Abstract
The adenosine A2A-dopamine D2 receptor heteromer is one of the most studied receptor heteromers. It has important implications for basal ganglia function and pathology. Recent studies using Bioluminescence and Sequential Resonance Energy Transfer techniques shed light on the role of Ca2+ in the modulation of the quaternary structure of the A2A-D2 receptor heteromer, which was found to depend on the binding of calmodulin (CaM) to the carboxy terminus of the A2A receptor in the A2A-D2 receptor heteromer. Importantly, the changes in quaternary structure correlate with changes in function. A Ca2+/CaM-dependent modulation of MAPK signaling upon agonist treatment could only be observed in cells expressing A2A-D2 receptor heteromers. These studies provide a first example of a Ca2+-mediated modulation of the quaternary structure and function of a receptor heteromer.
Introduction
Ca2+ plays an important role in the physiology of higher order organisms and is involved in the regulation of many cellular events. Various stimuli, such as membrane depolarization or binding of ligands to plasma transmembrane receptors trigger Ca2+-channel opening, which results in a significant influx of Ca2+ into the cytosol. Then, Ca2+-binding proteins act as sensors and mediators of the initial Ca2+ signal. CaM is a small (17 kDa), highly conserved, soluble, intracellular, acidic Ca2+-binding protein that is considered to be a major transducer of Ca2+-mediated signals in mammalian cells [1]. A series of recent studies have revealed that CaM can directly interact with intracellular domains of G protein-coupled receptors (GPCRs). CaM’s roles in the interaction with GPCR function are very diverse. It was initially found that CaM binds to the carboxy-terminus of metabotropic glutamate receptors (mGlu5 and mGlu7 receptors) altering their phosphorylation [2,3]. CaM has also been shown to bind to the third intracellular loop (3IL) of the μ opioid receptor and the dopamine D2 receptor at overlapping regions required for Gi/o protein activation, thereby compromising the receptor Gi/o protein-mediated activation [4–6•]. Furthermore, CaM can be directly involved in receptor signaling, as in the recently described G protein-independent and arrestin-dependent MAPK activation by the 5-HT2C receptor, which depends on the direct interaction of CaM with the carboxy-terminus of the receptor [7].
The adenosine A2A-dopamine D2 receptor heteromer is one of the most studied receptor heteromers [8•]. In the brain, A2A and D2 receptors are highly expressed in one type of striatal neuron, the GABAergic enkephalinergic neuron [9,10]. This type of neuron constitutes almost half the neuronal population in the striatum and its malfunction plays a key role in the pathogenesis of basal ganglia disorders (such as Parkinson’s disease and Huntington’s chorea) and most probably in obsessive-compulsive disorders, schizophrenia and drug addiction [11]. The A2A-D2 receptor heteromer plays an important role in the modulation of the activity of the GABAergic enkephalinergic neuron [9–12••] and therefore could be targeted in the development of drugs for these neuropsychiatric disorders. Here we review recent data that demonstrates that calmodulin (CaM) oligomerizes with the A2A-D2 receptor heteromer and modulates its function in a Ca2+-dependent manner.
Electrostatic interactions involved in CaM binding to A2A and D2 receptors
It has been suggested that the binding sites of CaM in different proteins are often formed by amphiphilic domains, with hydrophobic residues interspersed with several positively charged ones [1]. Based on these characteristics, the introduction of the human A2A receptor sequence in the Internet-based search program “Calmodulin Target Data Base” (Cancer Institute, Ontario, 2002; http://calcium.uhnres.utoronto.ca/ctdb/ctdb/sequence.html) disclosed the existence of a very likely binding site in the proximal portion of the carboxy-terminus of the A2A receptor. The sequence, 291RIREFRQTFR300, contains several arginines (Arg; positively charged residues), 1 Isoleucine and 2 Phenylalanine residues (hydrophobic residues). In fact, in vitro, this A2A receptor epitope formed multiple stable non-covalent complexes with CaM [13•]. However, a more detailed analysis indicated the particular involvement of electrostatic interactions. A close look at the amino acid sequence of CaM shows that 32.5% of its residues contain a negative charge [13•]. Furthermore, CaM has several serine (Ser) and threonine (Thr) residues susceptible of phosphorylation by casein kinases located in the vicinity of acidic residues [13•]. A mass spectrometric analysis of an enzymatic digest of the CaM-A2A receptor epitope non-covalent complexes indicated the involvement of strong electrostatic interactions between the acid motifs of CaM and the Arg residues (particularly the RIR sequence) of the A2A receptor epitope [13•]. A proteomics approach demonstrated the existence of CaM-A2A receptor interactions in rat brain tissue and a direct interaction between both proteins was demonstrated by BRET experiments in transfected mammalian cells [14•]. An important negative control was that the A2A receptor mutated in its putative CaM binding motif located in the carboxi-terminus (with the sequence 291AIREFAQTFA300) did not interact with CaM [14•].
Electrostatic interactions also play a key role in the binding of CaM to the D2 receptor. A mass spectrometric analysis indicated that CaM binds to an Arg-rich epitope from the amino-terminal part of the long 3IL of the D2 receptor [13•]. Importantly, the Arg-rich D2 receptor motif is also involved with the activation of Gi/o protein and with the heteromerization with adenosine A2A receptor (see below). Binding of CaM to the Arg-rich motif does not disturb Gi/o recognition but impedes the D2 receptor-induced activation of Gα and this effect is Ca+ dependent [6•]. Thus, Ca2+/CaM blocks Gi/o protein activation by D2 receptor in a non-competitive manner [6•]. But since the Arg-rich motif of the D2 receptor is also involved in A2A-D2 receptor heteromerization, it was interesting to know if CaM could also bind to the D2 receptor in the A2A-D2 receptor heteromer.
Intermolecular interactions in the A2A-D2 receptor heteromer
A2A-D2 receptor heteromerization has been demonstrated in mammalian transfected cells with co-immunoprecipitation and fluorescence and bioluminescence resonance energy transfer techniques (FRET and BRET, respectively) (reviewed in ref. [8•]). By using computerized modeling, pull-down techniques and mass spectrometric analysis, it was shown that A2A-D2 receptor heteromerization depends on an electrostatic interaction between the Arg-rich epitope located in the amino-terminal portion of the 3IL of the D2 receptor and a single phosphate group from a casein kinase phosphorylable Ser localized in the distal portion of the carboxy-terminus of the A2A receptor [15]. Studies in vitro with peptides corresponding to both epitopes demonstrated that the Arg-phosphate interaction possesses a “covalent-like” stability. Hence, these bonds could withstand fragmentation by mass spectrometric collision-induced dissociation at energies similar to those that fragment covalent bonds [16]. The Arg-phosphate electrostatic interaction between epitopes located in intracellular domains is obviously not the only interaction responsible for A2A-D2 receptor heteromerization. Thus, a significant but not complete reduction of BRET is observed when transfected cells express mutated D2 receptors that lack the key amino acids involved in the Arg-phosphate interaction [15], indicating that other receptor domains are also involved. Most probably, intramembrane domains play an important role in A2A-D2 receptor heteromerization, as it has been demonstrated for other GPCR homomers and heteromers [17,18]. Nevertheless, the significant modification of BRET with mutated receptors indicates that the Arg-phosphate interaction is necessary to provide the final quaternary structure of the heteromer, which in fact determines its function. Patch-clamp experiments in identified GABAergic enkephalinergic neurons demonstrated that disruption of the Arg-phosphate interaction in A2A-D2 receptor heteromers (by intracellular addition of small peptides with the same sequence than the receptor epitopes involved in the Arg-phosphate interaction) completely eliminates the ability of the A2A receptor to antagonistically modulate the D2 receptor-mediated inhibition of neuronal excitability [12••].
The just mentioned antagonistic interaction between A2A and D2 receptors is most probably related to the existence of an allosteric modulation in the A2A-D2 receptor heteromer. This kind of allosteric modulation, in which binding of a ligand to one of the receptor units in the receptor heteromer changes the binding properties of another receptor unit seems to be a common biochemical characteristic of receptor heteromers [19,20•]. An antagonistic A2A-D2 receptor interaction has been demonstrated in many different membrane preparations from different transfected mammalian cells and from rat and human striatal tissues and implies the ability of A2A receptor stimulation to change the binding characteristics (decrease the affinity) of the D2 receptor for agonists [8•,10,19]. In experiments with chimeric D1/D2 receptors, the A2A receptor could still modulate the binding characteristics of a D2 receptor with the 6th transmembrane domains of the D1 receptor, but the modulation disappeared with a D2 receptor with the 3IL and 5th and 6th transmembrane domains of the D1 receptor [21]. These results indicate that the epitope (or epitopes) of the D2 receptor involved in the antagonistic A2A-D2 receptor interaction might be located somewhere in the 3IL or 5th transmembrane. Experiments using more detailed mutants are in progress to demonstrate that the epitope, in fact, corresponds to the Arg-rich domain of the 3IL.
In addition to the allosteric modulation in the A2A-D2 receptor heteromer, A2A and D2 receptors can also interact at the second messenger level, and this has been well demonstrated both in cell culture and in the brain. In this case, however, it is the stimulation of D2 receptor that counteracts the effects of A2A receptor stimulation. A2A receptor, through its coupling to Gs/olf proteins, can potentially stimulate adenyl-cyclase, with phosphorylation of several PKA substrates, such as DARPP-32, CREB and AMPA receptors and the consequent increase in the expression of different genes, such as c-fos or preproenkephalin in the GABAergic enkephalinergic neuron [8•,9,10,19]. D2 receptor, on the other hand, can potentially couple to Gi/o proteins and counteract the ability of A2A receptor stimulation to signal through the cAMP/PKA cascade [8•,9,10,19]. It might sound counterintuitive, but both types of antagonistic A2A-D2 receptor interactions coexist in the same cells and, in fact, they do coexist in the GABAergic enkephalinergic neurons [8•,9,10,19]. Thus, co-stimulation of A2A and D2 receptors implies a simultaneous A2A receptor-mediated inhibition of the D2 receptor-mediated modulation of neuronal excitability and a D2 receptor-mediated inhibition of the A2A receptor-mediated modulation of gene expression, which provides a clear example of a functional dissociation between neuronal excitability and gene expression. This apparently incompatible coexistence of reciprocal antagonistic A2A-D2 receptor interactions could be explained by the presence in the same cell of A2A and D2 receptors forming and not forming A2A-D2 receptor heteromers. The antagonistic A2A-D2 receptor interaction at the second messenger level depends on the activation of Gi/o proteins, which requires the Arg-rich domain not being interacting with either CaM or the A2A receptor.
Role of CaM in A2A-D2 receptor heteromer function
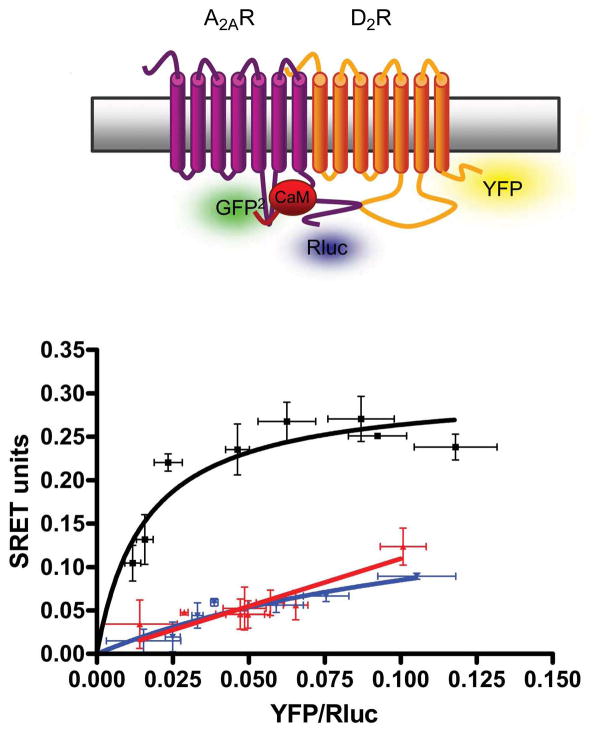
The existence of CaM-A2A-D2 receptor oligomerization has been detected by the recently introduced Sequential-BRET-FRET (SRET) technique, which allows the identification of oligomers formed by three different proteins [22••,23]. In SRET2, the oxidation of a Renilla Luciferase (RLuc) substrate by an RLuc fusion protein triggers the excitation of the acceptor GFP2 by BRET2 and subsequent energy transfer to the acceptor YFP by FRET [22••]. SRET2 was obtained with the fusion proteins A2ARluc, CaMGFP2 and D2YFP when adding DeepBlueC as Rluc substrate, indicating that the two acceptor/donor pairs, A2ARluc-CaMGFP2 and CaMGFP2-D2YFP were well oriented at distances of less than 10 nM [14•] (Figure 1). But, how are these three proteins arranged? We know that CaM binds to A2A and D2 receptors when not forming heteromers. Does CaM bind to both receptors in the A2A-D2 receptor heteromer? Does CaM disrupt the heteromerization or does it change the quaternary structure of the A2A-D2 receptor heteromer and therefore its function? Which Ca2+-dependend functional changes does CaM convey in the A2A-D2 receptor heteromer?
Figure 1. SRET for CaM, A2A receptor and D2 receptor in living cells.
SRET saturation curves performed in HEK-293 cells expressing A2A-Rluc (0.75 μg of cDNA), CaMGFP2 (0.6 μg of cDNA) and increasing amounts of D2YFP (0.5 to 5 μg of the cDNA). Net SRET was obtained by monitoring the YFP fluorescence emission after DeepBlueC addition, with subtraction of the value obtained with cells expressing the same amount of A2A-Rluc and CaMGFP2. Significant net SRET was detected for A2ARluc-CaMGFP2-D2YFP coupling, while negligible or linear net SRET was obtained in cells expressing equivalent amounts (equivalent fluorescence and luminescence units) of A2ARluc, CaMGFP2 and 5HT2BYFP (red curve), or A1RRluc, CaMGFP2 and D2RYFP (blue curve) as negative controls. Values, expressed as net SRET, represent means ± S.E.M. of 5 independent experiments performed in triplicate. At the top, scheme depicting the expressed proteins in the SRET assay (modified from ref. [14•]).
As mentioned above, the Arg-rich domain of the amino-terminal portion of the 3IL of the D2 receptor is a binding site for both CaM and for a phosphorylated Ser localized in the distal portion of the carboxy-terminus of the A2A receptor. BRET competition experiments demonstrated that increasing the expression of the D2 receptor does not modify the binding of CaM to the A2A receptor. Also, increasing the expression of CaM does not modify A2A-D2 receptor heteromerization. In contrast, increasing amounts of A2A receptor led to significant reduction in the BRET signal due to the interaction between the D2 receptor and CaM [14•]. Overall these results indicate that CaM cannot compete with the A2A receptor for its binding to the D2 receptor but that binds to the A2A receptor in the A2A-D2 receptor heteromer.
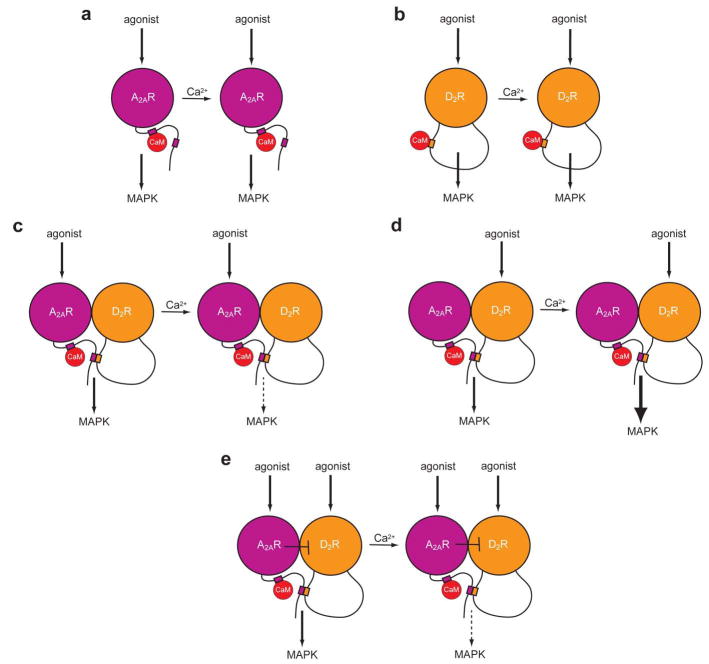
The increase in intracellular Ca2+ (with ionomycin treatment) in cells expressing A2A and D2 receptors produced modifications in the BRET signal due to the interaction between A2A and D2 receptors [14•], which implies alterations in the quaternary structure of the A2A-D2 receptor heteromer. Importantly, Ca2+ exerted a selective modulation of A2A-D2 receptor heteromer-mediated activation of MAPK pathway. First, in cells transfected with either A2A or D2 receptors, agonist-induced activation of MAPK pathway (ERK1/2 phosphorylation) was not modified by increasing intracellular Ca2+ (Figure 2a,b). At least for the D2 receptor, this suggests that MAPK signaling, in HEK-293T cells [6•,14•], is not dependent on the activation of Gi/o protein, since it has been previously shown that the Gi/o protein-dependent signaling efficiency of the D2 receptor is regulated (decreased) by a rise in Ca2+ via CaM [6•]. Second, in cells expressing both receptors, Ca2+ significantly decreased A2A receptor agonist-induced ERK1/2 phosphorylation (Figure 2c) whereas it significantly increased D2 receptor agonist-induced ERK1/2 phosphorylation [14•] (Figure 2d). Third, co-activation of A2A and D2 receptors in cells co-expressing both receptors led to the same qualitative response than activation of A2A receptors alone, with a significant Ca2+-dependent decrease of agonist-induced ERK1/2 phosphorylation [14•] (Figure 2e). The effects of Ca2+ were dependent on the intracellular levels of CaM [14•], indicating that CaM selectively transduces Ca2+-dependent changes of MAPK signaling in the A2A-D2 receptor heteromer. In summary, the results indicate that in the absence of Ca2+ the A2A-D2 receptor heteromer produces very similar effects on MAPK signaling upon stimulation of one of both receptor units, while binding of Ca2+ to CaM produces a very different qualitative response, reducing and enhancing the effects of the stimulation of A2A and D2 receptors, respectively. Additionally, with co-activation of the A2A receptor, the ability of Ca2+ to increase the effect of D2 receptor stimulation on ERK1/2 phosphorylation is lost. This could be related to the allosteric interaction in the A2A-D2 receptor heteromer, with A2A receptor stimulation antagonizing the effects of D2 receptor stimulation (Figure 2e).
Figure 2. Selective Ca2+/CaM-mediated modulation of A2A-D2 receptor heteromer function.
Scheme showing the motifs of the C-terminus of the A2A receptor and the 3IL of the D2 receptor involved in the binding of CaM and in A2A-D2 receptor heteromerizacion, as well as the selective Ca2+/CaM-mediated modulation of MAPK activation in the A2A-D2 receptor heteromer (see text). (a, b) When not forming heteromers, CaM binds to the proximal portion of the carboxy-terminus of the A2A receptor and to the Arg-rich domain localized in the amino-terminus of the 3IL of the D2 receptor. Under these conditions the effects of the stimulation of either receptor on MAPK activation does not change in the presence or absence of Ca2+. (c–e) When forming heteromers, the distal portion of the A2A receptor binds to the Arg-rich domain of the D2 receptor, which cannot bind to CaM. In the A2A-D2 receptor heteromer, the binding of Ca2+ to CaM modifies its quaternary structure and decreases the effects of stimulation of A2A receptor (c) and increases the effects of stimulation of the D2 receptor (d) on MAPK activation. Co-activation of A2A and D2 receptors in cells co-expressing both receptors led to the same qualitative response than activation of A2A receptors alone, probably due to the ability of A2A receptor stimulation to antagonize allosterically the effects induced by D2 receptor stimulation in the A2A-D2 receptor heteromer (e). Receptors are shown as monomers for the sake of simplicity, but they are most probably forming homomers when not forming heteromers. Also for schematic purposes, the main portion of each receptor unit is represented by a sphere and only the long 3IL of the D2 receptor and the long tail of the A2A receptor are explicitly shown.
We still need to determine which molecular mechanisms are involved in the Ca2+/CaM-modulated, A2A-D2 receptor heteromer-mediated MAPK signaling. Which are the G proteins involved? Recent studies strongly suggest that the minimal signal unit for class A GPCRs is composed of two receptors and a single G protein and that probably applies to both GPCR homomers and heteromers [24••]. We then need to figure out which is the G protein that binds to the A2A-D2 receptor heteromer and if it changes in the presence of CaM. We already mentioned that D2 receptor signals through MAPK by a Gi/o protein-independent mechanism in cells only expressing D2 receptors and, most probably, Gi/o proteins are not involved either in MAPK signaling by the A2A-D2 receptor heteromer. Thus, the Arg-rich domain of the D2 receptor required for Gi/o protein activation is bound to the C-terminus of the A2A receptor in the A2A-D2 receptor heteromer (see above). Also, we have preliminary data suggesting that the Gs/olf/PKA pathway, commonly used by the A2A receptor, is not involved either. One open possibility is the involvement of Gq, as it has been shown for the D1-D2 receptor heteromer [25]. But it is also probable we are dealing with a CaM-dependent, G protein-independent MAPK activation, as recently reported for the 5-HT2c receptor [7].
Conclusions
Receptor heteromers constitute an expanding new area of research which is changing previous concepts of receptor pharmacology. A receptor heteromer is defined as a macromolecular complex composed of at least two (functional) receptor units with biochemical properties that are demonstrably different from those of its individual components [20•]. This definition underscores the fact that the receptor heteromer constitutes a new functional unit and a previously unforeseen number of emerging properties have already been described for several different receptor heteromers. Those properties include changes in ligand recognition, allosteric interactions, G-protein coupling switching and changes in the pattern of activation of signaling pathways and receptor internalization [20•].
Here we reviewed a new biochemical property of the receptor heteromer formed by adenosine A2A and dopamine D2 receptors, which has important implications for basal ganglia function and dysfunction [9–11]. This property consists of a specific Ca2+-mediated modulation of MAPK signaling, which depends on the binding of CaM to the proximal portion of the carboxy-terminus of the A2A receptor in the A2A-D2 receptor heteromer. This is associated with changes in the quaternary structure of the A2A-D2 receptor heteromer, as demonstrated by RET techniques. Experiments with mutated receptors are in progress to determine which receptor domains are key determinants for the establishment of the appropriate Ca2+/CaM-dependent functional and structural changes of the A2A-D2 receptor heteromers.
Acknowledgments
Work supported by the intramural funds of the National Institute on Drug Abuse, the Spanish Ministry of Education and Science (grant SAF2006-05481)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sergi Ferré, Email: sferre@intra.nida.nih.gov.
Amina S. Woods, Email: awoods@intra.nida.nih.gov.
Gemma Navarro, Email: gnavarro@ub.edu.
Marisol Aymerich, Email: maymerich@unav.es.
Carme Lluís, Email: clluis@ub.edu.
Rafael Franco, Email: rfranco@ub.edu.
References
- 1.Vetter SW, Leclerc E. Novel aspects of calmodulin target recognition and activation. Eur J Biochem. 2003;270:404–414. doi: 10.1046/j.1432-1033.2003.03414.x. [DOI] [PubMed] [Google Scholar]
- 2.Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J Biol Chem. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J Biol Chem. 1999;274:27573–27577. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sadée W, Quillan JM. Calmodulin binding to G protein-coupling domain of opioid receptors. J Biol Chem. 1999;274:22081–22088. doi: 10.1074/jbc.274.31.22081. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Tolbert LM, Carlson KW, Sadée W. Nuclear Ca2+/calmodulin translocation activated by mu-opioid (OP3) receptor. J Neurochem. 2000;74:1418–1425. doi: 10.1046/j.1471-4159.2000.0741418.x. [DOI] [PubMed] [Google Scholar]
- 6 •.Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–32680. doi: 10.1074/jbc.M002780200. This study clearly demonstrates that the D2 receptor binds CaM in the N-terminal part of the third intracellular loop, which allows intracellular Ca2+ to modulate a Gi/o protein-dependent D2 receptor-mediated signaling. [DOI] [PubMed] [Google Scholar]
- 7.Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 •.Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–74. doi: 10.2174/138161208784480108. An updated review about the multiple interactions between A2A and D2 receptors with reference to receptor heteromerization and to the apparently incompatible co-existence of reciprocal interactions, which seem to depend on the co-existence of separate pools of A2A and D2 receptors forming and not forming A2A-D2 receptor heteromers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferré S, Agnati LF, Ciruela F, Lluis C, Woods AS, Fuxe K, Franco R. Neurotransmitter receptor heteromers and their integrative role in ‘local modules’: the striatal spine module. Brain Res Rev. 2007;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 12 ••.Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. Patch-clamp experiments in identified GABAergic enkephalinergic neurons which show the complete disruption of the A2A receptor-mediated modulation of D2 receptor function with the application of peptides with the same sequence than epitopes involved in A2A-D2 receptor heteromerization. This study provides strong evidence in situ for a specific functional property of the A2A-D2 receptor heteromer in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 •.Woods AS, Marcellino D, Jackson SN, Franco R, Ferré S, Agnati LF, Fuxe K. How calmodulin interacts with the adenosine A(2A) and the dopamine D(2) receptors. J Proteome Res. 2008;7:3428–3434. doi: 10.1021/pr8001782. This study with mass spectrometry techniques demonstrates for the first time an important role of electrostatic interactions in the intermolecular interactions between CaM and epitopes of the A2A and D2 receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 •.Navarro G, Aymerich MS, Marcellino D, Cortes A, Casado V, Mallol J, Canela EI, Agnati LF, Woods AS, Fuxe K, et al. Interactions between calmodulin, adenosine A2A and dopamine D2 receptors. J Biol Chem. 2009 doi: 10.1074/jbc.M109.034231. By using Bioluminescence and Sequential Resonance Energy Transfer techniques, evidence is provided for oligomerization of CaM, A2A and D2 receptors. The study also provides an example of re-organization of signaling protein molecules as a consequence of receptor heteromerization and of specific (Ca2+-dependent) signaling by the receptor heteromer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciruela F, Burgueno J, Casado V, Canals M, Marcelino D, Goldberg SR, Bader M, Fuxe K, Agnati LF, Lluis C, et al. Combining Mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- 16.Woods AS, Ferré S. The amazing stability of the arginine-phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20 •.Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. Most recent recommendations for nomenclature of receptor heteromers and criteria for their identification in native tissues. Some definitions are also established, including allosteric interaction in the receptor heteromer, which is described as an intermolecular interaction by which binding of a ligand to one of the receptor units in the receptor heteromer changes the binding properties of another receptor unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torvinen M, Kozell LB, Neve KA, Agnati LF, Fuxe K. Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci. 2004;24:173–180. doi: 10.1385/JMN:24:2:173. [DOI] [PubMed] [Google Scholar]
- 22 ••.Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods. 2008;5:727–733. doi: 10.1038/nmeth.1229. New method to determine the existence of three interacting proteins in the plasma membrane of living mammalian cells. First demonstration of heterotrimerization of different GPCRs, A2A, D2 and cannabinoid CB1 receptors. [DOI] [PubMed] [Google Scholar]
- 23.Cabello N, Gandía J, Bertarelli DC, Watanabe M, Lluís C, Franco R, Ferré S, Luján R, Ciruela F. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J Neurochem. 2009;109:1497–1507. doi: 10.1111/j.1471-4159.2009.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 ••.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. This is and elegant study that shows that the minimal signaling unit of the D2 receptor is a receptor homomer with two protomers and a single G protein. It also shows that such a dimer is maximally activated by agonist binding to a single protomer and that agonist binding to the second protomer inhibits signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashid AJ, O’Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci. 2007;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]