Abstract
Background
Low-income African American women face numerous barriers to mammography screening. We tested the efficacy of a combined interactive computer program and lay health advisor (LHA) intervention to increase mammography screening.
Methods
In this randomized, single blind study, participants were 181 African American female health center patients ages 41-75, ≤250% of poverty level with no breast cancer history and no screening mammogram in the past 15 months. They were assigned to either (a) a low dose comparison group consisting of a culturally appropriate mammography screening pamphlet or (b) interactive, tailored computer instruction at baseline and 4 monthly LHA counseling sessions. Self-reported screening data were collected at baseline and 6 months and verified by medical record.
Results
For intent-to-treat analysis of primary outcome (medical-record-verified mammography screening, available on all but two participants), the intervention group had increased screening to 51% (45/89) compared to 18% (16/90) for the comparison group at 6 months. When adjusted for employment status, disability, first-degree relatives with breast cancer, health insurance, and previous breast biopsies, the intervention group was three times more likely (adjusted relative risk [RR]=2.7 [95% CI: 1.8, 3.7], p<.0001) to get screened than the low dose comparison group. Similar results were found for self-reported mammography stage of screening adoption.
Conclusions
The combined intervention was efficacious in improving mammography screening in low-income African American women, with an unadjusted effect size (RR = 2.84) significantly higher (p < .05) than previous studies of each intervention alone.
Keywords: African Americans, mammography, intervention study, computer-assisted instruction, lay health advisors
Introduction
Although the incidence of breast cancer is lower in African American women than among non-Hispanic White women in the U.S., mortality among African American women remains disproportionately high.1 African American women are diagnosed at later stages and have poorer 5-year survival rates.2 Breast cancer is becoming an increasingly important public health problem in African Americans, and determining the effectiveness of culturally-appropriate interventions will provide important information for reducing the associated mortality.
Mammography screening remains an effective method for detecting breast cancer in early stages, which leads to increased survival rates.3 However, African American women experience numerous barriers to mammography screening. These barriers involve: (a) personal belief barriers such as inaccurate estimates of breast cancer risk (1-5), conflicting religious beliefs about screening and healing (6), and feeling no need for screening in the absence of symptoms (7); (b) personal fear barriers such as fatalistic view of cancer (2, 8-11), fear of breast cancer discovery (3, 11), fear of breast cancer treatment, (2, 3, 6, 11) physical discomfort with the mammogram procedure (2, 12), and embarrassment about the procedure (2, 5, 7); (c) healthcare provider barriers such as impersonal attitudes of health care providers and mammography technicians (2), distrust of the healthcare system (11), and lack of screening recommendation (3, 5, 7, 13); (d) personal need barriers such as limited income to pay for a mammogram (14), inadequate or no health insurance (14), and no transportation (7); and (e) time orientation and management barriers such as a present time orientation (15), being less focused on preventive health (12), having other pressing priorities (7), and forgetting to make and keep screening appointments (7).
Health messages tailored to cognitive and behavioral factors are effective in decreasing barriers to mammography screening (16). Tailored messages use data collected directly from the individual to provide highly individualized messages (17). Intervention trials to increase mammography screening show that tailored health messages can be effectively delivered using print materials (18, 19), phone counseling (20-22), or in-person counseling (23, 24). Interactive computer programs also are effective in delivering tailored health messages for increasing mammography screening. For example, Champion and colleagues (25) increased mammography screening to 40% in low-income African American women, non-adherent with mammography screening guidelines, who received an interactive tailored computer program.
Although tailored interactive computerized instruction efficiently gives women the individual knowledge they need to identify and overcome barriers, this approach does not provide the emotional and logistical support women may need to act on what they have learned and may not capture all relevant contextual information for the individual (16). A complementary approach is using lay health advisors or women who are indigenous to and trusted by the community (26). Lay health advisors address the contextual barriers to screening and abnormal mammography follow-up, enhance access to screening, and reinforce tailored messages (27, 28). They provide advice, education, emotional support, tangible aid, and advocacy (28, 29), and they serve as a bridge between the health care system and community (30). Previous studies show that lay health advisor interventions have increased mammography screening use in African American women by 10-43% in terms of absolute percentage points (30-35).
Consistent with the Task Force on Community Preventive Services recommendation for increasing breast cancer screening by mammography,4 both lay health advisors and tailored media interventions are effective one-on-one education strategies. Although we know that, as single interventions, lay health advisor and tailored interactive computer programs are effective, the efficacy of combining the two is unknown. Such information would guide future dissemination studies that implement multi-component interventions to increase mammography screening in under-screened women.
The purpose of our study, known as Project Mammography Outreach and Community Health Awareness (MOCHA) by the community, was to determine the effect of a combined interactive computer instruction and lay health advisor intervention on mammography screening in low-income African American women. As shown in figure 1, the conceptual model that guided this study was based upon the Health Belief Model (HBM) (36), Extended Parallel Process Model (EPPM) (37), and the Transtheoretical Model (TTM) (38). According to the HBM, health action is determined by an individual's perceived susceptibility to a particular health condition, perceived benefits in reducing the threat of the health condition, perceived barriers to taking the health action, and perceived self-efficacy or confidence in carrying out the health action. Studies have found significant correlations between these constructs and breast cancer screening behavior (36, 39, 40). The EPPM incorporates fear and fatalism constructs which have shown that fear of cancer and perceived inevitable death from cancer negatively affect mammography screening behavior in African American women (25, 41). The TTM posits that health behavior change involves a series of stages from not considering the health action to adopting and sustaining the behavior. We hypothesized that (1) six months after intervention delivery, mammography adherence would be significantly greater for the combined intervention group than the low dose comparison group and (2) six months after intervention delivery, movement forward in mammography stage of adoption would be significantly greater for the combined intervention group than the low dose comparison group.
Figure 1.

Conceptual Model for Study
Materials and Methods
Setting and Study Participants
Eligible participants were recruited from November 2006 through June 2008, and participants were administered surveys at baseline (i.e., at time of randomization) and 6 months after baseline. The sample consisted of patients from Citizens Health Center, a federally qualified health center located in an urban neighborhood of Indianapolis, IN. The health center provides primary health care services to low-income residents in 19 census tracts. In 2006, the health center served 10,919 patients of whom 80% were non-Hispanic Black.
Participant eligibility included being an African American female, age 41-75, at or below 250% of the federally designated poverty level, and no mammogram within the last 15 months. Participant income relative to poverty level was determined by asking participants their total household income and number of persons residing in the household. Poverty level was calculated using US Census Bureau's 2006 federal poverty guidelines. Patients with a history of breast cancer were excluded.
Recruitment
All participant recruitment was done in one health center. Strategies included letters from staff sent to potentially eligible women through a review of medical records, direct staff contact during clinic appointments, clinic flyers, and the mentioning of the study to potential participants by other patients in the same health center.
Staff provided names of patients who indicated an interest in the study to trained research assistants. The research assistants contacted potential participants by phone to further explain the details of the study, determine eligibility to participate in the study, and schedule an appointment at the health center to enroll in the study.
During the appointment, eligibility was confirmed, consent forms to participate in the study were signed, a release form for medical record information on mammography screening verification was signed, and administration of a baseline survey was completed. After completion of the survey and verification of mammography status, the participants were randomized, using stratified random assignment, to the intervention group or the low dose comparison group. Specifically, participants were randomized within three age groups (41-50, 51-60, and 61-75) using a separate computer- generated randomized list (created by a statistician) for each age group. Participants were allocated to the next available ID number on the list, within each age group, on entry into the trial. The allocation sequence was not concealed from the interventionists who assigned participants; however, these interventionists were trained to strictly follow the sequence without deviation. The data collectors were blinded, but the interventionists and participants were not blinded, to group assignment. The success of blinding was not evaluated.
Sample size was based upon estimating the effect size of a difference in two proportions. We expected the low dose comparison group to display 20% adherence to mammography screening guidelines, and we expected the intervention group to exhibit a minimum of 40% adherence which was a conservative estimate given that 40% adherence was shown by Champion and colleagues (25) for interactive computer alone. We desired sample sizes large enough so that the width of a two-sided 95% confidence interval for the difference between two proportions was less than a width of .15. A sample size of 80 in each group met this criterion by yielding a width of .14. Approximately 10 women were recruited in each group beyond the original goal to allow for attrition.
This study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board.
Baseline and Follow-up Survey
Structured interview questions were read to each participant by trained research assistants who were blinded to group assignment. These data were collected at the health center on the same day immediately following participant enrollment in the study and again at 6 months at a convenient location for the participant, including by telephone, in-person at home, or at the health center before or after an appointment. Each participant received a $25 gift certificate to one of four local businesses of her choice upon completion of each survey. Participants were informed that they would receive the gift certificate regardless of their screening status at the end of the study. Both surveys collected data on previous exposure to screening information and provider recommendation, barriers to screening, health and cultural beliefs, knowledge about breast cancer and screening, mammography screening history, and mammography stage of adoption. Additional items on the baseline survey addressed demographic and medical and family history. For this paper, we report demographics, family and medical history, previous exposure to screening information and provider recommendation, barriers to mammography screening, mammography stage of adoption, and mammography adherence.
Demographics and family and medical history
These data involved standard demographics (age, income, education, marital status, number of people in household, employment), health insurance (Medicare, commercial, or publicly funded plans), amount of coverage for mammograms, presence of regular healthcare provider, first-degree relatives with breast cancer, previous breast biopsies, and disability status.
Previous exposure to screening information and provider recommendation
These data related to previous exposure to mammogram information (e.g., newspaper, radio, magazine, Internet), previous provider mammography screening recommendation (e.g., never, within last 1-2 years), and receipt of screening reminder.
Barriers to mammography screening
Individual barriers were assessed by 35 items adapted from Champion's barriers scale (42) and Paskett's lay health advisor counseling intervention (33). A 5-item Likert response scale of strongly agree to strongly disagree was used. The barrier items addressed personal beliefs (e.g., you don't need a mammogram because God will take care of you), personal fears (e.g., being afraid of finding a breast problem would keep you from getting a mammogram), health care provider barriers (e.g., you don't need a mammogram because your doctor did not tell you that you need a mammogram), personal needs (e.g., no transportation would keep you from getting a mammogram), and time orientation and management (e.g., you would not have time to have a mammogram).
Mammography stage of adoption
Stage of mammography adoption was assessed with measures developed for mammography by Rakowski and colleagues (43) and included the following four questions: (1) Have you ever had a mammogram?; (2) When did you have your last mammogram?; (3) Are you planning to have a mammogram in the next six months?; and (4) Do you have an appointment scheduled for a mammogram?. Four stages of readiness for mammography screening adoption were used, including (1) pre-contemplation (i.e., no plan for a mammogram in the next six months), (2) contemplation (i.e., planning for a mammogram in the next six months), (3) preparation (i.e., has a scheduled appointment for a mammogram), and (4) action (is currently adherent). At baseline all women were non-adherent with screening and, therefore, not in the action stage.
Outcome measures
These measures included forward movement in mammography stage of adoption and mammography adherence. Forward stage movement was computed by stage of adoption at the 6-month post-baseline survey (1=precontemplation, 2=contemplation, 3=preparation; 4=action) minus stage of adoption at baseline (1=precontemplation, 2=contemplation, 3=preparation). The outcome of stage change was implemented as a dichotomous variable in the model: improvement (increased one, two, or three stages) versus non-improvement (remained the same or decreased one or two stages) because less than 25% of women decreased in stage or increased either one or three stages, that is most women either increased two stages or remained in the same stage.
Mammography adherence was measured by self-report and medical record verification of receipt of a screening mammogram within 6 months of the baseline survey.
Combined Intervention Group
The combined interactive computer program and lay health advisor intervention was theory-based. The interactive computer program provided an algorithm of tailored messages guided by the Extended Parallel Process Model (37) to identify fear and fatalistic views of breast cancer; the Health Belief Model (36) to assess health beliefs, self-efficacy, and barriers to screening; and the Transtheoretical Model (38) to assess stage of readiness for mammography screening adoption. The computer program, which is described elsewhere (25), incorporated African American narrators and story tellers as well as video demonstration of the mammography screening procedure.
The lay health advisor intervention was adopted from the Forsyth County, NC, lay health advisor model (33). This model is based upon the PRECEDE/PROCEED Model (44) for assessment and planning, the Health Belief Model (36) for identifying health beliefs and barriers to screening, Social Learning Theory (45) for role modeling of lay advisors in message delivery and developing self-efficacy, and the PENIII Model (46) for socio-culturally appropriate program development. The barriers counseling addressed a total of 35 personal belief, personal fear, healthcare provider, personal need, and time orientation and management barriers. Each barrier had a scripted message. An informal group of community women reviewed the barriers for comprehensiveness and scripted messages for relevancy to the Indianapolis community. Minimum revisions were required. Lay health advisors also provided access-enhancing services, including referral to low or no cost mammograms, assistance with scheduling screening appointments, and assistance with transportation, including free bus passes and agency referrals.
The eight lay health advisors, recruited through word-of-mouth and the project's community advisory board, consisted of African American women who lived in or near the targeted community. They participated in two 8-hour training sessions to deliver scripted messages addressing barriers to screening, assessing progress participants made in overcoming existing and newly developed barriers, assisting in making screening appointments, arranging for transportation to screening facilities, and referring women to the clinic social worker for additional community resources. Two nurses (KMR and MM), who had previous experience in lay health advisor interventions and breast cancer screening, independently rated the skills performance of each advisor. Inter-rater reliability was established at or above 90% agreement for demonstration of competency in lay health advisor skills. Periodic audiotape evaluation of counseling sessions was performed to assure intervention fidelity throughout the study. Lay health advisors in this project received a small stipend.
A formal group of community advisors, including representatives from the community, government, faith-based organizations, a minority nursing organization, and health and social service agencies, were involved throughout the project. They referred potential lay health advisors to the project team, participated in a lay advisor recognition ceremony, identified sources for community resource referrals, and participated in data analysis interpretation and dissemination of results.
Each participant met with an assigned lay health advisor immediately following administration of the baseline interview at the health center and was given the computer program. The lay advisor then assessed the participant's understanding of the program and reviewed a printout of barriers (personal belief, personal fear, healthcare provider, personal need, and time orientation and management barriers) that the participant had identified from the baseline survey and any additional barriers of concern to the participant. The lay advisor provided tailored messages that addressed each barrier and engaged the participant in an open, trusting conversation about barriers resolution. Lastly, the advisor provided information on accessing mammography screening and provided a variety of options for screening sites. If the participant indicated an interest in making an appointment, the lay health advisor initiated the referral process.
Each lay health advisor contacted her assigned participant by phone again at 3-4, 7-8, and 13-14 weeks after the first intervention session to assess and provide counseling, role modeling, and encouragement for overcoming barriers and to determine progress towards getting screened. The counseling consisted of assessing progress in resolving barriers identified at baseline and providing scripted messages from the training curriculum that were tailored to these barriers. At 18 weeks following the first intervention session, the lay advisors mailed a post card tailored by stage of screening adoption to assigned participants in the intervention group, regardless of screening status. For example, if the participant reported to the lay health advisor that she had made an appointment for a mammogram, then a specific tailored post card message pointed out tasks to complete in arranging to keep the appointment.
Low dose comparison group
Participants assigned to the low dose comparison group received a culturally appropriate pamphlet about breast cancer and mammography screening and a recommendation from a lay health advisor to contact the clinic referral nurse to schedule a mammography screening appointment. As an attention control strategy, the participants also received a mailed post card with general nutrition information at 3-4, 7-8, 13-14, and 18 weeks following the baseline interview.
Statistical Methods/Data Analysis
For bivariate comparisons of the two groups on demographic and outcome variables, the two-sided Fisher's exact test was used for categorical variables and the two-sided Wilcoxon Rank sum test was used for continuous and ordinal measures. The two groups were compared on the binary outcomes, after adjusting for potentially confounding covariates using multivariable logistic regression models. Adjusted relative risks were approximated from the adjusted odds ratios estimated from logistic regression models using a formula provided by Zhang and Yu (47); this approximation is valid even when the outcome is not rare. For the purpose of reporting which individual barriers most frequently occurred, the ordinal barriers items were collapsed into binary item scores (presence = strongly agree, agree, or neutral; and absence = disagree or strongly disagree); neutral was included in the presence category to be consistent with the lay health intervention because the lay health advisors were instructed to counsel on any barrier item that was scored neutral, agree, or strongly agree.
Results
Participant Baseline Characteristics
Of the 659 potentially eligible women, 380 were unreachable. Of the 279 contacted, 98 were excluded because of not meeting inclusion criteria, failing to attend the enrollment appointment, or refusing to participate (Fig.2). The final sample consisted of 181 women, resulting in a 65% participation rate for the baseline interview.
Figure 2.
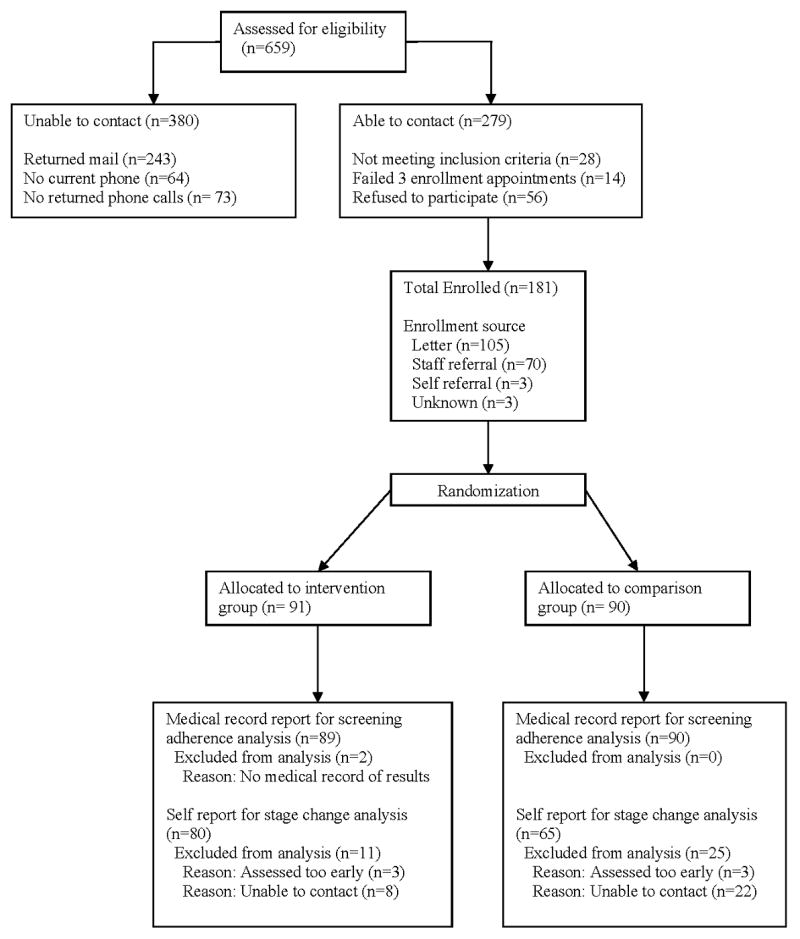
Flow of participants through trial
Table 1 shows that no differences existed between the two groups in demographic characteristics at the .05 level, except that the low dose comparison group was more likely to have health insurance (p = .05). At baseline, by chance alone, differences existed (p < .05) in the barriers selected by the two groups. A greater proportion of intervention group participants perceived that getting a mammogram would be inconvenient and that the technician was too rough with their breasts, whereas a greater proportion of the low dose comparison group perceived no need for a mammogram because God would take care of them. The most frequently reported barriers for the two groups in descending order included a belief that one could find a lump themselves without having a mammogram (n=52%), not able to afford a mammogram (n=46%), breast cancer treatment is worse than the cancer (n=39%), breast cancer treatment would cause a lot of problems (n=36%), forgetting to keep screening appointment (n=36%), and being treated rudely by mammography technician (n=32%).
Table 1.
Participant baseline characteristics
| Intervention N=89 |
Comparison N=90 |
p value | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | ||
| Age (years) | 51.3 | 7.3 | 51.2 | 7.1 | 0.96 |
| Education (highest grade completed) | 12.0 | 2.2 | 12.1 | 1.6 | 0.76 |
| Number of people in household | 2.3 | 2.0 | 2.3 | 2.0 | 0.59 |
| Income ($) | 10,984 | 10,080 | 10,405 | 7,389 | 0.98 |
| n | % | n | % | ||
| Employed (or retired or full time student) | 43 | 49.4 | 34 | 38.6 | 0.17 |
| Percentage of poverty level | 0.58 | ||||
| 0-50 | 23 | 26.1 | 21 | 23.3 | |
| 51-100 | 42 | 47.7 | 50 | 55.6 | |
| 101-250 | 23 | 26.1 | 19 | 21.1 | |
| Married or in long-term committed relationship | 13 | 14.9 | 17 | 19.1 | 0.55 |
| Exposure to mammogram info, mean (SD) no. sources | 2.6 | 1.9 | 2.7 | 1.8 | 0.89 |
| Have a regular doctor or nurse practitioner | 69 | 79.3 | 75 | 87.2 | 0.22 |
| Doctor ever suggested you have a mammogram | 64 | 73.6 | 64 | 72.7 | 1.00 |
| In last 1-2 yr, Dr or Nurse recommend mammogram | 48 | 55.8 | 47 | 54.0 | 0.88 |
| Received mammogram reminder | 33 | 37.9 | 32 | 36.4 | 0.88 |
| Have health insurance | 51 | 60.0 | 66 | 74.2 | 0.05 |
| Cost of mamm covered by health insurance | 0.95 | ||||
| All | 17 | 32.1 | 22 | 32.8 | |
| Some | 14 | 26.4 | 15 | 22.4 | |
| None | 2 | 3.8 | 2 | 3.0 | |
| Don't Know | 20 | 37.7 | 28 | 41.8 | |
| Disabled | 18 | 20.7 | 27 | 30.7 | 0.17 |
| No. of first degree relatives ever had breast cancer | 0.07 | ||||
| None | 72 | 82.8 | 81 | 92.0 | |
| One, two or three | 15 | 17.2 | 7 | 8.0 | |
| No. previous biopsies | 0.08 | ||||
| None | 82 | 94.3 | 76 | 85.4 | |
| One or two | 5 | 5.7 | 13 | 14.6 | |
| Baseline stage | 0.88 | ||||
| Precontemplation | 1 | 1.1 | 1 | 1.1 | |
| Contemplation | 82 | 92.1 | 84 | 93.3 | |
| Preparation | 6 | 6.7 | 5 | 5.6 | |
NOTE: P value for age, education, number of people in household, income, and number of sources of exposure to mammography information is from two-sided Wilcoxon rank sum test using the normal approximation. P value for categorical variables is from two-sided Fisher's Exact test.
Intervention Outcomes
The analysis was intent-to-treat analysis where (1) all patients were included in the analysis according to their randomly assigned groups regardless of intervention adherence and (2) medical record data for the primary outcome, mammography adherence, was obtained on almost all persons who did not complete the final survey. Final data for the primary outcome, audit-verified mammography screening adherence, came from 179 women who had medical record screening data, for a response rate of 98% (n = 89) in the intervention group and 100% (n = 90) in the low dose comparison group. Two patients whose records could not be obtained were not interviewed at the final survey. Final data analysis for the secondary outcome, forward movement in mammography stage of adoption, was for 145 participants who completed the 6-month post-baseline interview, for a response rate of 88% (n = 80) in the intervention group and 72% (n = 65) in the low dose comparison group.
Women who did not complete the 6-month interview were not significantly different from the completers on key demographic variables (age, years of education, number in household, number of children, income, marital status, employment, disability), except that non-completers were less likely than completers to obtain a mammogram by 6 months according to medical record data (7% vs. 39%, respectively). Among the 145 women for whom both medical record and 6 month interview data were available, the comparison of gold standard medical records to self reported mammography adherence showed that two women (1.4%) under reported and 12 women over reported (8.3%) having had a mammogram sometime between the baseline and the 6 month interview.
The outcomes for mammography screening adherence and movement in mammography stage of adoption are shown in Table 2. A significantly higher proportion of women in the intervention group received a mammogram than in the comparison group (Table 2, 51% vs. 18%, respectively). The unadjusted relative risk (RR=2.8, 95% CI =1.7 to 4.6, p<.0001) indicated that women in the intervention group were almost three times more likely to get a mammogram than women in the low dose comparison group (Table 2). The unadjusted odds ratio (OR=4.7, 95% CI =2.4 to 9.4, p<.0001) indicated that the odds of getting a mammogram was almost five times greater for women in the intervention group compared to women in the low dose comparison group (Table 2). When adjusted for five covariates (employment, disability, any first-degree relatives with breast cancer (yes vs. no), previous breast biopsies (yes vs. no), and health insurance (yes vs. no), the results were very similar (not shown in table); women in the intervention group were nearly three times more likely to get screened than women in the low dose comparison group, with approximately four times the odds, and the difference remained highly significant (RR=2.7, 95% CI =1.8 to 3.7; OR=4.3, 95% CI =2.1 to 9.0, p<.0001).
Table 2.
Mammography Screening Adherence and Forward Movement in Stage of Screening Adoption by Group at 6 Month Follow-up
| Intervention n=89 |
Comparison n=90 |
Relative risk (95% CI) Odds ratio (95% CI) |
p value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Adherence at 6 months (medical record verified) | 45 | 50.6 | 16 | 17.8 | 2.8 (1.7, 4.6) | <.0001 | ||
| 4.7 (2.4, 9.4) | <.0001 | |||||||
| Stage changes | <.0001 | |||||||
| -2 (went back 2 stages) | 0 | 0 | 1 | 1.5 | ||||
| -1 (Went back 1 stage) | 2 | 2.5 | 7 | 10.8 | ||||
| 0 (Stayed the same) | 17 | 21.3 | 32 | 49.2 | ||||
| 1(Moved forward 1 stage) | 14 | 17.5 | 8 | 12.3 | ||||
| 2 (Moved forward 2 stages) | 46 | 57.5 | 17 | 26.2 | ||||
| 3 (Moved forward 3 stages) | 1 | 1.3 | 0 | 0 | ||||
| Forward stage movement at 6 month interview | 61 | 76.3 | 25 | 38.5 | 2.0 (1.4, 2.8) | <.0001 | ||
| 5.1 (2.5, 10.5) | <.0001 | |||||||
Note: p-value for Stage change is from two-sided Fisher exact test for collapsed variable into three categories (-2/-1/0 vs. 1 vs. 2/3). P value for forward stage movement is for forward (1, 2, 3) vs backward or stay the same, regarding stage change from baseline to 6 month interview (-2/-1/0). The sample size for stage changes at 6 months (T3) are Intervention (n=80) and Usual Care (n=65).
Significant differences in stage movement also existed between the two groups. A greater proportion of women in the comparison group than the intervention group did not have any stage change (49% vs. 21%) or moved one or more stages backward (12% vs. 3%) (Table 2). A greater proportion of women in the intervention group than the comparison group moved one or more stages forward (Table 2, 76% vs. 39%, p<.0001). The unadjusted relative risk (RR=2.0, 95% CI =1.4 to 2.8, p<.0001) indicated that women in the intervention group were two times more likely to move forward in screening adoption than women in the low dose comparison group (Table 2). The unadjusted odds ratio in forward movement was 5.1 (95% CI, 2.5, 10.5, p<.0001), indicating that the odds of forward stage movement was five times greater for women in the intervention group compared to women in the low dose comparison group (Table 2). When adjusted for five covariates, the results were very similar (not shown in table); RR=2.0, 95% CI =1.5 to 2.3; OR=4.9, 95% CI =2.3 to 10.4, p<.0001). There were no unanticipated adverse events or side effects in either study group.
Discussion
This paper reports the effect of an interactive computer program tailored to health beliefs, and mammography stage of screening adoption combined with a lay health advisor intervention on promoting mammography screening in low-income African American women in need of a mammogram. We compared the combined intervention group to a low dose comparison group that received a culturally appropriate pamphlet on breast cancer and mammography screening and information on procedures for getting screened. Mammography screening adherence and forward movement in stage of screening adoption were more improved in the combined intervention group.
Compared to the low dose comparison group, the intervention group significantly increased mammography screening adherence (18% vs. 51%, respectively). The effect of this intervention was greater than reported in prior studies that used tailored modalities in clinic-based settings (16, 32). Only one study was identified that used a theory-based interactive computer program intervention to increase mammography screening in low-income African American women (25). In that study, 344 women non-adherent with screening guidelines were recruited from both clinic and community settings and were randomly assigned to an interactive tailored computer program, a targeted video, or a culturally appropriate pamphlet. Women in the tailored computer group increased adherence by 40% compared to 24.6% for the video group and 32.1% for the pamphlet group (p=.037).
Similarly, clinic-based lay health advisor interventions with low income African American women had demonstrated varying effects compared to our study results. West and colleagues (35) used tailored phone counseling delivered by an indigenous African American community health care worker with low-income African American women who were non-adherent with screening guidelines and had not received a mammogram 6 months after receipt of a screening reminder letter. Mammography screening increased in women with no history of screening to 16%. In a tri-racial sample of 851 low-income women, Paskett and colleagues (32) found that a higher proportion of participants in a lay health advisor intervention group increased screening compared to the usual care group (42.5% vs. 27.3%, p<.001) and that the African American intervention group had a relative risk of 1.54 (p.=008) when compared to the African American comparison group.
We compared the relative risk of our study's combined intervention with the best relative risk reported in the literature among African Americans for a single lay health advisor intervention alone and a single interactive tailored computer program alone using a method shown by Altman and Bland (48). The combined intervention relative risk (RR = 2.84) was significantly greater than the single lay health advisor intervention alone relative to usual care (RR = 2.84 versus 1.54, p = .042) (32) or a single interactive computer alone relative to pamphlet (RR = 2.84 versus 1.24, p = .014) (25).
In regards to mammography stage of screening adoption, our findings showed that 76% of participants who received the combined intervention had forward stage movement and the intervention group was two times more likely to move forward in stage of readiness to adopt mammography screening compared to the low dose comparison group, even after adjusting for employment, disability, any first-degree relatives with breast cancer, and any previous biopsies. Champion and colleagues (25) found a 52% increase in forward stage movement (from pre-contemplation to contemplation and/or from contemplation to action) for African American women who received an interactive tailored computer program compared to 46.4% who received a culturally appropriate pamphlet on breast cancer and mammography screening. No studies were identified that assessed changes in specific stages of readiness for screening adoption and lay health advisor interventions.
Several limitations existed for this study. The low response rate may have introduced external validity bias, specifically, the screening effectiveness may have been overestimated in both groups because attending a study appointment at the clinic was required for enrollment. However, because of the experimental design (i.e., random assignment), the internal validity (i.e., differential effectiveness in the two groups, which was the main research question addressed) was likely not affected by this selection bias. The differential completion rate of the final survey between intervention and comparison groups may have introduced internal validity bias into the self-report screening rate comparison. However, because medical records were obtained for all but two participants, screening adherence results based on medical records, which was the primary outcome, was not affected by selection bias from differential drop out. Results of this study have limited generalizability since participants lived in one region of the United States and were urban residents.
The present study has several strengths. This study focused on a medically underserved population that experiences disparities in breast cancer mortality. The study used a randomized controlled trial design and tested a combination of evidenced-based interventions to promote mammography screening. Self-reported mammograms were validated by medical record review.
In conclusion, the combined interactive tailored computer and lay health advisor intervention was more efficacious than low dose in increasing mammography screening in low-income African American women. In addition, the combined intervention produced a screening percentage superior to those of either intervention alone reported in published studies. Future research should consider replication of this intervention across multiple community sites in differing urban and rural geographic locations to increase regular, routine mammography screening and forward mammography stage of adoption with various multiethnic underserved populations.
Acknowledgments
Grant support: 5K01CA111826 from the National Institutes of Health/ National Cancer Institute and Indiana University School of Nursing/Center for Enhancing Quality of Life in Chronic Illness
Footnotes
U.S. Cancer Statistics Working Group. United States cancer statistics: 1999-2005 incidence and mortality web-based report. 2009; Available from: http://apps.nccd.cdc.gov/uscs/.
American Cancer Society. Breast cancer facts and figures. Atlanta: American Cancer Society; 2007; Available from: http://www.cancer.org/downloads/STT/BCFF-Final.pdf.
U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. 2004; Available from: http://www.ahrq.gov/clinic/3rduspstf/breastcancer/.
Task Force on Community Preventive Services. The guide to community preventive services. 2009; Available from: http://www.thecommunityguide.org/cancer/screening/clientoriented/index.html.
References
- 1.Williams KP, Sheppard VB, Todem D, Mabiso A, Wulu JT, Jr, Hines RD. Family matters in mammography screening among African-American women age > 40. J Natl Med Assoc. 2008;100:508–15. doi: 10.1016/s0027-9684(15)31297-9. [DOI] [PubMed] [Google Scholar]
- 2.Thomas EC. African American women's breast memories, cancer beliefs, and screening behaviors. Cancer Nurs. 2004;27:295–302. doi: 10.1097/00002820-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Young RF, Severson RK. Breast cancer screening barriers and mammography completion in older minority women. Breast Cancer Res Treat. 2005;89:111–8. doi: 10.1007/s10549-004-1476-8. [DOI] [PubMed] [Google Scholar]
- 4.Calvocoressi L, Kasl SV, Lee CH, Stolar M, Claus EB, Jones BA. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40 to 79 years. Cancer Epidemiol Biomarkers Prev. 2004;13:2096–105. [PubMed] [Google Scholar]
- 5.Paskett ED, Tatum C, Rushing J, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004;101:2650–9. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregg J, Curry RH. Explanatory models for cancer among African-American women at two Atlanta neighborhood health centers: the implications for a cancer screening program. Soc Sci Med. 1994;39:519–26. doi: 10.1016/0277-9536(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Crump SR, Mayberry RM, Taylor BD, Barefield KP, Thomas PE. Factors related to noncompliance with screening mammogram appointments among low-income African-American women. J Natl Med Assoc. 2000;92:237–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips JM, Cohen MZ, Moses G. Breast cancer screening and African American women: fear, fatalism, and silence. Oncol Nurs Forum. 1999;26:561–71. [PubMed] [Google Scholar]
- 9.Bailey EJ, Erwin DO, Belin P. Using cultural beliefs and patterns to improve mammography utilization among African-American women: the Witness Project. J Natl Med Assoc. 2000;92:136–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Zollinger TW, Champion VL, Monahan PO, et al. Impact of personal characteristics on African American women's beliefs about breast cancer. Am J Health Promot. 2010 doi: 10.4278/ajhp.07031727. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler BA. Social processes used by African American women in making decisions about mammography screening. Journal of Nursing Scholarship. 2006;38:247–54. doi: 10.1111/j.1547-5069.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 12.Russell KM, Perkins SM, Zollinger TW, Champion VL. Sociocultural context of mammography screening use. Oncol Nurs Forum. 2006;33:105–12. doi: 10.1188/06.ONF.105-112. [DOI] [PubMed] [Google Scholar]
- 13.Garbers S, Chiasson MA. Breast cancer screening and health behaviors among African American and Caribbean Women in New York City. J Health Care Poor Underserved. 2006;17:37–46. doi: 10.1353/hpu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 14.Hegarty V, Burchett BM, Gold DT, Cohen HJ. Racial differences in use of cancer prevention services among older Americans. J Am Geriatr Soc. 2000;48:735–40. doi: 10.1111/j.1532-5415.2000.tb04746.x. [DOI] [PubMed] [Google Scholar]
- 15.Lukwago SN, Kreuter MW, Holt CL, Steger-May K, Bucholtz DC, Skinner CS. Sociocultural correlates of breast cancer knowledge and screening in urban African American women. Am J Public Health. 2003;93:1271–4. doi: 10.2105/ajph.93.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med. 2007;45:252–61. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring health messages: Customizing communication with computer technology. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 18.Clark MA, Rakowski W, Ehrich B, et al. The effect of a stage-matched and tailored intervention on repeat mammography(1) Am J Prev Med. 2002;22:1–7. doi: 10.1016/s0749-3797(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 19.Kreuter MW, Sugg-Skinner C, Holt CL, et al. Cultural tailoring for mammography and fruit and vegetable intake among low-income African-American women in urban public health centers. Prev Med. 2005;41:53–62. doi: 10.1016/j.ypmed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Carney PA, Harwood BG, Greene MA, Goodrich ME. Impact of a telephone counseling intervention on transitions in stage of change and adherence to interval mammography screening (United States) Cancer Causes Control. 2005;16:799–807. doi: 10.1007/s10552-005-2612-4. [DOI] [PubMed] [Google Scholar]
- 21.Luckmann R, Savageau JA, Clemow L, Stoddard AM, Costanza ME. A randomized trial of telephone counseling to promote screening mammography in two HMOs. Cancer Detect Prev. 2003;27:442–50. doi: 10.1016/j.cdp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Messina CR, Lane DS, Grimson R. Effectiveness of women's telephone counseling and physician education to improve mammography screening among women who underuse mammography. Ann Behav Med. 2002;24:279–89. doi: 10.1207/S15324796ABM2404_04. [DOI] [PubMed] [Google Scholar]
- 23.Champion VL, Skinner CS, Foster JL. The effects of standard care counseling or telephone/in-person counseling on beliefs, knowledge, and behavior related to mammography screening. Oncol Nurs Forum. 2000;27:1565–71. [PubMed] [Google Scholar]
- 24.Ciatto S, Rosselli Del Turco M, Burke P, Visioli C, Paci E, Zappa M. Comparison of standard and double reading and computer-aided detection (CAD) of interval cancers at prior negative screening mammograms: blind review. Br J Cancer. 2003;89:1645–9. doi: 10.1038/sj.bjc.6601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champion VL, Springston JK, Zollinger TW, et al. Comparison of three interventions to increase mammography screening in low income African American women. Cancer Detect Prev. 2006;30:535–44. doi: 10.1016/j.cdp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Simon CE. Breast cancer screening: cultural beliefs and diverse populations. Health Soc Work. 2006;31:36–43. doi: 10.1093/hsw/31.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Crump SR, Shipp MPL, McCray GG, et al. Abnormal mammogram follow-up: do community lay health advocates make a difference? Health Promotion Practice. 2008;9:140–8. doi: 10.1177/1524839907312806. [DOI] [PubMed] [Google Scholar]
- 28.Earp JA, Flax VL. What lay health advisors do: An evaluation of advisors' activities. Cancer Pract. 1999;7:16–21. doi: 10.1046/j.1523-5394.1999.07104.x. [DOI] [PubMed] [Google Scholar]
- 29.Eng E, Smith J. Natural helping functions of lay health advisors in breast cancer education. Breast Cancer Res Treat. 1995;35:23–9. doi: 10.1007/BF00694741. [DOI] [PubMed] [Google Scholar]
- 30.Earp J, Eng E, O'Malley M, et al. Increasing use of mammography among older, rural African American women: results for a community trial. Am J Public Health. 2002;92:646–54. doi: 10.2105/ajph.92.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erwin DO, Spatz TS, Stotts RC, Hollenberg JA. Increasing mammography practice by African American women. Cancer Pract. 1999;7:78–85. doi: 10.1046/j.1523-5394.1999.07204.x. [DOI] [PubMed] [Google Scholar]
- 32.Paskett E, Tatum C, Rushing J, et al. Randomized trial of an intervention to improve mammography utilization among a triracial rural population of women. J Natl Cancer Inst. 2006;98:1226–37. doi: 10.1093/jnci/djj333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paskett ED, Tatum CM, D'Agostino R, Jr, et al. Community-based interventions to improve breast and cervical cancer screening: Results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiol Biomarkers Prev. 1999;8:453–9. [PubMed] [Google Scholar]
- 34.Sung J, Blumenthal D, Coates R, Williams J, Alema-Mensah E, Liff J. Effect of a cancer screening intervention conducted by lay health workers among inner-city women. Am J Prev Med. 1997;13:51–7. [PubMed] [Google Scholar]
- 35.West DS, Greene P, Pulley L, et al. Stepped-care, community clinic interventions to promote mammography use among low-income rural African American women. Health Educ Behav. 2004;31:29S–44S. doi: 10.1177/1090198104266033. [DOI] [PubMed] [Google Scholar]
- 36.Janz NK, Champion VL, Strecher VJ. The health belief model. In: Glanz K, Rimer BK, Lewis EM, editors. Health behavior and health education: theory, research and practice. 3rd. San Francisco: Jossey-Bass; 2002. pp. 45–66. [Google Scholar]
- 37.Witte K. Fear control and danger control: a test of the extended parallel process model (EPPM) Communication Monographs. 1994;61:113–34. [Google Scholar]
- 38.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 39.Russell KM, Champion VL, Skinner CS. Psychosocial factors related to repeat mammography screening over 5 years in African American women. Cancer Nurs. 2006;29:236–43. doi: 10.1097/00002820-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Vernon SW, Laville EA, Jackson GL. Participation in breast screening programs: a review. Soc Sci Med. 1990;30:1107–18. doi: 10.1016/0277-9536(90)90297-6. [DOI] [PubMed] [Google Scholar]
- 41.Russell KM, Monahan P, Wagle A, Champion V. Differences in health and cultural beliefs by stage of mammography screening adoption in African American women. Cancer. 2007;109:386–95. doi: 10.1002/cncr.22359. [DOI] [PubMed] [Google Scholar]
- 42.Champion VL, Monahan PO, Springston JK, et al. Measuring mammography and breast cancer beliefs in African American women. J Health Psychol. 2008;13:827–37. doi: 10.1177/1359105308093867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakowski W, Ehrich B, Goldstein MG, et al. Increasing mammography among women aged 40-74 by use of a stage-matched, tailored intervention. Prev Med. 1998;27:748–56. doi: 10.1006/pmed.1998.0354. [DOI] [PubMed] [Google Scholar]
- 44.Green LW, Kreuter MW. Health promotion planning: An educational and environmental approach. Mountain View, CA: Mayfield; 1999. [Google Scholar]
- 45.Bandura A. Social learning theory. Prentice-Hall; Englewood Cliffs, NJ: 1977. [Google Scholar]
- 46.Airhihenbuwa CO. Health promotion and disease prevention strategies for African Americans: A conceptual model. In: Braithwaite RL, Taylor SE, editors. Health issues in the black community. San Francisco, CA: Jossey-Bass; 1993. pp. 267–77. [Google Scholar]
- 47.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds raio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 48.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. Br Med J. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]


