg three times a week from the time of vaccination to 2 weeks after CT26 challenge. The control group was untreated. Three weeks after vaccination, 1×106 live CT26 cells were injected sc. into these mice. Two and 1 day before, and 4, 7, 10, and 14 days after CT26 challenge, some vaccinated mice treated with 1D11 were also treated with 0.5 mg of anti-CD8 monoclonal antibody (2.43). Tumors were measured by a caliper gauge, and tumor size was determined as the product of tumor length (mm) × tumor width (mm). Five female BALB/c mice were used for each group per each experiment. Mice were euthanized when the tumor area exceeded 1 cm2, and data were represented as percent survival. (A) The results were pooled from six different experiments comparing vaccine with anti-TGF-β vs control antibody (n=30, each group). p<0.0001 by log-rank test between control and vaccine plus control antibody group. p<0.002 by log-rank test between vaccine plus control antibody group and vaccine plus anti-TGF-β monoclonal antibody group. (B) The results were pooled from two different experiments in which an anti-CD8-treated group was included. p<0.002 by log-rank test between control and vaccine plus control antibody group. p<0.005 by log-rank test between vaccine plus control antibody group and vaccine plus anti-TGF-β monoclonal antibody group.
Anti-tumor effect of anti-TGF-β antibodies in the s.c. CT26 tumor model is independent of CD4+CD25+ T regulatory cells
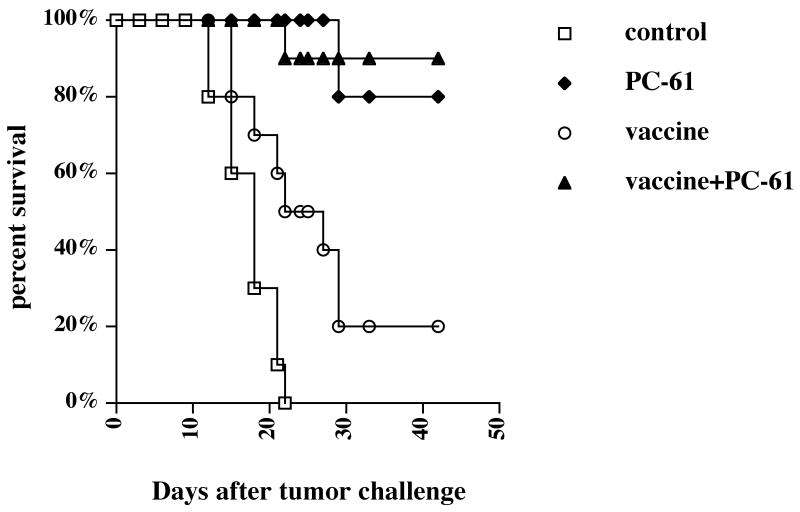
To determine whether the mechanism of the enhancement of tumor vaccine efficacy by anti-TGF-β Ab (1D11) was due to the abrogation of CD4+CD25+ T regulatory cells, we challenged un-vaccinated and vaccinated mice treated with anti-CD25 monoclonal antibodies (PC-61) with CT26 tumors. As shown in Fig.2, almost all vaccinated mice treated with PC-61 rejected tumors as did vaccinated mice treated with 1D11 in Fig.1. However, almost all un-vaccinated mice treated with PC-61 also rejected tumors, whereas no un-vaccinated mice treated with 1D11 showed any inhibition of tumor growth in Fig.1. These data suggested that depletion of CD4+CD25+ T regulatory cells enhanced anti-tumor immunity in the s.c. CT26 tumor model regardless of vaccination, consistent with Golgher et.al 18, so the mechanism of enhancement of tumor vaccine efficacy by the depletion of CD4+CD25+ T regulatory cells does not appear to be equivalent to that of 1D11.
Fig.2. Depletion of CD4+CD25+ T regulatory cells leads to tumor rejection in mice regardless of vaccination.
3 and 1 day prior to vaccination, 1 mg anti-CD25 mAb (PC-61) or rat IgG mAb was injected ip. into BALB/c mice, and then 1×105 irradiated (25,000 rad) CT26 cells as a vaccine were injected sc. into these mice. 3 weeks after vaccination, 1×106 live CT26 cells were injected sc. into these mice. Tumors were measured by a caliper gauge, and tumor size was determined as the product of tumor length (mm) × tumor width (mm). Five female BALB/c mice were used for each group per each experiment. Mice were euthanized when the tumor area exceeded 1 cm2, and the data were represented as percent survival. The results were pooled from two independent experiments. p<0.01 by log-rank test between control and vaccine alone group. p<0.0001 by log-rank test between control group and PC-61-treated groups with or without vaccination.
We also measured the number of T regulatory cells in spleens of un-vaccinated or vaccinated mice treated with 1D11 at the time of tumor challenge. As shown in Fig. 3A, we could not see any difference in the number of CD4+CD25+ T regulatory cells regardless of 1D11 inoculation at the time of tumor challenge. In most mice treated with vaccine and 1D11, regulatory cells could not be measured in tumors as no tumors were present to harvest. Therefore, to see whether 1D11 showed any impact on Treg cells in tumor-challenged mice, we examined the number of Treg cells in draining lymph nodes of tumors of mice treated with 1D11 or 13C4 without vaccine. As shown in Fig. 3B, there was no difference in the proportion of CD4+CD25+ Foxp3+ T regulatory cells in tumor draining lymph nodes between these two groups of mice. We also investigated the number of tumor infiltrating Treg cells in these mice (Fig. 3C). Consistent with the results of tumor draining lymph nodes, there was no difference in the proportion of CD4+CD25+ Foxp3+ T regulatory cells in tumors between these two groups of mice. Taken together, these data indicated that the enhancement of tumor vaccine efficacy by 1D11 may be independent of CD4+CD25+ T regulatory cells.
Fig.3. Enhancement of tumor vaccine efficacy by anti-TGF-β antibody is independent of CD4+CD25+ T regulatory cells.
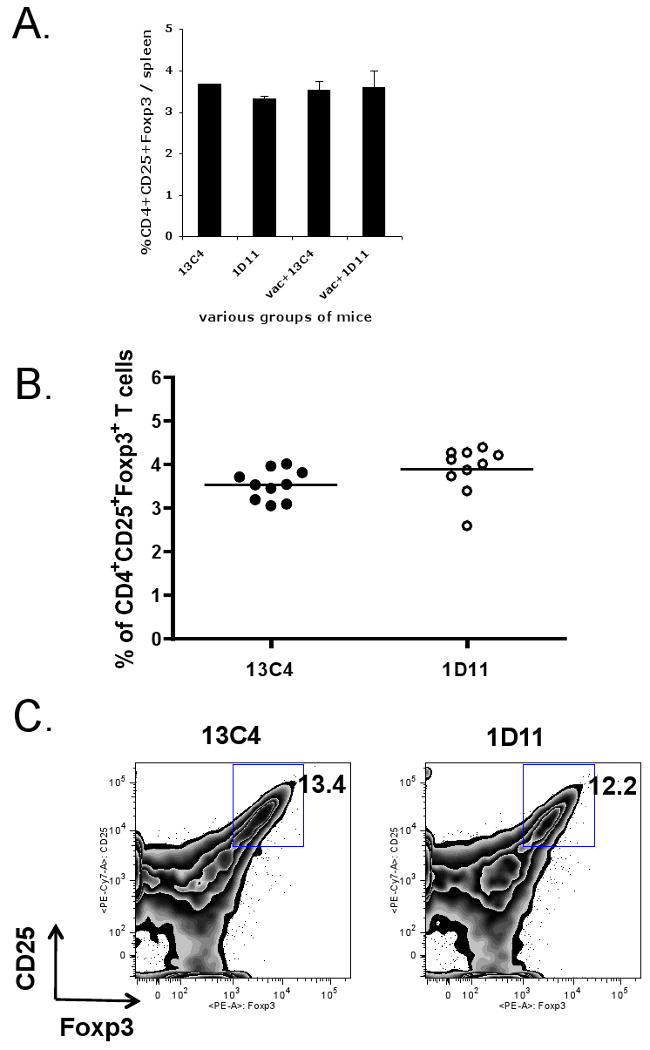
1×105 irradiated (25,000 rad) CT26 cells were injected sc. into BALB/c mice. Some vaccinated or unvaccinated mice were treated with 200 μg (at the time of vaccination and CT26 challenge) or 100 μg (other time points) 1D11 or 13C4 i.p. three times a week. 3 weeks after vaccination, spleens were removed from these mice. These splenocytes were stained for CD4 and CD25 followed by anti-Foxp3 intracellular staining and analyzed by flow cytometry. 4∼5 female mice were used for each group. A. % of CD4+ CD25+ Foxp3+ cells per spleen. B. 10 days after tumor injection, tumor draining lymph node cells were recovered and stained with anti-CD3, anti-CD4, anti-CD25, and anti-Foxp3 in 1D11- and 13C4-treated mice without the tumor vaccine. The proportions of CD3+CD4+CD25+Foxp3+ T cells were determined by flow cytometry. 10 female mice were used for each group. Each symbol represents one data point. Median is shown as bars. C. 10 days after tumor challenge, tumor infiltrating Treg cells were examined by flow cytometry. Tumor infiltrating lymphocytes were recovered from 5 pooled tumors as described in the materials and methods section, and stained with anti-CD3, anti-CD4, anti-CD25, and anti-Foxp3 in 1D11- and 13C4-treated mice without the tumor vaccine. Presented density plots were gated on the CD3+CD4+ population. These experiments were repeated at least twice with comparable results.
Abrogation of the NKT cell-IL-13-IL-4R-STAT-6 immunoregulatory pathway does not enhance vaccine efficacy
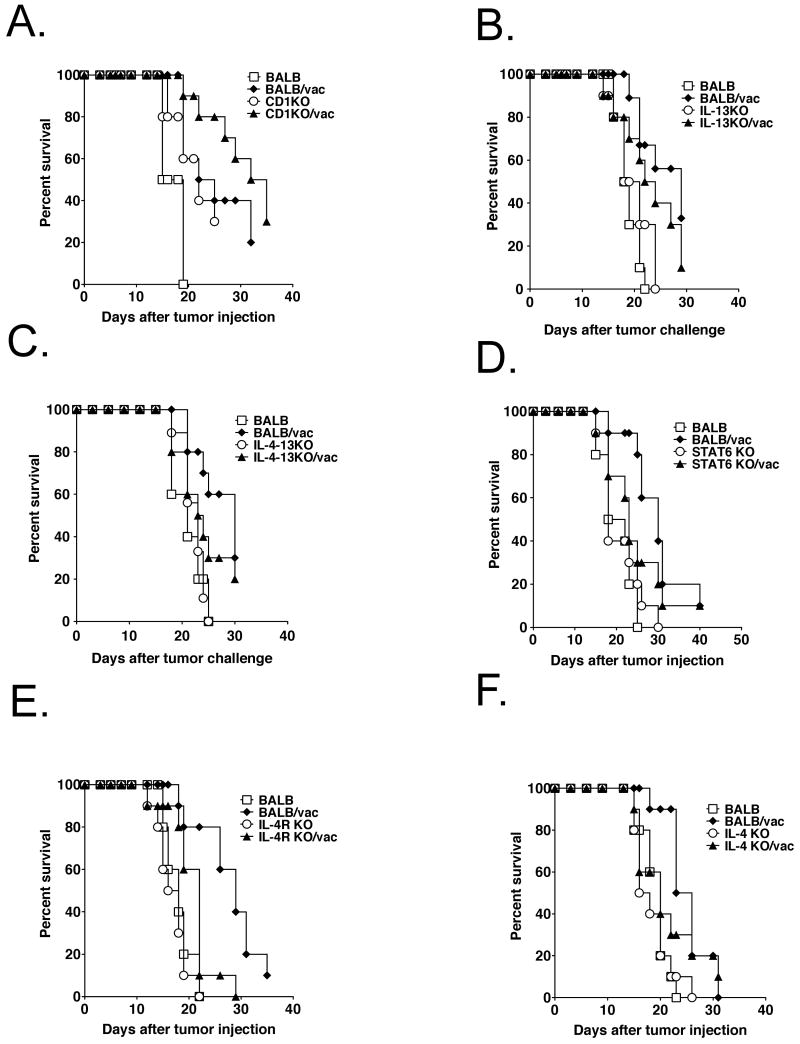
To determine whether the mechanism of the anti-TGF-β enhancement of tumor vaccine efficacy was due to abrogation of the NKT cell, IL-13, and IL-4R-STAT-6 immunoregulatory pathway that leads to TGF-β production, we injected CT26 tumor cells into un-vaccinated and vaccinated mice of various KO strains, lacking one of the components of the NKT cell, IL-13, and IL-4R-STAT-6 immunoregulatory pathway. We asked whether knocking out one of these genes in the absence of anti-TGF-β mimicked the effect of anti-TGF-β on vaccine efficacy. In accordance with some other tumor models, tumor growth was partially inhibited in un-vaccinated CD1KO mice, which lack NKT cells, compared to that in wild-type (wt) un-vaccinated BALB/c mice (Fig. 4A). However, enhancement of tumor vaccine efficacy (defined by the difference between vaccinated and unvaccinated mice) over that in w.t. vaccinated mice was not observed in vaccinated CD1KO mice (Fig. 4A). Surprisingly, no inhibition of the tumor growth was observed in unvaccinated IL-13KO, IL-4-13KO, IL-4KO, IL-4 receptor alpha KO, or STAT-6 KO mice compared to un-vaccinated BALB/c mice. Moreover, none of these vaccinated mice showed enhancement of vaccine efficacy (fig.4 B∼F). These data suggested that though abrogation of NKT cells showed partial inhibition of tumor growth, enhancement of tumor vaccine efficacy by 1D11 may be not the result of inhibiting the NKT cell, IL-13, and IL-4R-STAT-6 immunoregulatory pathway in the s.c. CT26 tumor model. Interestingly, the trend toward prolonged survival in the vaccinated wild-type mice was completely lost in the IL-4 KO, STAT-6 KO, and IL-4 -13 KO, but not in the IL-13 single KO mice although not statistically significant (p = 0.0894). Moreover, though vaccinated IL-4Rα KO mice showed prolonged survival compared to un-vaccinated IL-4Rα KO mice as in the case of wild-type mice, the impact of vaccination in IL-4Rα KO mice was much smaller than that in wild-type mice (p = 0.0116 between un-vaccinated and vaccinated IL-4Rα KO mice. p = 0.0002 between un-vaccinated and vaccinated wild-type mice). These results may be due to a requirement for IL-4 for CTL induction as reported by Schuler et.al29.
Fig. 4. Abrogation of the NKT cell-IL-13-IL-4R-STAT-6 immunoregulatory pathway does not enhance vaccine efficacy.
1×105 irradiated (25,000 rad) CT26 cells were injected sc. into wt. BALB/c, BALB-CD1KO (A), BALB- IL-13 KO (B), BALB-IL-4-13KO (C), BALB-STAT-6 KO (D), BALB-IL-4R KO (E), or BALB-IL-4 KO (F) mice. 3 weeks after vaccination, 1×106 live CT26 cells were injected sc. into these mice. Tumors were measured by a caliper gauge, and tumor size was determined as the product of tumor length (mm) × tumor width (mm). Five female BALB/c mice were used for each group per each experiment. Mice were euthanized when the tumor area exceeded 1 cm2, and data were represented as percent survival. The results were pooled from two independent experiments. p<0.001 in A and B, p<0.002 in C, p<0.0005 in D, E, and F by log-rank test between un-vaccinated BALB/c mice and vaccinated BALB/c mice. p<0.01 in A by log-rank test between un-vaccinated BALB/c mice and CD1KO mice. p<0.02 in E by log-rank test between un-vaccinated IL-4R KO mice and vaccinated IL-4R KO mice.
Discussion
Our previous study showed that systemic anti-TGF-β treatment protected mice from tumor recurrence in a sc. fibrosarcoma model and significantly reduced lung metastases in the i.v. CT26 tumor model 20. However, this treatment was not always sufficient for the complete rejection of tumors. To maximize the anti-tumor effect of TGF-β blockade, a combination immunotherapy with some vaccine is a very attractive way to enhance antitumor immunity. In the present study, we investigated the anti-tumor effect of anti-TGF-β Ab (1D11) combined with a whole cell vaccine in the sc. CT26 tumor model. The whole cell vaccine alone showed a significant delay of tumor growth, but the survival rate was still low (23%), suggesting that the whole cell vaccine was not sufficient for the rejection of tumors. Likewise, 1D11 alone did not show any anti-tumor effect against CT26. However, vaccination combined with 1D11 induced better protection from tumors, and the survival rate was much higher (67%) (Fig,1A and B). These data suggested that the blockade of TGF-β synergistically enhanced the whole cell vaccine efficacy in the prophylactic setting in the s.c CT26 tumor model. Since depletion of CD8+ T cells in vivo led to the complete loss of protection from tumors in vaccinated mice treated with 1D11(Fig.1B.), the protection was mediated by CD8+ T cells. Recently Kim et al., reported that a kinase inhibitor of TGF-β receptor (SM16) enhanced the therapeutic effect of a vaccine, which was administered when tumors became approximately 200 mm2 30. However, the effect of TGF-β blockade by antibody has different in vivo kinetics from that of the small chemical. Moreover, it was not clear whether we can observe any effect of TGF-β blockade in normal mice that do not have a high level of TGF-β as found in tumor bearing mice. In this study, we showed that at least TGF-β blockade by anti-TGF-β Ab significantly increased the efficacy of the whole cell vaccine in normal healthy mice. These results suggest that blockade of TGF-β may be an attractive strategy not only for cancer vaccines but also for other vaccines targeting T cell responses as well.
Recently, other investigators showed that regulatory T cells suppress tumor-specific CD8+ T cell cytotoxicity through TGF-β signals in vivo 15. Since TGF-β also maintains suppressor function and Foxp3 expression in CD4+CD25+ T regulatory cells 16 and TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells 17, we hypothesized that blockade of TGF-β may suppress CD4+CD25+T regulatory cells, and as a result, lead to the enhancement of anti-tumor immunity. At first, to see whether abrogation of T regulatory cells showed any anti-tumor effect against CT26, we injected CT26 tumor cells into un-vaccinated and vaccinated mice pre-treated with anti-CD25 antibody (PC-61). In accordance with other investigators' reports 18, 31, depletion of CD4+CD25+ T regulatory cells led to the almost complete rejection of tumors independent of vaccination (Fig.2). If the anti-tumor effect of 1D11 was associated with CD4+CD25+ T regulatory cells in this tumor model, 1D11 alone should show some anti-tumor effect against CT26. However, as shown in Fig.1A, 1D11 alone did not show any anti-tumor effect against CT26. These data suggested that the anti-tumor effect of 1D11 was not equivalent to that afforded by depletion of CD4+CD25+ T regulatory cells. Since T regulatory cells have multiple ways to inhibit immune responses other than TGF-β 32, 33, the anti-tumor impact of depleting of T regulatory cells may be much stronger than that of 1D11. We also measured the number of CD4+CD25+ T regulatory cells in 1D11-treated un-vaccinated or vaccinated mice. However, regardless of 1D11 treatment, the number of T regulatory cells was almost the same among these groups (Fig.3A). Moreover, the frequency of intratumoral T regulatory cells in tumors of 1D11- treated and untreated mice were very similar (Fig.3C), as were T reg numbers in tumor draining lymph nodes (Fig. 3B). Though we cannot exclude the possibility that the suppressive function of CD4+CD25+ T regulatory cells may be inhibited by 1D11 in vaccinated mice, these data suggested that the anti-tumor effect of 1D11 may be independent of CD4+CD25+ T regulatory cells.
Recently, we have shown that a new immunosuppressive mechanism involving NKT cells, IL-13, and IL-4R-STAT-6 leads to the suppression of CD8+ CTL function via TGF-β in several tumor models 19, 20. Therefore, we next investigated whether the anti-tumor effect of TGF-β blockade was due to the abrogation of the NKT cell, IL-13, and IL-4R-STAT-6 immunoregulatory pathway by using various gene-targeted mice. Though CD1KO mice manifested partially enhanced anti-tumor immunity against s.c. CT26 tumors analogous to the result in the i.v. CT26 model 21, they did not show any enhancement of tumor vaccine efficacy (Fig.4 A). These data suggested that the enhancement of tumor vaccine efficacy by 1D11 was independent of CD1d restricted NKT cells. Since we previously reported an osteosarcoma tumor model in which tumor rejection was mediated by the abrogation of CD1d restricted NKT cells, but independent of the IL-4R-STAT-6 immunoregulatory pathway or TGF-β, the immunological mechanism of the partial anti-tumor effect by the abrogation of CD1d restricted NKT cells in the s.c. CT26 may be similar to that in this osteosarcoma model 27. Similarly, when we challenged various vaccinated KO mice (IL-13KO, IL-4-IL-13 KO, IL-4KO, IL-4R KO, and STAT-6KO) with CT26 tumors sc, none of them showed either an anti-tumor effect or enhancement of tumor vaccine efficacy compared to wild-type vaccinated mice (Fig.4 B-F). These data suggested that the anti-tumor effect of 1D11 was not dependent solely on TGF-β from the NKT cell, IL-13 and IL-4R-STAT-6 immunoregulatory pathway.
Since CT26 tumor can activate the NKT cell, IL-13 and IL-4Rα-STAT6 immunoregulatory pathway in the i.v. challenge model 20, 34, the route of tumor administration and the organ microenvironment may be important to determine whether this pathway gets activated. Recently it has been shown that induction of IL-13Rα2 expression by IL-13 and TNF-α is necessary for the NKT cell, IL-13 and IL-4Rα-STAT6 immunoregulatory pathway to inhibit tumor-specific CTL function 35. Thus, it may be possible that in vivo TNF-α production is insufficient to induce IL-13Rα2 expression, with the result that such a counter-immunosurveillance mechanism is not activated in s.c. CT26 tumor model.
Overall, because the anti-TGF-β is effective when given with the prophylactic vaccine before injection of the tumor, it appears to improve the immunity induced by the vaccine independent of any inhibitory effects of TGF-β made by the tumor, which is not yet present. Thus, the source of TGF-β that is preventing optimal prophylactic vaccine efficacy must be the immune system itself. Because we have found that no single source of TGF-β, whether T reg cells or myeloid cells activated by NKT cells, is sufficient to explain the need to block TGF-β to maximize vaccine efficacy, we expect that there are likely multiple sources of TGF-β that dampen the vaccine response, all of which are inhibited by blockade of TGF-β. It is this action at the downstream end of many sources of suppressive cytokine that may account for the effectiveness of this treatment.
It has been reported that T helper cell type-1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in IL-4 KO mice in the s.c. CT26 tumor model 36. In this study, it was shown that generation of tumor-associated CTLs required IL-4 from CD8+ T cells 29. In accordance with these observations, IL-4KO, IL-4-IL-13 double KO, and STAT-6KO mice did not show any trend toward partial protection by vaccine alone in contrast to WT and IL-13 single KO mice (Fig. 4). It has been also reported that the presence of CD4+ T cell help restores the development of CD8+ T cell responses against influenza infection in IL-4Rα KO mice 37. In accordance with these observations, in our study, vaccinated IL-4Rα KO mice showed prolonged survival after CT26 challenge compared to un-vaccinated IL-4Rα KO mice, as in the case of wild-type mice. However, the impact of vaccination in IL-4Rα KO mice was much smaller than that in wild-type mice (approximately a 5-day prolongation of median survival, p = 0.0116, between un-vaccinated and vaccinated IL-4Rα KO mice vs approximately an 11-day prolongation, p = 0.0002, between un-vaccinated and vaccinated wild-type mice). These data suggested a requirement for IL-4 in CD8-dependent vaccine efficacy.
Since 1D11 showed strong impact on tumor growth in vivo only when inoculated with a tumor vaccine (Fig.1A), TGF-β may be associated with the termination of expanding tumor-specific CD8+ T cells induced by the tumor vaccine. Consistent with this speculation, other investigators recently identified a novel role for TGF-β as a key inducer of apoptosis of the short lived effector CD8+ T cells during acute immune response after Listeria infection, and showed that such TGF-β–mediated apoptosis of this effector subpopulation occurred during clonal expansion and contraction 38. Therefore, it may be possible that the enhancement of a tumor vaccine by 1D11 is accomplished by the inhibition of apoptosis of tumor-specific CD8+ T cells via the neutralization of TGF-β possibly produced by effector T cells, Treg cells or antigen-presenting cells.
Recently, It has been reported that TGF-β and IL-6 together induce the differentiation of Th17 cells from naïve cells 39, and that TGF-β plays a crucial role for inducing IL-17 producing CD8+ T cells in tumor-bearing mice, whose IL-17 contributes to promote tumor growth 40. Therefore, it may be possible that blockade of TGF-β enhances tumor vaccine efficacy through preventing the induction of IL-17 producing Th17 cells indispensable for the survival of tumors. These issues warrant further investigation.
In summary, the blockade of TGF-β by anti-TGF-β synergistically enhanced tumor vaccine efficacy, and the protection was mediated by CD8+ T cells. The anti-tumor effect of the blockade of TGF-β was independent of both CD4+CD25+ T regulatory cells and the NKT cell, IL-13, and IL-4R-STAT-6 immunoregulatory pathway. Nevertheless, concerns have been expressed regarding the safety of inhibiting TGF-β experimental animals and humans given the pleiotropic nature of this cytokine. 1D11 is one of the most effective antibodies for the purpose of systemic inhibition of TGF-β 41. Since immune-mediated disease and even lethality was associated with the genetic ablation or inhibition of TGF-β signaling in mice, it was unclear if inhibiting this pathway to treat cancer would lead to unaccepted side effects when delivered for a sustained period in vivo 42-45. However, it has been recently shown that a lifetime exposure to systemic soluble TGF-β1, TGF-β3, or pan-TGF-β inhibitors in mouse models did not result in significant adverse effects 46, 47. These animal studies have shown that antibody-mediated TGF-β-specific inhibition does not result in the types of serious biological abnormalities that have been observed in TGF-β knockout mice. Consistent with these previous reports, no apparent adverse effects were noted in the mice treated with anti-TGF-β in the current study. Furthermore, a human pan neutralizing anti-TGF-beta antibody (Fresolimumab) has been reported to be well tolerated when evaluated as a single agent in a phase 1 clinical trial in patients with malignant melanoma48. Taken together, these results suggest that antibody-mediated inhibition of TGF-β provides a plausible clinical approach to enhance the efficacy of cancer immunotherapy strategies.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The National Cancer Institute has a Cooperative Research and Development Agreement with the Genzyme Corporation.
Footnotes
SL and JM are employees of the Genzyme Corporation, and thus may have the conflict of interest.
References
- 1.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16551245.
- 2.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83(12):4167–71. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2424019.
- 3.Desruisseau S, Ghazarossian-Ragni E, Chinot O, Martin PM. Divergent effect of TGFbeta1 on growth and proteolytic modulation of human prostatic-cancer cell lines. Int J Cancer. 1996;66(6):796–801. doi: 10.1002/(SICI)1097-0215(19960611)66:6<796::AID-IJC15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8647652.
- 4.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8(1):21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9174661.
- 5.Ananth S, Knebelmann B, Gruning W, Dhanabal M, Walz G, Stillman IE, Sukhatme VP. Transforming growth factor beta1 is a target for the von Hippel-Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res. 1999;59(9):2210–6. [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10232610.
- 6.Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999;17(2):607–14. doi: 10.1200/JCO.1999.17.2.607. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10080606.
- 7.Yan Z, Deng X, Friedman E. Oncogenic Ki-ras confers a more aggressive colon cancer phenotype through modification of transforming growth factor-beta receptor III. J Biol Chem. 2001;276(2):1555–63. doi: 10.1074/jbc.M004553200. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11029459.
- 8.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16286245.
- 9.Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174(9):5215–23. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15843517
- 10.Weber F, Byrne SN, Le S, Brown DA, Breit SN, Scolyer RA, Halliday GM. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother. 2005;54(9):898–906. doi: 10.1007/s00262-004-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15776284
- 11.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176(9):5637–43. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16622033
- 12.Chen W, Jin W, Cook M, Weiner HL, Wahl SM. Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J Immunol. 1998;161(11):6297–304. [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9834119
- 13.Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14(2):85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12651220
- 14.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18(2):120–7. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16464609
- 15.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-{beta} signals in vivo. Proc Natl Acad Sci U S A. 2005;102(2):419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15623559.
- 16.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-{beta}1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15809351.
- 17.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173(11):6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15557141.
- 18.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267–75. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12555672.
- 19.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunology. 2000;1(6):515–20. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 20.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, et al. Transforming Growth Factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. International J of Cancer. 2004;114(1):80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 22.Won J, Kim H, Park EJ, Hong Y, Kim SJ, Yun Y. Tumorigenicity of mouse thymoma is suppressed by soluble type II transforming growth factor beta receptor therapy. Cancer Res. 1999;59(6):1273–7. [PubMed] [Google Scholar]
- 23.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92(6):2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoefer M, Anderer FA. Anti-(transforming growth factor beta) antibodies with predefined specificity inhibit metastasis of highly tumorigenic human xenotransplants in nu/nu mice. Cancer Immunol Immunother. 1995;41(5):302–8. doi: 10.1007/BF01517218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109(12):1551–59. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7(10):1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=11590434.
- 27.Terabe M, Khanna C, Bose S, Melchionda F, Mendoza A, Mackall CL, Helman L, Berzofsky JA. CD1d-restricted NKT cells can down-regulate tumor immunosurveillance independent of IL-4R-STAT6 or TGF-β. Cancer Research. 2006;66(7):3869–75. doi: 10.1158/0008-5472.CAN-05-3421. [DOI] [PubMed] [Google Scholar]
- 28.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167(11):6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11714814.
- 29.Schuler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J Exp Med. 2001;194(12):1767–75. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11748278.
- 30.Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X, Corbley M, Kaiser LR, Ling L, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68(24):10247–56. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19074893
- 31.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171(11):5931–9. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14634104.
- 32.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18285775.
- 33.Vignali D. How many mechanisms do regulatory T cells need? Eur J Immunol. 2008;38(4):908–11. doi: 10.1002/eji.200738114. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18395857.
- 34.Ambrosino E, Berzofsky JA, Terabe M. Regulation of tumor immunity: the role of NKT cells. Expert Opin Biol Ther. 2008;8(6):725–34. doi: 10.1517/14712598.8.6.725. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18476784
- 35.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68(9):3467–75. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18451175
- 36.Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 1999;189(5):803–10. doi: 10.1084/jem.189.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0010049944.
- 37.Marsland BJ, Schmitz N, Kopf M. IL-4Ralpha signaling is important for CD8+ T cell cytotoxicity in the absence of CD4+ T cell help. Eur J Immunol. 2005;35(5):1391–8. doi: 10.1002/eji.200425768. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15816016
- 38.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31(1):131–44. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19604492.
- 39.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16648838
- 40.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, et al. An Anti-Transforming Growth Factor {beta} Antibody Suppresses Metastasis via Cooperative Effects on Multiple Cell Compartments. Cancer Res. 2008;68(10):3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18483268
- 41.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L, Allen JB. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142(5):1536–41. [PubMed] [Google Scholar]
- 42.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-b1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–0. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10714683.
- 44.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8421714.
- 45.Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100(2):560–8. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12091349.
- 46.Ruzek MC, Hawes M, Pratt B, McPherson J, Ledbetter S, Richards SM, Garman RD. Minimal effects on immune parameters following chronic anti-TGF-beta monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol. 2003;25(2):235–57. doi: 10.1081/iph-120020473. [DOI] [PubMed] [Google Scholar]; Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12784916.
- 47.Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109(12):1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olencki TE, Dezube BJ, Hsu FJ, Reiss M, Berzofsky JA. Phase I/II Study of GC1008: A human anti-transforming growth factor-beta (TGFb) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC) J Clin Oncol. 2008;26(15S Part I of II) doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]