Abstract
Background
Many patients treated with hemodialysis remain anemic despite exogenous erythropoietin therapy, suggesting the anemia experienced by these patients is multifactorial in etiology. Iron deficiency, infection, inflammation, and malnutrition have been implicated in this process. Additionally, secondary hyperparathyroidism has been associated with anemia in adults, but little data exists on this topic in children.
Study Design
Cross-sectional, retrospective.
Setting & Participants
Children treated in hemodialysis centers (N=588) within the Center for Medicare & Medicaid Services’ (CMS) 2002 Clinical Performance Measures (CPM) Project.
Predictor
Intact parathyroid hormone (iPTH) assessed in October, November, and December 2001 and categorized as quintiles.
Outcomes & Measurements
Achievement of a serum hemoglobin ≥11 g/dl was assessed by Poisson regression adjusting for sex, age, race, dialysis vintage, vascular access type, single-pool Kt/V, serum albumin, normalized protein catabolic rate (nPCR), calcium-phosphorus product, and erythropoietin alpha dose.
Results
Using the second quintile (iPTH 103–224 pg/ml) as the reference quintile, there was no association between quintile of iPTH and achievement of the hemoglobin goal: 1st quintile prevalence ratio (95% confidence interval) 1.0 (0.9, 1.2); 3rd quintile 0.95 (0.8, 1.1); 4th quintile 0.99 (0.8, 1.2); 5th quintile 0.97 (0.8, 1.1). Only serum albumin ≥ 3.5 g/dl (Bromocresol Green assay method) or ≥ 3.2 g/dl (Bromocresol Purple assay method) was significantly associated with meeting the hemoglobin goal 1.4 (1.2, 1.6).
Limitations
The simultaneous collection of iPTH and hemoglobin limits causal inference. Iron stores and iron therapy are potential confounders not accounted for in this study.
Conclusions
In the largest study on this topic in children, there was no association found between iPTH levels and achievement of a hemoglobin ≥ 11g/dl. Serum albumin was strongly associated with achievement of the hemoglobin goal.
Index Words: secondary hyperparathyroidism, anemia, hemodialysis
Introduction
Patients requiring dialysis often develop anemia. Anemia is associated with impairments in cardiac function, cognition, exercise capacity, and decreased quality of life (1–4). Studies in both adults and children with chronic kidney disease (CKD) and end-stage renal disease (ESRD) have shown that serum hemoglobin levels <11 g/dl have been associated with increased morbidity and mortality (1,3,4,5). Determining factors that contribute to anemia in these patients may limit such morbidity and mortality in this population. The anemia experienced by patients with CKD/ESRD is thought to be primarily due to inadequate endogenous erythropoietin production (1). However, many patients remain anemic despite exogenous erythropoiesis stimulating agents (ESAs), suggesting a multifactorial process (6–9). Iron deficiency, infection, malnutrition, inflammation, and secondary hyperparathyroidism have been implicated as potential contributing factors in anemia (6–10, 11). Several studies in adults treated by dialysis have linked refractory anemia to higher intact parathyroid hormone (iPTH) levels. There is a paucity of data on this topic in children treated with dialysis.
To evaluate the association between secondary hyperparathyroidism and anemia in children requiring hemodialysis, a cross-sectional analysis of data from the Centers for Medicare & Medicaid Services’ (CMS) Clinical Performance Measures (CPM) 2002 Project Special Data Collection was performed. This data represents the largest available collection of iPTH values in children on dialysis. We hypothesized that increasing degree of secondary hyperparathyroidism was associated with lower serum hemoglobin in this population.
Methods
Sample
CMS’ CPM Project performs yearly assessments of the quality of care provided to ESRD patients and included children beginning in the year 2000. For the 2002 project year only, a special data collection was conducted to include calcium, phosphorus, and iPTH levels. The data collection and sample methods of CMS’ CPM Project have been described previously (12). In brief, clinical parameters and demographic data were obtained on all patients less than 18 years of age maintained on in-center hemodialysis during the data collection period: October, November, and December of 2001 (12). Patients eligible to remain in the sample for analysis had at least one measurement of serum albumin, hemoglobin, phosphorus, calcium, weight, erythropoietin alpha dose, and paired pre- and post-dialysis blood urea nitrogen (BUN) submitted. Patients were excluded if serum iPTH units were not documented.
Data Analysis
Corrected calcium was calculated using the Modified Orrell formula (13). Single-pooled Kt/V was calculated using the Daugirdas II formula (14). Normalized protein catabolic rate (nPCR) was calculated from available data (15). Erythropoietin alpha dose was analyzed as units per kilogram per week.
For the descriptive data analysis, mean values were calculated for age, dialysis vintage, spKt/V, iPTH, serum albumin, nPCR, calcium-phosphorus product, serum ferritin, and erythropoietin alpha dose using data from all three collection periods. Patients were categorized by serum hemoglobin <11 g/dl or ≥ 11 g/dl. Patients were also categorized by calcium-phosphorus product of <55 mg2/dl2 or ≥ 55 mg2/dl2 for patients ≥12 years old and < 65 mg2/dl2 or ≥ 65 mg2/dl2 for patients <12 years old based on National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) recommendations and targets (1,16). Patients were also classified by serum albumin <3.5 (bromocresol green assay) or <3.2 g/dl (bromocresol purple assay method) versus ≥ 3.5 (bromocresol green) or ≥ 3.2 g/dl (bromocresol purple) based on serum albumin threshold levels historically reported in the CPM Project (17). Additionally, nPCR was categorized as <1.0 or ≥ 1.0 for those ≥ 12 years old and <1.3 or ≥ 1.3 for younger patients based on thresholds used in previous studies (18). Erythropoietin alpha (units/kg/week) and iPTH (pg/ml) were evaluated as quintiles. Activated Vitamin D use was recorded for each of the three data collection periods; however no information regarding dose was collected. Therefore, activated vitamin D use was evaluated in a dichotomous manner in the descriptive analysis. The type of vascular access was also examined in a dichotomous manner comparing patients with a venous catheter to those with either an arteriovenous graft or fistula. Race was categorized as “White,” “Black,” or “Other.” The outcome variable was defined as obtaining a serum hemoglobin of ≥11g/dl or <11 g/dl based on KDOQI recommendations (1).
Chi-squared and t-tests were performed to assess any associations between the various patient characteristics and outcome. Collinearity was assessed using the variance inflation factor method.
For each patient, there were three data collection periods and therefore, three possible iPTH values. Many of the patients meeting inclusion criteria were missing at least one of the three iPTH values. Therefore, multiple imputation was performed to complete the missing iPTH data. Multiple imputation is a common statistical approach to limit bias and maximize precision in the context of missing data. This statistical approach completes missing values for a given variable based on other covariates, specifically the covariates shown to predict whether the variable to be imputed (iPTH in this case) is missing (19). To identify predictors of missing iPTH values, patients were first divided into those missing iPTH values and those not missing iPTH values for each collection period. A binary outcome variable was created for each month of data collection based on this distinction. A multiple logistic regression analysis was performed for each month. Variables with less than five percent of values missing at a given collection period served as the independent variables in the analysis. These variables included age, race, sex, type of vascular access, dialysis vintage, and albumin. Missing data was imputed based on variables identified as being associated with increased odds of missing iPTH values as well as iPTH levels measured at other collection periods when available using multiple imputation by chained equations analysis (20). Given that the non-imputed iPTH values were severely right skewed, intact PTH was loge transformed prior to imputation. Five imputed datasets were generated in this process. The bivariate and multivariate Poisson analyses described below were performed on each imputed dataset with iPTH categorized as quintiles. These quintiles were based on iPTH values on the arithmetic scale. Standard methods of imputation were used to combine the results from the five imputed datasets.
Bivariate Poisson analysis was conducted to evaluate an association between iPTH quintile and achievement of the existing KDOQI hemoglobin goal (1). The Poisson regression method was chosen to estimate prevalence ratios and 95% confidence intervals (CI) in this cross sectional analysis (21,22). The iPTH and hemoglobin values from each collection period were treated as independent observations in the Poisson analysis. The lack of independence between observations coming from the same patient was corrected for with robust standard errors. Robust standard errors were also used to address the tendency for the errors in the Poisson model to overestimate the prevalence ratio standard errors when applied to binomial data. A similar analysis evaluating iPTH as a binary exposure (≥ 300 pg/ml versus <300 pg/ml) was conducted. Additionally, another bivariate analysis compared achievement of the hemoglobin goal among patients with iPTH values persistently in the highest quintile (in all three monthly collection periods) or with iPTH values that increased across monthly data collection periods from October to December, to that of patients with iPTH values persistently in the lowest quintile or with decreasing iPTH values across all three months.
Multivariate Poisson analysis evaluating the association between quintiles of iPTH and attainment of the hemoglobin goal, adjusting for sex, age, race, dialysis vintage, vascular access type, dialysis adequacy, serum albumin, calcium-phosphorus product, nPCR status, and erythropoietin alpha dose was then performed (22). Ferritin was not included as an independent variable in the primary analysis due to a large amount of missing values in each collection period. However, a separate analysis including ferritin as an independent variable was also performed. All analyses were performed in both the imputed and non-imputed datasets.
Intercooled STATA 9 statistical package was used to conduct all analyses (Stata Statistical Software, version 9.1. StataCorp LP, College Station, TX). P-values of less than 0.05 were considered statistically significant.
Results
Of the 764 eligible patients enrolled in the 2002 CPM project, 588 (77%) met the minimum data requirements for inclusion in our study. One hundred seven patients had no date of birth documented, 37 patients had unclear iPTH units, 21 had no erythropoietin alpha dose documented, 6 were missing values for weight, and the albumin assay method was unclear in 5 patients. The remaining patients had iPTH units recorded in pg/ml. Prior to imputation, 528 patients (90%) had at least one iPTH value recorded. One-hundred-forty-eight (25%) patients had iPTH values for all three collection periods, 104 (18%) patients had values for only two of the collections, and 276 (47%) had iPTH values for only one collection period. For the October data collection, 341 (58%) patient had iPTH values available. For the November and December data collections, 291 (49%) and 296 (50%) patients had iPTH values recorded, respectively. The normal ranges for each assay were included in the data collection. The median range was 10–65 pg/ml. Seventy-five percent of lower limit values were between 10–15 pg/ml while 68% of upper limit values were between 65–75 pg/ml.
Excluded patients were more likely to be at least 13 years old (85% vs. 70%, p<0.001), have dialysis vintage ≥ 1year, (86% vs. 66% p<0.001), have arteriovenous fistulas or grafts rather than catheters (78% vs. 45% p<0.001), have higher mean iPTH levels (522 pg/ml vs. 402 pg/ml p=0.04), meet the spKt/V (94% vs. 87% p=0.009), hemoglobin (86% vs. 62% p<0.001) and nPCR (85% vs. 52% p<0.001) goals. Excluded patients were less likely to meet the albumin threshold (28% vs. 83% p<0.001) and calcium-phosphorus targets (21% vs. 45% p<0.001). Since quintiles are defined by the included data, it is not surprising that the percentage of patients in each quintile differed between included and excluded patients.
Table 1 compares demographic and clinical characteristics of the study population by iPTH quintile. The mean age was lowest in the first iPTH quintile. There were a higher percentage of patients with dialysis vintage of at least one year in the fifth quintile when compared to other quintiles. Patients in the fifth quintile also had the highest median ferritin values. Patients in higher quintiles were more likely to receive activated vitamin D supplementation than were patients in the two lowest iPTH quintiles.
Table 1.
Demographic and clinical patient characteristics stratified by iPTH quintile*.
| Patient Characteristics** |
Total*** | iPTH quintile 1 |
iPTH quintile 2 |
iPTH quintile 3 |
iPTH quintile 4 |
iPTH quintile 5 |
p- value**** |
|---|---|---|---|---|---|---|---|
| Sex | 0.1 | ||||||
| Female | 253 (43) | 57 (54) | 44 (42) | 41 (39) | 41 (39) | 42 (40) | |
| Male | 335 (57) | 48 (46) | 62 (58) | 65 (61) | 65 (61) | 63 (60) | |
| Age in years, mean (SD) | 13.89 (3.93) |
12.97 (4.68) |
13.83 (3.80) |
14.26 (3.31) |
14.68 (3.17) |
13.70 (4.36) |
0.02 |
| Age, years | 0.1 | ||||||
| <13 | 177 (30) | 39 (37) | 37 (35) | 28 (26) | 24 (23) | 32 (30) | |
| ≥13 | 411 (70) | 66 (63) | 69 (65) | 78 (74) | 82 (77) | 73 (70) | |
| Race | 0.1 | ||||||
| White | 289 (49) | 59 (56) | 58 (55) | 45 (43) | 44 (42) | 44 (42) | |
| Black | 232 (40) | 34 (33) | 40 (38) | 48 (45) | 49 (47) | 49 (47) | |
| Other | 67 (11) | 12 (11) | 8 (7) | 13 (12) | 12 (11) | 12 (11) | |
| Dialysis Vintage in years Mean (SD) |
3.20 (3.53) |
3.01 (3.34) |
2.45 (3.12) |
3.46 (3.66) |
3.41 (3.75) |
3.71 (3.69) |
0.09 |
| Dialysis Vintage | 0.04 | ||||||
| <1 year | 197 (34) | 39 (37) | 41 (39) | 32 (30) | 34 (33) | 21 (20) | |
| ≥1 year | 385 (66) | 66 (63) | 64 (61) | 73 (70) | 70 (67) | 82 (80) | |
| Vascular Access | 0.6 | ||||||
| Fistula/Graft | 265 (45) | 42 (40) | 52 (49) | 47 (45) | 52 (49) | 50 (48) | |
| Catheter | 322 (55) | 63 (60) | 54 (51) | 58 (55) | 54 (51) | 55 (52) | |
| spKt/V, median (IQR) | 1.54 (0.44) |
1.58 (0.44) |
1.51 (0.47) |
1.51 (0.45) |
1.49 (0.44) |
1.55 (0.41) |
0.6 |
| spKt/V | 0.2 | ||||||
| <1.2 | 79 (14) | 10 (10) | 18 (17) | 15 (14) | 19 (18) | 10 (10) | |
| ≥1.2 | 499 (86) | 92 (90) | 86 (83) | 89 (86) | 86 (82) | 94 (90) | |
| iPTH in pg/ml, median (IQR) | 329 (578) |
65 (46) |
171 (74) |
329 (101) |
632 (203) |
1310 (675) |
<0.001 |
| Hb in g/dl, median (IQR) | 11.4 (2) |
11.5 (2.1) |
11.5 (1.5) |
11.4 (2.0) |
11.6 (1.6) |
11.5 (2.2) |
0.9 |
| Hb in g/dl | 0.7 | ||||||
| <11 | 224 (38) | 38 (36) | 36 (34) | 37 (35) | 33 (31) | 43 (41) | |
| ≥11 | 364 (62) | 67 (64) | 70 (66) | 69 (65) | 73 (69) | 62 (59) | |
| Albumin in g/dl, median (IQR) | 3.83 (0.63) |
3.75 (0.64) |
3.80 (0.57) |
3.80 (0.70) |
4.00 (0.57) |
3.87 (0.53) |
0.1 |
| Albumin, g/dl | 0.2 | ||||||
| <3.2 (BCP) or <3.5(BCG) | 102 (17) | 19 (18) | 23 (22) | 15 (14) | 12 (11) | 14 (13) | |
| ≥3.2 (BCP) or ≥3.5(BCG) | 486 (83) | 86 (82) | 83 (78) | 91 (86) | 94 (89) | 91 (87) | |
| nPCR, mean (SD) | 1.12 (0.44) |
1.10 (0.33) |
1.07 (0.30) |
1.06 (0.30) |
1.12 (0.30) |
1.23 (0.77) |
0.05 |
| nPCR | 0.2 | ||||||
| <1.0(≥12yrs) or <1.3(<12yrs) | 283 (49) | 56 (55) | 55 (53) | 56 (54) | 43 (41) | 47 (45) | |
| ≥1.0(≥12yrs) or≥1.3(<12yrs) | 295 (51) | 45 (45) | 49 (47) | 48 (46) | 62 (59) | 57 (55) | |
| Ca × P, mean (SD) | 60.08 (16.05) |
59.20 (15.67) |
59.75 (14.90) |
58.95 (16.63) |
61.47 (16.93) |
61.03 (16.11) |
0.7 |
| Ca × P | 0.7 | ||||||
| <55(≥12yrs)or<65(<12yrs) | 263 (47) | 49 (49) | 49 (49) | 52 (50) | 43 (41) | 45 (43) | |
| ≥55(≥12yrs)or≥65(<12yrs) | 301 (53) | 52 (51) | 51 (51) | 53 (50) | 61 (59) | 59 (57) | |
| Ferritin in ng/ml, median (IQR) |
279 (468) |
248 (443) |
214 (389) |
259 (466) |
327 (474) |
395 (411) |
0.02 |
| Erythropoietin α (units/kg/week), median (IQR) |
278 (280) |
308 (292) |
260 (269) |
258 (249) |
235 (214) |
310 (317) |
0.3 |
| Activated vitamin D supplementation |
396 (76) | 65 (63) | 76 (72) | 83 (82) | 88 (85) | 84 (81) | <0.001 |
Raw data iPTH quintile 1: ≤108 pg/ml; quintile 2: >108–≤246 pg/ml; quintile 3: >246 – ≤431 pg/ml; quintile 4: <431 – ≤808 pg/ml; quintile 5: >808 pg/ml.
Values presented as N(%) or median (interquartile range).
Column may not add up to 588 due to missing data.
P value applies to X2 and ANOVA F-tests comparing patient characteristics between iPTH quintiles.
Conversion factors for units: serum hemoglobin in g/dl to g/L, ×10; serum albumin in g/dl to g/L, ×10; serum calcium in mg/dl to mmol/L, ×0.2495; serum phosphorus in mg/dl to mmol/L, ×0.3229. No conversion necessary for serum ferritin in ng/ml and µg/L and intact PTH in pg/ml and ng/ml.
Values in the table reflect averages across all three data collection periods.
Abbreviations: BCG, bromocresol green; BCP, bromocresol purple; spKt/V, single-pool Kt/V.
Trajectories of iPTH were evaluated for the study population over time. Among patients with an iPTH value recorded at all three collection periods, 18.2% had iPTH values that monotonically increased with time, 21.6% had iPTH values that monotonically decreased with time, and 60.2% had iPTH values that either increased then decreased, decreased then increased, or plateaued at some point during their trajectory.
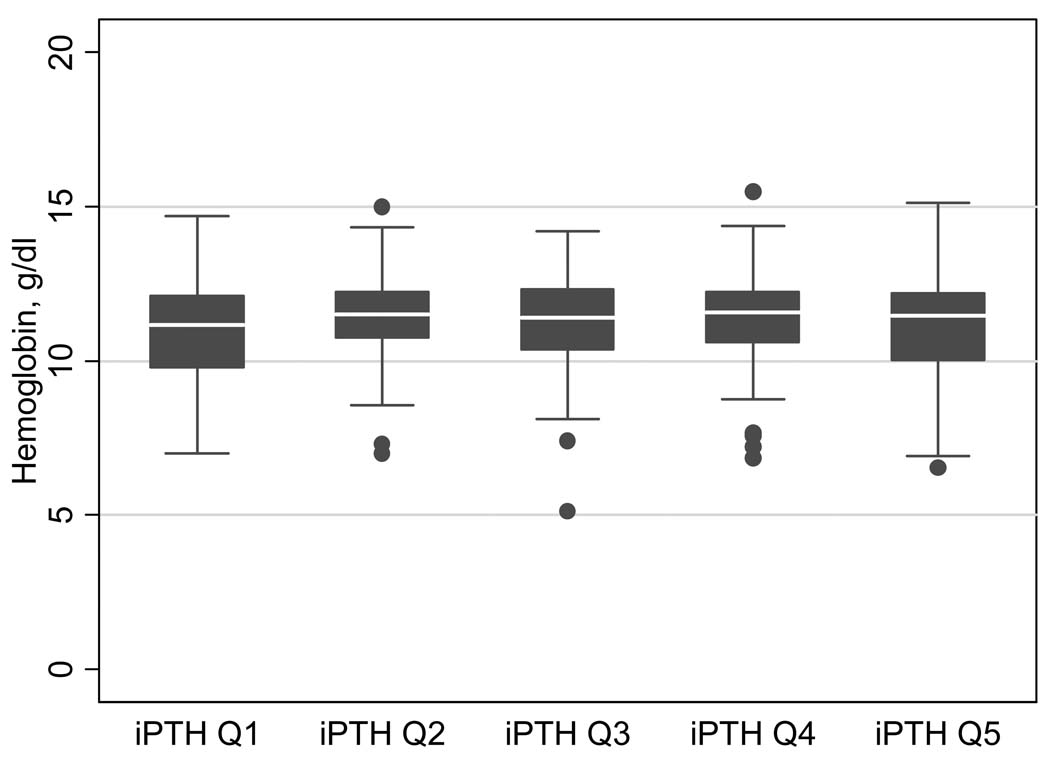
The median hemoglobin stratified by iPTH quintiles is shown in Figure 1. Fifty-eight percent, 61% and 64% of patients met the hemoglobin goal in the October, November and December collection periods, respectively. When the hemoglobin values were averaged across all three months, 62% of patients met the hemoglobin goal. Patients with serum hemoglobin ≥ 11g/dl were more likely to have an arteriovenous fistula or graft, to have longer dialysis vintage, to be at least 13 years old, and to have serum albumin levels ≥ 3.5 g/dl (BCG) or ≥ 3.2 g/dl (BCP). Patients meeting the hemoglobin goal had a median erythropoietin alpha dose 129 units/kg/week lower than patients not meeting the hemoglobin goal.
Figure 1.
Median and interquartile range of hemoglobin levels stratified by iPTH quintiles. Raw data for PTH quintiles: 1, ≤108 pg/ml; 2, >108–≤246 pg/ml; 3, >246 – ≤431 pg/ml; 4, <431 – ≤808 pg/ml; 5, >808 pg/ml.
Conversion factor for serum hemoglobin in g/dl to g/L, ×10; no conversion necessary for intact PTH in pg/ml and ng/ml.
Table 2 shows the results of the bivariate Poisson analysis performed to evaluate the relationship between iPTH quintile and achievement of the hemoglobin threshold. The second quintile was chosen as the clinically pertinent reference category as it included values within, but not above the KDOQI target iPTH range of 200–300 pg/ml for patients with ESRD (16). There was not a decreased likelihood of achieving the hemoglobin goal based on: 1) increasing iPTH quintile, 2) iPTH ≥ 300 pg/ml versus < 300 pg/ml, or 3) increasing/highest iPTH quintile versus decreasing/lowest iPTH quintile.
Table 2.
Bivariate associations between iPTH level and achieving the KDOQI recommended serum hemoglobin level of 11 g/dl or greater.
| Patient Characteristics | Unadjusted Prevalence Ratio (95% CI) |
p-value |
|---|---|---|
| IPTH quintile* | ||
| 1 | 0.95 (0.8, 1.1) | 0.5 |
| 2 | Reference | - |
| 3 | 0.95 (0.8, 1.2) | 0.5 |
| 4 | 1.02 (0.9, 1.2) | 0.8 |
| 5 | 0.95 (0.8, 1.1) | 0.5 |
| iPTH, pg/ml | ||
| <300 | Reference | |
| ≥300 | 1.01 (0.9, 1.1) | 0.8 |
| IPTH level | ||
| Q5 or ↑ across all 3 collection periods |
0.98 (0.7, 1.3) | 0.9 |
| Q1 or ↓ across all 3 collection periods |
Reference | |
Average serum values defining quintiles (Q) across all 5 imputed datasets: Q1, <103 pg/ml; Q2, >103–224 pg/ml; Q3, >224–416 pg/ml; Q4, >416–823 pg/ml; Q5, >823 pg/ml.
Conversion factor for serum hemoglobin in g/dl to g/L, ×10; no conversion necessary for intact PTH in pg/ml and ng/ml.
The results from the multivariate Poisson regression examining the association between quintile of iPTH and reaching a hemoglobin level of ≥ 11 g/dl are presented in table 3. This analysis adjusted for sex, age, race, dialysis vintage, vascular access type, adequacy, serum albumin, calcium-phosphorus product, nPCR, and Erythropoietin alpha dose. There was no collinearity. In this analysis, there was no association found between iPTH levels and achievement of a serum hemoglobin of ≥ 11g/dl. Examining the effect of iPTH as a continuous predictor did not change this lack of an association. However, serum albumin levels ≥ 3.5 (BCG) or ≥ 3.2 (BCP) were strongly associated with achievement of the hemoglobin goal (prevalence ratio, 1.4, 95%CI 1.2, 1.6). No evidence of an interaction between erythropoietin alpha dose and iPTH as a continuous and quintile predictor was found.
Table 3.
Adjusted associations between demographic and clinical patient characteristics and achieving the KDOQI recommended serum hemoglobin level of 11 g/dl or greater.
| Patient Characteristics | Adjusted Prevalence Ratio * (95% CI) |
p-value |
|---|---|---|
| Gender | ||
| Female | Reference | |
| Male | 0.98 (0.9, 1.1) | 0.8 |
| Race | ||
| White | Reference | |
| Black | 1.0 (0.9, 1.1) | 0.8 |
| Other | 1.1 (0.9, 1.2) | 0.5 |
| spKt/V | ||
| <1.2 | Reference | |
| ≥1.2 | 1.05 (0.9, 1.2) | 0.5 |
| iPTH quintile** | ||
| 1 | 1.0 (0.9, 1.2) | 0.9 |
| 2 | Reference | - |
| 3 | 0.95 (0.8, 1.1) | 0.5 |
| 4 | 0.99 (0.8, 1.2) | 0.9 |
| 5 | 0.97 (0.8, 1.1) | 0.7 |
| Albumin, g/dl | ||
| <3.2(BCP) or <3.5(BCG) | Reference | |
| ≥3.2(BCP) or ≥3.5(BCG) | 1.4 (1.2, 1.6) | <0.001 |
| nPCR | ||
| <1.0(≥12yrs) or <1.3(<12yrs) | Reference | |
| ≥1.0(≥12yrs) or ≥1.3(<12yrs) | 1.0 (0.9, 1.1) | 0.4 |
| Ca × P | ||
| <55(≥12yrs)/<65(<12yrs) | Reference | |
| ≥55(≥12yrs)/ ≥65(<12yrs) | 1.1 (1.0, 1.2) | 0.2 |
| Erythropoietin α quintile*** | ||
| 1 | ||
| 2 | Reference | |
| 3 | 1.0 (0.9, 1.2) | 0.6 |
| 4 | 0.8 (0.7, 0.9) | 0.001 |
| 5 | 0.8 (0.7, 0.9) | <0.001 |
| 0.6 (0.5, 0.7) | <0.001 | |
Adjusted for all covariates in table, age, dialysis vintage, and type of vascular access
The average serum values defining quintiles across all 5 imputed datasets: quintile 1 (<103 pg/ml), quintile 2 (>103–224 pg/ml), quintile 3 (>224–416 pg/ml), quintile 4 (>416–823 pg/ml) quintile 5 (>823 pg/ml)
Erythropoietin alpha quintile 1 (<128 units/kg/week), quintile 2 (>128–218 units/kg/week), quintile 3 (>218–332 units/kg/week), quintile 4 (>332–500 units/kg/week), quintile 5 (>500 units/kg/week)
Conversion factors for units: serum hemoglobin in g/dl to g/L, ×10; serum albumin in g/dl to g/L, ×10; serum calcium in mg/dl to mmol/L, ×0.2495; serum phosphorus in mg/dl to mmol/L, ×0.3229. No conversion factor necessary for intact PTH in pg/ml and ng/ml.
Abbreviations:
Abbreviations: BCG, bromocresol green; BCP, bromocresol purple; spKt/V, single-pool Kt/V.
Ferritin values were not included in the primary analysis due to the large amount of missing data. Including ferritin in the model did not significantly affect the results. Using iPTH quintile 2 as the reference category, the analysis that included ferritin yielded the following results: 1st quintile prevalence ratio (95% CI) 0.95 (0.8,1.2); 3rd quintile 0.9 (0.8, 1.1); 4th quintile 0.99 (0.8, 1.2); and 5th quintile 0.96 (0.8, 1.2). Analyses in the non-imputed dataset yielded results that did not significantly differ from those presented from the imputed dataset.
Discussion
It is well established that untreated anemia can lead to decreased quality of life, impairments in cardiac function, cognition and exercise capacity, as well as increased mortality in patients treated by dialysis (1–5). There is also emerging evidence that anemia may contribute to worsening kidney disease as a part of the cardiorenal anemia syndrome (4). Identifying factors contributing to anemia in children on dialysis could impact the associated morbidity and mortality in these patients. Several studies have identified secondary hyperparathyroidism as one such factor in adults treated by dialysis (6,7,8,9). In 1978, Zingraff and colleagues first reported an improvement in hematocrit, hemoglobin, and red blood cell count in 18 chronic hemodialysis patients undergoing parathyroidectomy. In the subset of patients undergoing pre- and post-parathyroidectomy bone marrow biopsies, the degree of bone marrow fibrosis improved after this procedure (10). Since that time, there have been other reports corroborating these findings (7,9). More recently, Coen and colleagues found that patients undergoing successful parathyroidectomy had an increase in serum hemoglobin despite a decrease in the ESA dose (23).
Animal and in vitro studies suggest that hyperparathyroidism interferes with both red blood cell production and survival. In an animal model of hyperparathyroidism, Drueke and Eckardt found reduced red cell production. Parathyroidectomy resulted in increased red cell iron incorporation in these animals, signifying increased red blood cell production (9). There has also been conflicting evidence of a possible direct inhibitory effect of parathyroid hormone on erythropoiesis, as well as reports of increased red cell osmotic fragility in the presence of high iPTH levels, leading to decreased red blood cell lifespan (9).
Few studies on this topic have been conducted in children treated by dialysis, and those that exist have been limited by small sample size. Seeherunvong and colleagues studied 23 children on hemodialysis and found that the 4 children who required higher doses of ESAs had higher serum iPTH levels than those maintained on the standard dose (24). Belsha and Berry demonstrated that among 17 children receiving either peritoneal or hemodialysis, those not responding to standard doses of ESAs had higher serum iPTH levels than those that did respond (25). In our analysis, strengthened by a much larger sample size, we hypothesized that higher iPTH levels would be associated with failure to achieve a goal hemoglobin of ≥ 11g/dl. However, a statistically significant relationship between iPTH quintile and achievement of the hemoglobin goal was not found.
There are several possible explanations for the lack of association between iPTH and anemia in this study. It may be that more extreme secondary hyperparathyroidism, such as iPTH levels above approximately 800 pg/ml (represented by the 5th quintile in this study), may be needed before there is an impact on anemia. The relatively small number of patients (n=117) in the study population with this level of secondary hyperparathyroidism may limit detection of such an association. Many participants had missing iPTH values. We attempted to address the missing data with multiple imputation, but it is possible that not all predictors of missing iPTH were measured and included in the multiple imputation analysis. As such, misclassification may have resulted, biasing the results toward the null hypothesis of no association. Intact PTH and hemoglobin values were measured at each collection period and may not represent hemoglobin or iPTH levels of the previous days, weeks, or months which may further contribute to misclassification bias. Additionally, the slight variation in normal ranges for iPTH values, suggesting more than one assay was used, may also contribute to misclassification bias. Missing values for potential measured confounders such as ferritin as well as unmeasured confounders may have resulted in residual confounding bias.
Not all factors known to contribute to anemia could be accounted for in this study. Measures of iron stores and iron dose were missing an excessive amount of data and therefore were not included in this study. These factors could potentially modify an association between secondary hyperparathyroidism and anemia. The effect of iPTH on serum hemoglobin possibly may be more pronounced in patients who are iron deficient as compared to those who are not. We were also unable to account for inflammation which has been associated with anemia and hyporesponsiveness to ESAs (11). Future studies would benefit from inclusion of markers of iron stores and inflammation.
Low albumin has been repeatedly linked with anemia, and our results support such an association (26–29). Low albumin is known to result from both poor nutrition and inflammation (11, 30, 31). Further study may reveal whether malnutrition, inflammation, or another process predicts low serum albumin and its subsequent contribution to anemia.
This study is limited by the cross-sectional nature of the analysis where hemoglobin, albumin, iPTH, and other covariates were examined simultaneously. Thus results cannot be interpreted as causal. In addition, the values measured at each collection period may not represent hemoglobin or iPTH levels of the previous days, weeks, or months, which further limits causal inference. However, when comparing patients with increasing and decreasing iPTH trends over the collection periods, there was no significant difference in achievement of the hemoglobin goals at the final data collection period. Also, the differences between included and excluded patients may potentially limit the generalizability of these results. Additionally, children on peritoneal dialysis were not evaluated in this analysis, which may further impact generalizability.
In our study, the largest study to date on this topic in children treated with hemodialysis, iPTH levels were not significantly associated with ability to achieve a threshold level of hemoglobin. The lack of a statistically significant association between intact PTH levels and anemia in this study does not rule out the possibility that secondary hyperparathyroidism contributes to anemia, and does not diminish the importance of treating secondary hyperparathyroidism in these patients.
Acknowledgements
Support: Dr Smith is supported by NIH grant 5T32DK007732, a Ruth L. Kirschstein National Research Service Award, and a Renal Disease Epidemiology Training Grant. Dr Fadrowski is supported by NIH/NIEH grant K23ES016514, and a National Kidney Foundation Young Investigator Grant. Dr Howe is supported by NIH/NIDA grant 5F31DA022114-02, and a Ruth L. Kirschstein National Research Award Predoctoral Fellowship. Dr Fivush is supported by NIH grant U01DK074082. Dr Neu is supported by a NephCure Foundation Grant, a grant for Protocol 20050256 from Amgen, Inc., and an Investigator-Initiated Grant from Merck & Co., Inc. Dr Furth is supported by NIH/NIDDK (National Institutes of Diabetes ***) grant K24DR078737-01, NIH/NIDDK grant UL1 RR025005, NIH/NIDDK U01 DK066174, Montefiore Medical Center Prime Grant U01 DK63549, and a grant for Protocol #85-036 from Genetech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Furth serves as a consultant for Johnson & Johnson. The remaining authors declare that they have no relevant financial interests.
References
- 1.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease: 2007 Update of Hemoglobin Target. Am J Kidney Dis. 2007;50(3):471–529. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Mitsnefes MM, Daniels SR, Schwartz SM, Meer RA, Khoury P, Strife CF. Severe left ventricular hypertrophy in pediatric dialysis: prevalence and predictors. Pediatr nephrol. 2000;14:898–902. doi: 10.1007/s004670000303. [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G. The importance of early treatment of the anemia of chronic kidney disease. Nephrol Dial Transplant. 2001;16 suppl 5:45–49. doi: 10.1093/ndt/16.suppl_5.45. [DOI] [PubMed] [Google Scholar]
- 4.Dowling T. Prevalence, etiology, and consequences of anemia and clinical and economic benefits of anemia correction in patients with chronic kidney disease: an overview. Am J Health-Syst Pharm. 2007;64 suppl 8:S3–S7. doi: 10.2146/ajhp070181. [DOI] [PubMed] [Google Scholar]
- 5.Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S. Association of mortality and hospitalization with achievement of adult hemoglobin targets in adolescents maintained on hemodialysis. J Am Soc Nephro. 2006;17:2878–2885. doi: 10.1681/ASN.2005111215. [DOI] [PubMed] [Google Scholar]
- 6.Drueke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16:25–28. doi: 10.1093/ndt/16.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 7.Tutal E, Sezer S, Afsar B, Arat Z, Ozdemir FN, Haberal M. Additional effects of hyperparathyroidism on inflammatory status and rHuEpo requirements in hemodialysis patients. Transplantation Proceedings. 2006;38:2807–2812. doi: 10.1016/j.transproceed.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 8.Brancaccio D, Cozzolino M, Gallieni M. Hyperparathyroidism and anemia in uremic subjects: a combined therapeutic approach. J Am Soc Nephrol. 2004;15 Suppl 1:S21–S24. doi: 10.1097/01.asn.0000093369.09194.12. [DOI] [PubMed] [Google Scholar]
- 9.Drueke T, Eckardt K. Role of secondary hyperparathyroidism in erythropoietin resistance of chronic renal failure patients. Nephrol Dial Transplant. 2002;17:28–31. doi: 10.1093/ndt/17.suppl_5.28. [DOI] [PubMed] [Google Scholar]
- 10.Zingraff J, Drueke T, Marie P, Man NK, Jungers P, Bordier P. Anemia and secondary hyperparathyroidism. Arch Intern Med. 1978;138:1650–1652. [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, McAllister C, Lenn R, Lee G, Nissenson A, Kopple J. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 12.2002 Annual Report: ESRD Clinical Performance measures Project. Am J Kidney Dis. 2003;42 suppl 2:S1–S96. doi: 10.1016/s0272-6386(03)00520-1. YSIS supplement to CPM data collection S1–S2. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson RN, Alexander KH, Minard G, Croce MA, Brown RO. Accuracy of methods to estimate ionized and “corrected” serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. J Parenter Enteral Nutr. 2004;28:133–141. doi: 10.1177/0148607104028003133. [DOI] [PubMed] [Google Scholar]
- 14.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein AL. Prescribing and monitoring hemodialysis. In: Warady BA, Schaefer FS, Fine RN, Alexander SR, editors. Pediatric Dialysis. Boston, MA: Kluwer Academic Publishers; 2004. pp. 135–145. [Google Scholar]
- 16.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am J Kidney Dis. 2003;42 suppl 3:S1–S170. [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. Baltimore, MD: Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of Clinical Standards & Quality; 2005 Annual Report, End Stage Renal Disease Clinical Performance Measures Project. 2005 December;
- 18.Juarez-Congelosi M, Orellana P, Goldstein S. Normalized protein catabolic rate versus serum albumin as a nutrition status marker in pediatric patients receiving hemodialysis. J Ren Nutr. 2007;17(4):269–274. doi: 10.1053/j.jrn.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Finkle W. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 20.Royston P. Multiple imputation of missing values: Update of ice. Stata Journal. 2004;5(4):527–536. [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2003;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Coen G, Calabria S, Bellingham G, et al. Parathyroidectomy in Chronic Renal Failure: Short and Long-Term Results in Parathyroid Function, Blood pressure, and Anemia. Nephron. 2001;88:149–155. doi: 10.1159/000045976. [DOI] [PubMed] [Google Scholar]
- 24.Seeherunvong W, Rubio L, Abitbol C, et al. Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Peds. 2001;138(No 5):710–712. doi: 10.1067/mpd.2001.112246. [DOI] [PubMed] [Google Scholar]
- 25.Belsha C, Berry P. Effect of hyperparathyroidism on response to erythropoietin in children on dialysis. Pediatr Nephrol. 1998;12:298–303. doi: 10.1007/s004670050458. [DOI] [PubMed] [Google Scholar]
- 26.Wei M, Bargman J, Oreopoulos D. Factors related to erythropoietin hypo-responsiveness in patients on chronic peritoneal dialysis. Int Urol Nephrol. 2007;39:935–940. doi: 10.1007/s11255-007-9226-6. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal R, Davis J, Smith L. Serum albumin is strongly associated with erythropoietin sensitivity in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:98–104. doi: 10.2215/CJN.03330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonelli M, Blake P, Muirhead N. Predictors of erythropoietin responsiveness in chronic hemodialysis patients. ASAIO. 2001;47(1):82–85. doi: 10.1097/00002480-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Madore F, Lowrie E, Brugnara C, et al. Anemia in hemodialysis patients: variables affecting this outcome predictor. J Am Soc Nephrol. 1997;8(12):1921–1929. doi: 10.1681/ASN.V8121921. [DOI] [PubMed] [Google Scholar]
- 30.Paniichi V, Manca-Rizza G, Paoletti S, et al. Effects on inflammatory and nutritional markers of haemodiafiltration with online regeneration of ultrafiltrate (HFR) vs online haemodiafiltration; a cross-over randomized multicentre trial. Nephr Dial Transplant. 2006;21:756–762. doi: 10.1093/ndt/gfi189. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho KT, Silva MI, Bregman R. Nutritional profile of patients with chronic kidney failure. J Ren Nutr. 2004;14:97–100. doi: 10.1053/j.jrn.2004.01.009. [DOI] [PubMed] [Google Scholar]