Abstract
Digestion of blood meal proteins by midgut proteases provides anautogenous mosquitoes with the nutrients required to complete the gonotrophic cycle. Inhibition of protein digestion in the midgut of blood feeding mosquitoes could therefore provide a strategy for population control. Based on recent reports indicating that the mechanism and regulation of protein digestion in blood fed female Aedes aegypti mosquitoes is more complex than previously thought, we used a robust RNAi knockdown method to investigate the role of four highly expressed midgut serine proteases in blood meal metabolism. We show by Western blotting that the early phase trypsin protein (AaET) is maximally expressed at 3 h post blood meal (PBM), and that AaET is not required for the protein expression of three late phase serine proteases, AaLT (late trypsin), AaSPVI (5G1), and AaSPVII. Using the trypsin substrate analog BApNA to analyze in vitro enzyme activity in midgut extracts from single mosquitoes, we found that knockdown of AaSPVI expression caused a 77.6% decrease in late phase trypsin-like activity, whereas, knockdown of AaLT and AaSPVII expression had no significant effect on BApNA activity. In contrast, injection of AaLT, AaSPVI, and AaSPVII dsRNA inhibited degradation of endogenous serum albumin protein using an in vivo protease assay, as well as, significantly decreased egg production in both the first and second gonotrophic cycles (p<0.001). These results demonstrate that AaLT, AaSPVI, and AaSPVII all contribute to blood protein digestion and oocyte maturation, even though AaSPVI is the only abundant midgut late phase serine protease that appears to function as a classic trypsin enzyme.
Keywords: blood meal, digestion, fecundity, RNAi
1. INTRODUCTION
Anautogenous mosquito species require a blood meal to obtain the necessary nutrients for oocyte maturation and egg production. This blood feeding behavior has facilitated the evolution of blood-borne pathogens that exploit mosquitoes as biological vectors. The three primary mosquito vectors responsible for the transmission of human pathogens belong to the Anopheles (malaria plasmodia), Aedes (Dengue and yellow fever viruses), and Culex (West Nile virus) genera, with two species, Anopheles gambiae and Aedes aegypti, accounting for the vast majority of worldwide mosquito-borne diseases (Marquardt 2005). The most effective approach to slowing the spread of mosquito-borne diseases is to control mosquito populations in areas of high pathogen transmission. Indeed, the worldwide increase in Dengue virus transmission by Ae. aegypti in recent years, particularly in Mexico (Cuddehe 2009) and Southeast Asia (Kyle et al., 2008), have been attributed in large part to the spread of the vector mosquito into more urban areas. Because of mosquito resistance to conventional insecticides, and the collateral damage that insecticides can cause to other organisms, there is a need to explore new strategies for vector control. One idea is to develop mosquito-selective small molecule inhibitors that block blood meal metabolism, and as a result, disrupt reproductive processes and significantly reduce fecundity (Scaraffia et al., 2008, Isoe et al., 2009).
The dry weight of a typical 2µl mosquito blood meal consists almost entirely of protein (~500 µg), of which 80% consists of three proteins; hemoglobin (~330 µg), serum albumin (~50 µg), and immunoglobulin (~15 µg). Proteolytic enzymes secreted into the midgut lumen after feeding are responsible for rapidly degrading these blood meal proteins into oligopeptides and amino acids that are converted into other carbon-based metabolites. These protein-derived nutrients are primarily used for maternal energy needs during the gonotrophic cycle, with ~25% being utilized for egg protein and egg lipid synthesis (Zhou et al., 2004).
The two major classes of secreted proteases in the midgut of blood fed Ae. aegypti mosquitoes are endoproteases, represented by trypsin-like (Felix 1991, Barillas-Mury et al., 1993, Kalhok et al., 1993) and chymotrypsin-like (Jiang et al., 1997, Bian et al., 2008) serine proteases, and exopeptidases that function as aminopeptidases (Noriega et al., 2002, Billingsley et al., 1992) and carboxypeptidases (Edwards et al., 2000, Isoe et al., 2009). Studies in the 1990s showed that blood-feeding induces a biphasic increase in midgut trypsin-like activity in Ae. aegypti based on in vitro enzyme assays using the trypsin substrate analog BApNA (Felix 1991, Noriega et al., 1996a). The early phase of digestion consists of a modest, but reproducible, increase in trypsin activity from 0–6 h post blood meal (PBM), followed by the late phase of digestion that is characterized by a large increase in trypsin activity, beginning 12–18 h PBM. A serine protease, named early trypsin, was cloned and characterized by two groups and proposed to be responsible for trypsin-like activity in the early phase (Kalhok et al., 1993, Noriega et al., 1996b). Two other genes were also cloned at about the same time, one named late trypsin (Barillas-Mury et al., 1991), and the other referred to as 5G1 (Kalhok et al., 1993). Based on the expression pattern of the late trypsin and 5G1 genes in blood fed mosquitoes, it was proposed that one or both could be contributing to the late phase trypsin activity identified in the BApNA assays. Subsequent expression studies of the early and late trypsin genes showed that early trypsin is regulated at the level of protein synthesis (Noriega et al., 1996a, Brandon et al., 2008), whereas, the late trypsin gene is regulated at the transcriptional level (Barillas-Mury et al., 1993). Moreover, based on studies using a commercial soy bean extract that contained trypsin inhibiting activity, it was proposed that early trypsin enzyme activity is required for late trypsin gene transcription in the midgut of Ae. aegypti (Barillas-Mury et al., 1995, Noriega et al., 1999).
Successful application of RNAi technology to knockdown gene expression in adult female mosquitoes (Blandin et al., 2002), and completion of the Ae. aegypti genome sequence (Nene et al., 2007), led to two discoveries suggesting that the functional role of early trypsin and late trypsin in Ae. aegypti blood meal metabolism is more complex than previously thought. First, Lu et al. (2006) reported that soy bean extracts used in the original early and late trypsin expression studies, were likely contaminated with a toxic compound that interferes with mosquito metabolism and is unrelated to the trypsin inhibiting activity of the plant extract. Based on the finding that more specific trypsin inhibitors of early phase digestion did not block late trypsin gene expression, and on similar results from RNAi experiments that knocked down the level of early trypsin transcripts, they proposed that early trypsin protein expression is not required for transcription of the late trypsin gene. Second, Brackney et al. (Brackney et al., 2008) showed that knocking down late trypsin transcript levels in Ae. aegypti midguts with dsRNA had no effect on the level of trypsin-like enzyme activity during the late phase of digestion using the in vitro BApNA cleaving assay. Moreover, when they knocked down expression of 5G1, the other late phase trypsin-like gene that had been identified (Kalhok et al., 1993), they observed a significant reduction in the level of BApNA activity in midgut extracts prepared at 24 h PBM. These surprising results suggested that 5G1, not late trypsin, is most likely the major late phase trypsin in Ae. aegypti.
Since the discovery and analysis of midgut serine proteases in Ae. aegypti predated completion of the genome sequence, we thought it was possible that previously uncharacterized serine proteases may also be involved in blood protein digestion. To this end, we recently completed an extensive bioinformatic and quantitative gene expression analysis of midgut serine protease genes in blood fed Ae. aegypti and found two additional highly-induced midgut serine protease transcripts (Brackney et al., submitted). One gene is called AaSPI, which has sequence similarity to late trypsin and the serine collagenase family of endoproteases, and the other, AaSPVII, is homologous to 5G1 and appears to be a trypsin-like serine protease. In addition, we identified a 389 kb region in the Ae. aegypti genome that encodes the 5G1 gene, along with another five highly conserved 5G1 gene family members that likely arose by gene duplication and share >90% amino acid sequence identity in their coding sequences.
In these studies, we have used an optimized RNAi knockdown method to analyze the endoprotease function of early trypsin (AaET), and three late phase serine proteases referred to as AaLT (late trypsin), AaSPVI (5G1 gene family), and AaSPVII. Based on in vitro and in vivo enzyme assays, and fecundity studies, we conclude that all three late phase serine proteases play a significant role in blood meal metabolism.
2. MATERIALS AND METHODS
2.1. Rearing mosquitoes
Ae. aegypti mosquitoes from the Rockefeller strain were used in all experiments. Rearing conditions were at 25°C, 80% relative humidity, and a 16 h light: 8 h dark. Mosquitoes were maintained on 10% sucrose solution ad libitum.
2.2. Double-strand RNA (dsRNA) synthesis
The gene-specific dsRNA was designed, synthesized, and injected as described in Supplemental materials and methods. Briefly, the T7 RNA polymerase promoter sequence (TAATACGACTCACTATAGGGAGA) was added to the 5’ end of each dsRNA primer (Supplemental Table 1). PCR was carried out using GoTaq Green Master Mix (Promega, Madison, WI) and template cDNA derived from oligo dT-primed mosquito RNA from whole mosquitoes or midgut tissues. The PCR products from this reaction were electrophoresed in 1.0% agarose gel, purified using GFX Gel Band Purification Kit (GE Healthcare, Piscataway, NJ), and cloned into the pGEM-T easy vector (Promega). The plasmid DNA inserts were sequenced using an ABI 377 automated sequencer (Applied Biosystems, Foster City, CA) at the Genomics Analysis Technology Core facility (The University of Arizona, Tucson, AZ). In order to synthesize dsRNA for injection experiments, fresh PCR product was first synthesized using the same T7-gene specific primers and the sequenced-verified plasmid DNA as a template. In vitro dsRNA synthesis was performed using the MEGAscript RNAi Kit (Ambion, Austin, TX) in an overnight reaction at 37°C following the manufacturer’s instructions. After purification, a portion of the final eluate was used to calculate the RNA yield and for agarose gel electrophoresis to validate dsRNA size and integrity. The remainder was ethanol precipitated using sodium acetate overnight at −20°C. The recovered dsRNA was resuspended in nuclease-free water at 6.0 µg/ µl and stored at −80°C. Cold-anesthetized two-day old female mosquitoes were injected into the right mesothoracic spiracle (see Supplemental figure 1) with 1.2 µg of dsRNA using a Nanoject II microinjector (Drummond Scientific Company, Broomall, PA).
2.3. Blood feeding
Four-day old adult females (48 h post-injection of dsRNA) were artificially fed on bovine blood purchased from Pel-Freez Arkansas LLC (Rogers, AR) that contained 5 mM ATP. Only fully engorged females examined with a dissecting microscope were used in the experimental and control groups.
2.4. Measuring knockdown efficiency of dsRNA
Knockdown efficiency of each injected dsRNA was verified by real-time quantitative PCR (QRT-PCR). Total RNA was isolated from individual dissected midguts using TRIzol Reagent (Invitrogen, Carlsbad, CA) as described in Supplemental materials and methods. Dissection and total RNA isolation was performed at 6 h post-blood meal (PBM) for AaET dsRNA-injected mosquitoes, and at 24 h PBM for AaSPVI, AaSPVII, and AaLT dsRNA-injected mosquitoes. RNA yields from individual dissected midguts were determined spectroscopically at OD260 nm. All RNA samples were treated with DNase I to remove any residual genomic DNA, and cDNA was synthesized from 250 ng of total RNA using reverse transcriptase and oligo-dT. QRT-PCR was performed using FastStart Universal SYBR Green Master Mix (Applied Biosystems) by Real-Time PCR (7300 Real-Time PCR System, Applied Biosystems). Ribosomal protein S7 (RPS7) transcript levels were used as an internal control for normalization of mRNA yields in all samples. Primers used for the QRT-PCR are listed in Supplemental Table 1. The knockdown efficiency of gene-specific dsRNA was determined using mosquitoes injected with Fluc (firefly luciferase) dsRNA as a control.
2.5. Preparation of protein extracts
Midgut or whole abdomen tissues of cold - anesthetized mosquitoes were dissected in 1X PBS under a dissecting microscope. Individual tissue samples were manually homogenized with a pellet pestle (Kimbell-Kontes, Vineway, NJ) in a microcentrifuge tube containing 50µl of a homogenization buffer that was compatible with the in vitro BApNA enzyme assay (50 mM Tris-HCl pH 8.0, and 10 mM CaCl2). The homogenates were microcentrifuged at 4°C for 5 min. and the supernatant transferred to a new tube. Protein extracts used for BApNA enzymatic assays were flash frozen in liquid nitrogen and stored at −80°C until assayed, whereas, the protein extracts used for Western blot analysis were first heat-denatured and then stored at −80°C until use.
2.6. Western blotting
Rabbit polyclonal antibodies were prepared by GenScript Corporation (Piscataway, NJ) using antigenic peptides corresponding to AaET (TIRAGSTDRTNGGI), AaSPVII (NPSESRDVLR), and AaLT (GAVDFEDTTNDGRV). The AaSPVI rabbit polyclonal antibody was generated by Proteintech Group Inc (Chicago, IL) based on an antigenic peptide that is identical in all six AaSPVI gene family members (APVKLPQKDAPVNEGT). The anti-bovine serum albumin (BSA) polyclonal antibody was obtained from Gallus Immunotech (Cary, NC), and the anti-GAPDH antibody was obtained from Abcam (Cambridge, MA). Western blot analysis was performed by SDS–PAGE using a 10% acrylamide separation gel and a 3% stacking gel. The resolved proteins were electrophoretically transferred to a nitrocellulose membrane (Odyssey Nitrocellulose, LI-COR Biosciences, Lincoln, Nebraska). The membranes were blocked with 4% nonfat dry milk, and then incubated with each primary antibody in 4% skim milk in PBS saline (pH 7.5) containing 0.1% Tween 20. The dilutions of the primary antibodies were as follows; AaET (1:200), AaSPVI (1:1,000), AaSPVII (1:1,000), AaLT (1:200), BSA (1:1,000), and GAPDH (1:2,000). The secondary antibodies were either IRDye 800CW goat anti-rabbit secondary antibody (1:10,000, LI-COR), or IRDye 680 donkey anti-chicken secondary antibody (1:10,000, LI-COR) for one hour. The immunoreactive protein bands were visualized with an Odyssey infrared imaging system (LI-COR). The Western blot quantitative analysis for anti-BSA antibody was performed using NIH ImageJ software.
2.7. BApNA assay
Mosquito midguts were dissected in phosphate buffer (PBS, 10 mM Sodium Phosphate Buffer pH 7.2) and placed in microcentrifuge tubes containing ice-cold reaction buffer (50 mM Tris-HCl pH 8.0, 10 mM CaCl2). Midguts were homogenized on ice with a pellet pestle, followed by centrifugation at 20,800 g at 4°C. The supernatant was flash frozen in liquid N2, and the midgut extracts were stored at −80°C until use. The synthetic colorimetric substrate, Nα-benzoyl-D,L-arginine-p-nitroanilide hydrochloride (BApNA) (MP Biomedicals, Solon, OH, #100090), was used to measure midgut extract trypsin-like activity based on the method of Erlanger et al. (Erlanger et al., 1961). Total activity of midgut extracts was monitored using the CARY WinUV Enzyme Kinetics Application on the CARY 50 UV-visible spectrophotometer (Varian Medical Systems, Palo Alto, CA). Reaction rates were determined at 25°C from linear portions of the A405nm versus time plots using the extinction coefficient ε405nm = 8800 M−1 cm−1 and assuming steady-state conditions. All reaction mixtures (1.0 ml) contained 50 mM TRIS-HCl pH 8.0, 10 mM CaCl2, 1 mM BApNA, and one midgut equivalent (25µl) of midgut extract. Due to the blood content of the mosquito midgut, the spectrophotometer was first blanked to each reaction sample without BApNA using a quartz cuvette (1 cm path length), followed immediately by the addition of BApNA to initiate the reaction. One unit of enzyme activity was defined as 1 micromolar of p - nitroaniline produced per minute.
2.8. Measuring fecundity and viability
To determine whether dsRNA directed against the targeted serine proteases had a significant effect on reproductive capacity, mated and blood fed dsRNA-injected mosquitoes were individually transferred 48 h PBM into a netted scintillation vial containing an oviposition paper in 5 ml distilled water. The gravid mosquitoes were allowed to oviposit for the next 24–48 hours, at which point the paper was retrieved and stored in the insectary under humid conditions for up to one week. Subsequently, a second gonotrophic cycle was initiated by blood feeding the same mosquitoes that had been kept separately in netted scintillation vials and maintained on water following the first blood meal. Only the fully engorged females were kept and allowed to lay eggs at 48 h PBM using the same protocol as before. The number of eggs laid per female on the oviposition paper were then counted using a dissecting microscope. To determine egg viability, the oviposition paper was cut into equal pieces and the number of eggs retained on a subsection of paper was determined, usually 25–35 eggs/ paper section. The paper was submerged in 2.0 ml of 0.2% dissolved mosquito larval food in 12 well cell culture plates, and the hatched larvae were recorded 24 hours later as a percentage of the total number of starting eggs in the well.
2.9. Statistical analysis
Data were statistically analyzed by either one-way ANOVA or unpaired Student's t test with mean values ± SEM using GraphPad Prism statistical software package, version 5.0b for Mac OS X (version 5.0b for Mac OS X, GraphPad Software Inc, San Diego, CA). Asterisks indicate significant differences (*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant).
3. RESULTS
3.1. Knockdown of serine protease expression in individual mosquitoes using dsRNA
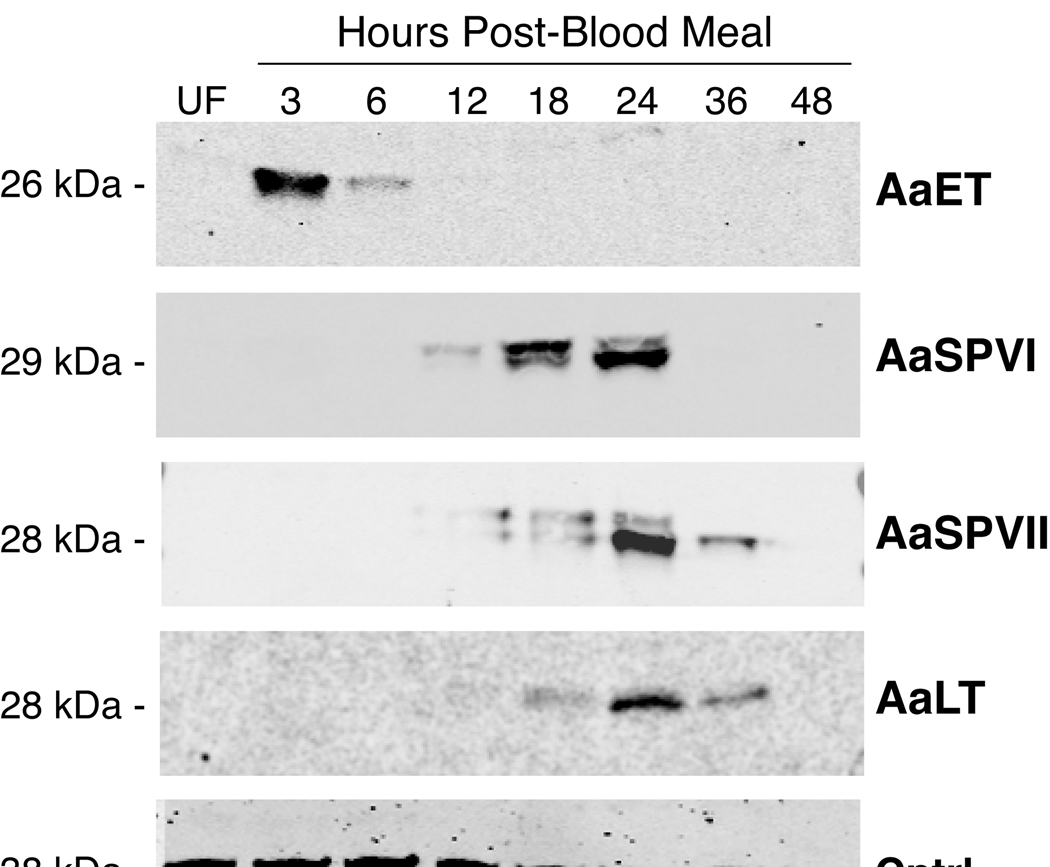
Antibodies generated against AaET, AaLT, AaSPVI, and AaSPVII peptides were used in Western blots to analyze expression of these four serine proteases in midgut extracts isolated 3–48 h PBM. The results in figure 1 show that AaET protein levels increase rapidly in the midgut after blood feeding, with maximal expression at 3 h PBM. AaET protein levels were undetectable by 12 h PBM, which is consistent with the role of this protease in the early phase of digestion (Felix 1991). In contrast, induced expression of the late phase serine protease proteins, AaSPVI, AaSPVII, and AaLT, was not observed until 12 h PBM, with peak expression at 24 h PBM. The doublet band seen in the AaSPVI and AaSPVII Western blots most likely corresponds to the zymogen and mature forms of the proteins based on predicted molecular weights. We confirmed that expression of AaET, AaSPVI, AaSPVII, and AaLT proteins is limited to the midgut of blood fed mosquitoes based on Western blots using protein extracts prepared from whole abdomen, midgut, and carcass (data not shown).
Figure 1.
Representative western blot of serine protease protein expression in the midgut during blood meal digestion. Each lane contains one midgut tissue equivalent of protein extract. Predicted molecular weights for each protein are indicated based on electrophoresed molecular weight standards (not shown) and the number of amino acid residues in the mature form of the serine protease. The serine protease antibody used in each Western blot is listed on the right side, the loading control is a protein detected by an anti-HspBP1 antibody (Raynes et al., 2000).
Since pooled tissue samples can be misleading with regard to individual RNAi knockdown efficiencies, we optimized dsRNA-mediated knockdown in individual mosquitoes as described in Supplemental materials and methods. As shown in figure 2A, results from three independent dsRNA experiments demonstrate that injection of AaSPVI dsRNA (1.2 µg) into the right mesothoracic spiracle causes a >98% knockdown of AaSPVI transcript levels. Moreover, this level of knockdown was achieved in 35 out of 35 injected mosquitoes from three different cohorts (Table 1). Similar high levels of reproducible knockdown were also observed in mosquitoes injected with dsRNA directed against the AaET, AaSPVII and AaLT gene transcripts (Table 1). It can be seen that 100% of AaET and AaSPVII dsRNA-injected mosquitoes exhibited ~98% knockdown, with 92% of AaLT dsRNA-injected mosquitoes (23 of 25) displaying an average of 96% knockdown.
Figure 2.
RNAi knockdown efficiency in the midgut of individual blood fed mosquitoes injected with AaSPVI dsRNA. A) AaSPVI transcript levels in Fluc and AaSPVI dsRNA-injected mosquitoes at 24 h PBM as determined by QRT-PCR. Results from three different experiments using separate cohorts of mosquitoes are shown. B) Representative Western blot of midgut extracts isolated from individual mosquitoes 24 h PBM that had been injected with Fluc (3 mosquitoes) or AaSPVI (six mosquitoes) dsRNA and analyzed with AaSPVI or GAPDH antibodies. The dark area between lanes 3 and 4 is portion of a background spot from the filter and not a protein band.
Table 1.
RNAi knockdown efficiencies and in vitro (BApNA cleavage) and in vivo (BSA degradation) serine protease activity in dsRNA injected mosquitoes.
| dsRNA | RNAia |
BApNA activity (µM/min, Mean±SEM) |
BSAc | |||
|---|---|---|---|---|---|---|
| injection | Nb | K.D. (%) | 18 h PBM | 24 h PBM | 36 h PBM | (%) |
| Fluc | 24 | - | 10.8 ± 1.3 | 11.8 ± 1.1 | 12.6 ± 2.4 | 3.7 |
| AaET | 15/15 | 97 | 10.3 ± 2.0 | 12.3 ± 1.9 | 8.2 ± 2.2 | 16.0*** |
| AaSPVI | 35/35 | 98 | 2.8 ± 0.4** | 3.2 ± 0.1*** | 2.9 ± 0.4** | 14.2*** |
| AaSPVII | 25/25 | 98 | 9.4 ± 1.8 | 11.1 ± 3.2 | 13.1 ± 1.9 | 9.1* |
| AaLT | 23/25 | 96 | 13.2 ± 0.9 | 17.8 ± 2.7 | 17.1 ± 3.8 | 10.2* |
Knockdown (K.D.) efficiency of each serine protease was determined in comparison with dsRNA-Fluc injected mosquitoes
The left number indicates mosquitoes with > 90% knockdown, and the right number shows the total number of injected mosquitoes examined.
Intact BSA (70 kDa) in the midgut was measured at 24 h PBM, and the percentage remaining was calculated using 41 µg of BSA as the initial amount ingested based on multiple experiments.
Significance levels are indicated as at P < 0.05,
P < 0.01,
P < 0.001 using unpaired student t-test.
To determine if the decreased levels of protease gene transcripts observed by QRT-PCR analysis corresponded to decreased protein expression, we performed Western blot analysis using midgut protein extracts prepared from individual mosquitoes. As shown in figure 2B for AaSPVI protein expression, all six AaSPVI dsRNA-injected mosquitoes lacked detectable AaSPVI protein at 24 h PBM as compared to the Fluc controls. Similar results were found using Western blots to analyze individual protein extracts from the AaET, AaSPVII and AaLT dsRNA-injected mosquitoes (data not shown).
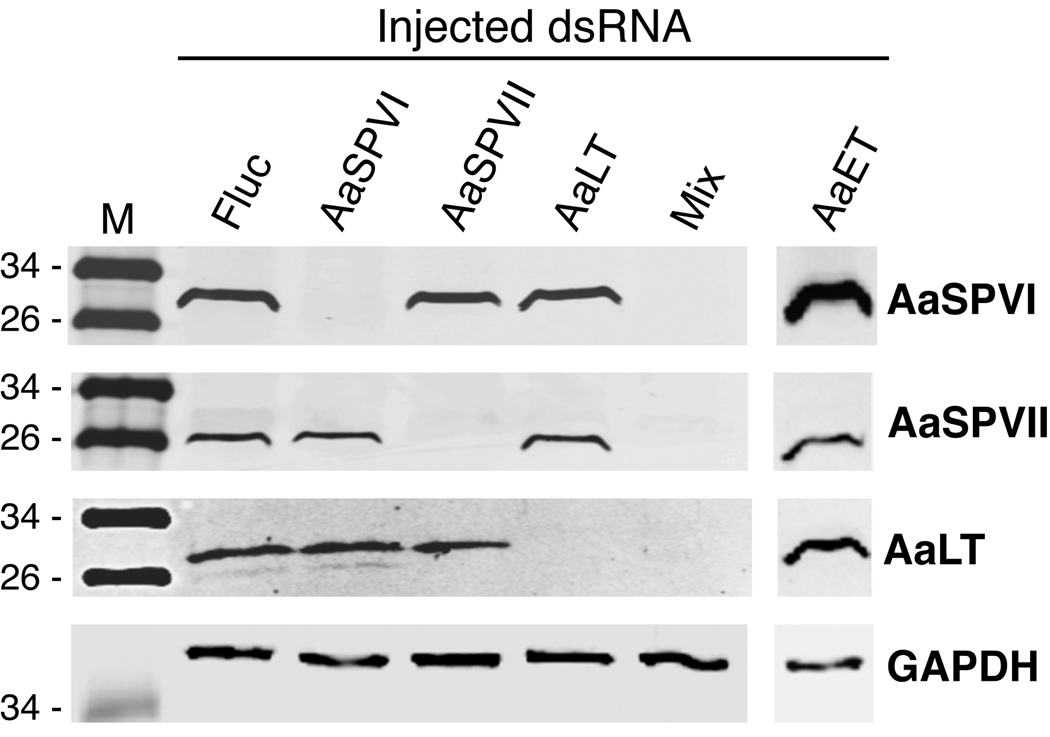
To confirm that the dsRNA targeted regions we chose were gene-specific, we analyzed protein extracts from the different dsRNA-injected mosquitoes at 24 h PBM using the four protease antibodies. As shown in figure 3, Western blot analysis revealed that each protease dsRNA target sequence led to decreased expression of only the cognate protease. For example, AaSPVI dsRNA-injected mosquitoes showed the same level of AaET, AaSPVII, and AaLT protein expression as the Fluc controls. Similarly, mosquitoes injected with AaET, AaSPVII, and AaLT dsRNA, only showed reduced expression of the corresponding protein (figure 3). In particular, knocking down AaET protein expression had no effect on the expression of AaSPVI, AaSPVII, or AaLT proteins, demonstrating that AaET does not control expression of these late phase proteases. Lastly, the data in figure 3 show that injecting mosquitoes with an equal mixture of AaSPVI, AaSPVII, and AaLT dsRNA sequences (400 ng of each), is sufficient to knockdown expression of all three proteins.
Figure 3.
Representative Western blot showing the target specificity of AaSPVI, AaSPVII, AaLT, and AaET dsRNA species. Midgut extracts were prepared from a pool of five mosquitoes that were harvested 24 h PBM and analyzed with the antibodies shown on the right side. Each lane contains ~0.5 midgut tissue equivalent of protein extract. Mix refers to an injected sample containing 400 ng each of AaSPVI, AaSPVII, and AaLT dsRNA.
3.2. AaSPVI is the major trypsin-like serine protease expressed during the late phase of digestion
In order to understand how the function of AaET, AaSPVI, AaSPVII, and AaLT serine proteases contribute to blood meal digestion in the mosquito midgut, we measured the amount of trypsin-like activity in midgut extracts from uninjected and dsRNA-injected mosquitoes using the substrate analog BApNA and a quantitative enzyme kinetic assay. As shown in figure 4A for uninjected blood fed mosquitoes, there is an initial increase in total BApNA activity during the first 8 h PBM that corresponds to the early phase of digestion. Following a 10 hour period in which there is no significant increase in BApNA activity (8–18 h PBM), enzyme activity levels increase 2–4 fold in the late phase of digestion until reaching a peak at 30 h PBM.
Figure 4.
Total BApNA activity in individual mosquito midguts as determined by quantitative enzyme kinetic analysis using steady state assumptions. Results are from a minimum of three experiments using 3 mosquitoes for each time point. The mean ± SEM are shown for A) uninjected mosquitoes, B) Fluc (solid line) and AaSPVI (dotted line) dsRNA-injected mosquitoes, and C) Fluc (solid line) and AaET (dotted line) dsRNA-injected mosquitoes. Asterisks indicate significant differences between the serine protease dsRNA and Fluc dsRNA-injected mosquitoes at the same time points (** P < 0.01; *** P < 0.001).
Based on results from uninjected mosquitoes, we next used the BApNA assay to quantitate trypsin-like activity in midgut extracts from dsRNA-injected mosquitoes. As seen in figure 4B, AaSPVI dsRNA-injected mosquitoes showed no significant difference in total BApNA activity at 12 h or 48 h PBM compared to Fluc dsRNA-injected mosquitoes, however, the level of BApNA activity at 18, 24, 30, 36, and 42 h PBM was dramatically reduced. Area under the curve analysis of the data in figure 4B, revealed that AaSPVI accounts for 77.6% of the trypsin-like activity during the entire digestion period. When we used the same molecular genetic and biochemical analysis to quantitate the contribution of AaET to total trypsin-like activity in the midgut, we found a 72% reduction in BApNA activity at the 6 h PBM time point, which contributed to a 49.7% decrease in BApNA activity from 0–9 h PBM based on area under the curve analysis (figure 4C). These AaET results are consistent with the Western blot in figure 3 showing no effect of AaET dsRNA injections on late phase serine protease expression, as well as, recent reports indicating that AaET protease activity is not required for AaSPVI gene expression in the late phase (Brackney et al., 2008).
In a separate experiment, we measured the levels of BApNA activity at 18, 24, and 36 h PBM in midgut extracts from AaSPVI, AaLT, and AaSPVII dsRNA-injected mosquitoes. As shown in table 1, the only significant difference in total BApNA activity between Fluc dsRNA-injected mosquitoes, and mosquitoes injected with protease-specific dsRNA, was in the AaSPVI dsRNA-injected mosquitoes at 24 h PBM (P<0.001), and at 18 and 36 h PBM (P<0.01). These data confirm that AaLT does not exhibit trypsin-like activity, and moreover, that AaSPVII is either not a trypsin-like serine protease, or does not make a significant contribution to late phase blood meal digestion.
3.3. Quantifying BSA degradation as a measure of in vivo serine protease activity
The in vitro BApNA activity assay is useful for measuring trypsin-like activity in mosquito midgut extracts, but it cannot be used to quantitate in vivo enzyme activity, nor can it detect the activity of serine proteases that lack trypsin-like substrate specificities. Therefore, we developed a quantitative in vivo protease digestion assay that measures the degradation of endogenous bovine serum albumin (BSA) protein in the blood meal. Although hemoglobin is the major protein in the blood meal, we chose to develop a quantitative in vivo BSA digestion assay because BSA digestion by trypsin has been well-characterized (Peters et al., 1975), and economical high affinity chicken anti-BSA antibodies are commercially available. This in vivo BSA digestion assay uses quantitative Western blotting to detect intact 70 kDa BSA protein present in the midgut lumen at various times PBM. The amount of intact BSA in the protein extracts is determined using a chemically-labeled secondary antibody that can be detected by infrared scanning of the Western blot filter. To quantitate these data, the image intensity values are calibrated using known amounts of purified BSA protein.
As shown in figure 5A, the anti-BSA antibody detects intact BSA in blood fed, but not unfed, mosquitoes, and the amount of intact BSA protein decreases over time after feeding. The early phase of digestion (0–6 h PBM) produces a major BSA degradation product that corresponds to trypsin cleavage at a previously mapped site (Peters et al., 1975). This initial degradation is followed by the appearance at 12 h PBM of additional BSA cleavage products and a steady decrease in the amount of intact BSA out to 36 h PBM. Based on a BSA standard curve, we determined that ~40 µg of BSA is present in the midgut right after feeding, which is close to the predicted amount of BSA (~50 µg) in a 2 µl blood meal. Figure 5B shows the amount of intact BSA present in each of the extracts analyzed by Western blotting in figure 5A. One indication that this in vivo BSA degradation assay is an accurate measure of early and late phase digestion in the mosquito midgut, is the observed rapid degradation of intact BSA between 0–6 h PBM which corresponds to the early phase. After a lag period between 6 and 12 h PBM, the late phase of digestion begins at 18 h PBM and continues until 30 h PBM when <5% of the ingested BSA remains intact.
Figure 5.
Representative Western blot of in vivo BSA degradation in uninjected mosquitoes as a function of time after blood feeding. A) Western blot analysis of endogenous levels of intact BSA (70 kD) in mosquito midguts isolated from 0.5 to 48 h PBM. Each lane contains ~0.1 midgut tissue equivalent of protein extract. B) Quantitative analysis of the Western blot in (A) using infrared scanning and image density analysis as described in Materials and Methods. The amount of BSA per midgut was determined using a BSA standard curve generated with known amounts of purified BSA that were analyzed by the same Western blot and image analysis method.
We next used the in vivo BSA degradation assay to quantitate the amount of intact BSA in the midguts of dsRNA-injected mosquitoes at 24 h PBM. Figure 6A shows a representative BSA Western blot using midgut extracts from mosquitoes that were injected with Fluc dsRNA, or with dsRNA directed against the four serine proteases, singly or in combination (mix of AaSPVI, AaSPVII and AaLT dsRNA). The results show that each of the serine protease dsRNA-injected mosquitoes displayed less BSA degradation at 24 h PBM than did the Fluc dsRNA-injected mosquito. The AaET dsRNA-injected mosquito had the lowest amount of cleavage at the preferred trypsin site compared to the other dsRNA-injected mosquitoes, suggesting that this initial cleavage event may be mediated by AaET.
Figure 6.
Quantitative analysis of in vivo BSA degradation in dsRNA-injected mosquitoes at 24 h PBM. A) Western blot analysis of endogenous levels of intact BSA (70 kD) in individual mosquito midguts injected with the dsRNA shown. Mix refers to an injected sample containing 400 ng each of AaSPVI, AaSPVII, and AaLT dsRNA. Each lane contains ~0.1 midgut tissue equivalent of protein extract. Purified BSA (20 µg) was loaded as a control. B) Quantitative analysis of in vivo BSA degradation in pooled midguts from dsRNA-injected mosquitoes at 24 h PBM. The amount of BSA per midgut was determined using a BSA standard curve generated with known amounts of purified BSA that were analyzed by the same Western blot and image analysis method. The mean ± SEM were calculated from a minimum of 3 samples.
To quantitate the effect of dsRNA knockdown on in vivo BSA degradation, we analyzed midgut extracts from pooled mosquitoes at 24 h PBM and plotted the data in figure 6B. We found that mosquitoes injected with Fluc dsRNA contained only 3.7% of the amount of intact BSA at 24 h PBM compared to the amount present immediately after feeding. In contrast, all four of the serine protease dsRNA-injected mosquitoes retained significantly more intact BSA at this same time point, with 9.1% to 16.0% of the intact BSA remaining (Table 1). Consistent with results from the in vitro BApNA assay, knocking down AaSPVI expression had the largest effect on inhibiting in vivo BSA degradation amongst the three late phase proteases. Importantly however, injection of AaSPVII and AaLT dsRNA also led to a significant increase in the amount of intact BSA at 24 h PBM, suggesting that the in vitro BApNA assay underestimates the contribution of these two serine proteases to blood meal digestion. The AaET dsRNA-injected mosquitoes retained the same high level of intact BSA as the AaSPVI dsRNA-injected mosquitoes, 16.0% and 14.2%, respectively, indicating that AaET-mediated in vivo degradation of BSA during the early digestion phase may be important for promoting BSA digestion during the late phase.
3.4. Loss of late phase serine protease function significantly decreases fecundity
If inhibiting midgut serine protease activity in blood fed mosquitoes were to be a viable strategy for mosquito control, then loss of protease expression should result in a significant decrease in mosquito viability or fecundity. Since we observed no significant effect on adult mortality in serine protease dsRNA-injected mosquitoes versus Fluc dsRNA-injected mosquitoes (data not shown), we tested the possibility that late phase serine protease activity was required for maximal fecundity. For these experiments, uninjected and dsRNA-injected mosquitoes were allowed to mate, and then 48 h PBM, the ovaries were examined for evidence of oocyte development. As shown in figure 7, the ovaries of AaSPVI dsRNA-injected mosquitoes were smaller and contained fewer oocytes than ovaries from either uninjected or Fluc dsRNA-injected mosquitoes. The same was true of mosquitoes that were injected with a mix of AaSPVI, AaSPVII, and AaLT dsRNA. A reduced number of oocytes was also apparent in the ovaries of AaSPVII and AaLT dsRNA-injected mosquitoes, however, the effect was less pronounced. A similar inhibition of oocyte maturation was observed in uninjected mosquitoes that had been fed a diluted blood meal that contained 60% or less the amount of protein in a normal blood meal (figure 7B). These feeding data suggest that the reduced number of oocytes in serine protease dsRNA-injected mosquitoes may be due to decreased availability of protein-derived nutrients that must be obtained from the blood meal prior to the onset of oviposition.
Figure 7.
Representative photographs showing the effect of dsRNA injections or diluted blood meals on oocyte maturation. A) Representative ovaries from uninjected (None) or dsRNA-injected mosquitoes that were dissected 48 h PBM. Mix refers to an injected sample containing 400 ng each of AaSPVI, AaSPVII, and AaLT dsRNA. B) Representative ovaries from uninjected mosquitoes that were fed blood diluted with feeding buffer (100 mM NaHCO3, 150 mM NaCl, pH 7.0) to 20%, 40%, 60%, or 80%. Mosquitoes were dissected 68 h PBM to ensure that oocyte maturation was complete. Only fully engorged females that had been examined by microscopy right after feeding were used in the experiment.
To determine if serine protease dsRNA injection had an effect on egg production, mated and blood fed mosquitoes were transferred at 48 h PBM to individual netted scintillation vials containing water and egg laying paper. Although direct validation of knockdown efficiency could not be done on individual mosquitoes used for fecundity experiments, our previous results showed that dsRNA injection resulted in a >95% decrease in RNA and protein levels in nearly 100% of the mosquitoes dissected (see Table 1). As shown in figure 8A and Table 2, Fluc dsRNA-injected mosquitoes laid an average of 120.0 ± 2.2 eggs during the first gonotrophic cycle, whereas, the mixed dsRNA-injected mosquitoes (AaSPVI, AaSPVII, AaLT), laid only 82.6 ± 2.3 eggs/ mosquito, representing a reduction in fecundity of 31.2%. Indeed, significant reductions in fecundity (P<0.001), were also observed in mosquitoes injected with AaSPVII (26.0%), AaLT (25.5%), or AaSPVI (17.2%) dsRNA, indicating that late phase serine protease activity is required for maximal fecundity. As shown in Table 2, we found no significant difference in larval viability between the serine protease dsRNA-injected mosquitoes and the Fluc dsRNA-injected mosquitoes, indicating that even though fewer eggs were laid, embryogenesis was normal.
Figure 8.
Effect of serine protease dsRNA injection on fecundity in individual mosquitoes during the first and second gonotrophic cycles. A) Number of eggs oviposited during the first gonotrophic cycle in dsRNA-injected mosquitoes at 96 h PBM. Mix refers to an injected sample containing 400 ng each of AaSPVI, AaSPVII, and AaLT dsRNA. Mosquitoes were kept in separate vials containing egg oviposition paper and water. B) Number of eggs oviposited during the second gonotrophic cycle in dsRNA-injected mosquitoes using the same method as in the first gonotrophic cycle. The second blood meal feeding was 4 days after the first blood meal feeding, without sugar feeding in between. Each dot represents the number of eggs oviposited by an individual mosquito. The mean ± SEM are shown as horizontal lines.
Table 2.
Summary of fecundity data from individual dsRNA injected mosquitoes. Mosquitoes were maintained on water only during the period of time separating the two blood feedings.
| First gonotrophic cycle | Second gonotrophic cycle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| dsRNA | N | Eggs laid Mean ± SEM |
Reduction (%) |
N | Viability (%) |
N | Eggs laid Mean ± SEM |
Reduction (%) |
N | Viability (%) |
| Fluc | 51 | 120.0 ± 2.2 | - | 10 | 94.0 | 19 | 99.0 ± 2.6 | - | 6 | 97.2 |
| AaSPVI | 48 | 99.3 ± 3.2*** | 17.2 | 9 | 92.2 | 21 | 72.3 ± 5.7*** | 27.0 | 6 | 98.3 |
| AaSPVII | 44 | 88.8 ± 3.5*** | 26.0 | 9 | 86.7 | 20 | 72.1 ± 3.8*** | 27.2 | 6 | 96.7 |
| AaLT | 41 | 89.4 ± 3.3*** | 25.5 | 10 | 91.3 | 17 | 63.4 ± 5.8*** | 36.0 | 6 | 88.9 |
| Mix | 38 | 82.6 ± 2.3*** | 31.2 | 9 | 91.9 | 27 | 72.4 ± 4.1*** | 26.9 | 6 | 95.6 |
N: Number of mosquitoes tested.
Indicates egg numbers significantly different from dsRNA-Fluc injected mosquitoes (P < 0.001 in all cases). Mix refers to injection with a mixture of AaSPVI, AaSPVII, and AaLT dsRNA using 400 ng of each.
Zhou et al. (2004) used metabolic labeling studies to show that Ae. aegypti females store some of the nutrients obtained from the first blood meal for use in the second gonotrophic cycle. Therefore, it was possible that nutrient deficiencies resulting from reduced serine protease expression during the first gonotrophic cycle could affect fecundity in the second gonotrophic cycle. To test this idea, the same dsRNA-injected mosquitoes that were blood fed and analyzed for fecundity during the first gonotrophic cycle, were maintained for another 48 h in separate scintillation vials on water only (no sugar was provided), and then blood fed a second time. As shown in Table 2, even though the number of eggs laid by the Fluc dsRNA-injected mosquitoes were 18% lower in the second gonotrophic cycle (99.0 ± 2.6 eggs/ mosquito), the mosquitoes injected singly, or in combination, with serine protease dsRNA, all showed a significant decrease in egg production (P<0.001). The values ranged from a decrease in fecundity of 26.9% in the mosquitoes injected with the mix dsRNA (72.4 ± 4.1 eggs/ mosquito), to 36.0% in the AaLT dsRNA-injected mosquitoes (63.4 ± 5.8 eggs/ mosquito). Again, there was no significant difference between the dsRNA-injected mosquitoes with regard to larval viability (Table 2).
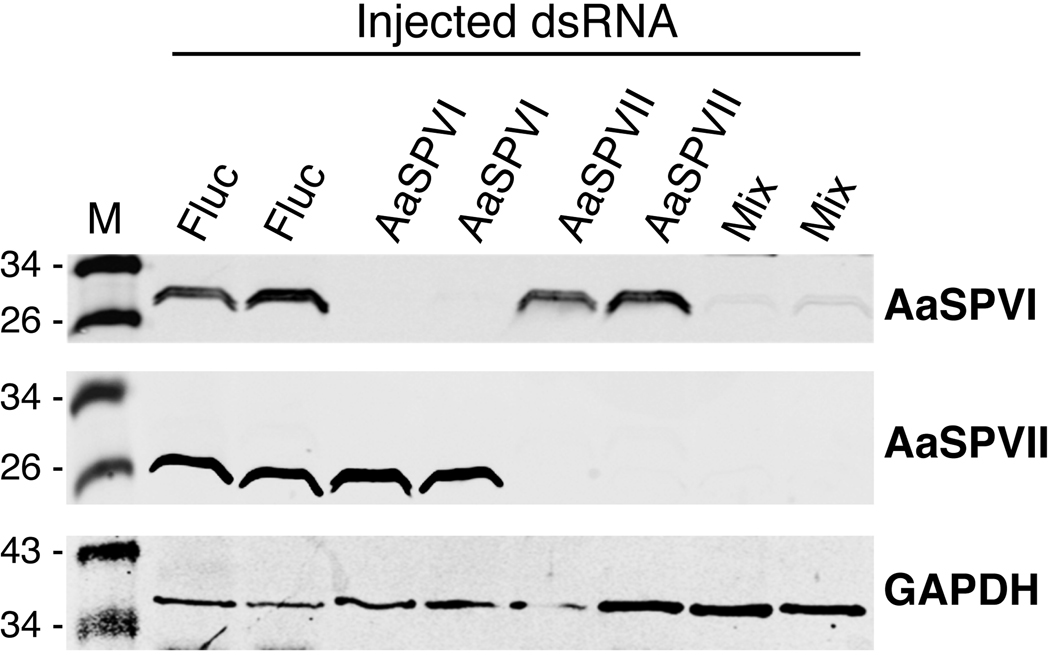
To determine if this reduced fecundity in the second gonotrophic was due to decreased levels of serine protease expression, the midguts of representative Fluc, AaSPVI, AaSPVII, and Mix dsRNA-injected mosquitoes were dissected 24 h PBM and analyzed by Western blotting. Surprisingly, the level of AaSPVI and AaSPVII protein expression was still knocked down in the mosquitoes we examined (figure 9), despite the fact that 8 days had passed from the time the dsRNA was injected on day 2 post-eclosion. Although we did not analyze levels of AaLT protein expression by Western blotting using protein extracts for from AaLT dsRNA-injected mosquitoes isolated after the second blood feeding, it is likely that expression of AaLT proteins was also very low based on the significant reduction in fecundity that was observed (Table 2).
Figure 9.
Representative Western blot showing that AaSPVI and AaSPVII protein levels were knocked down in midguts of individual mosquitoes for as long as 8 days post - injection and for a period of time that included two gonotrophic cycles. Mix refers to an injected sample containing 400 ng each of AaSPVI, AaSPVII, and AaLT dsRNA.
4. DISCUSSION
Following the initial isolation and characterization of AaLT nearly 20 years ago (Barillas-Mury et al., 1991), the AaLT gene was thought to encode the only major serine protease expressed in the late phase of mosquito midgut digestion (Noriega et al., 1999). However, two other midgut serine protease gene sequences, early trypsin and 5G1, were also cloned, and both appeared to be trypsin-like serine proteases (Kalhok et al., 1993) (Barillas-Mury et al., 1995). More recently, bioinformatic analysis led to the identification of two other highly-induced midgut serine protease genes in blood fed Ae. aegypti, AaSPI, which shares sequence homology with AaLT, and AaSPVII, which looks most like a trypsin-like serine protease (Brackney et al., submitted). Since our initial knockdown experiments using AaSPI dsRNA did not result in any obvious defects in blood protein digestion (JI and RM, unpublished data), we report here the results of functional studies focused on the other four highly expressed midgut serine protease genes, AaET, AaLT, AaSPVI, and AaSPVII.
One of the most surprising findings was the observation that dsRNA-mediated knockdown of a single late phase serine protease resulted in significant reductions in egg production (Table 2). This was true for both AaLT and AaSPVII, as well as, the AaSPVI gene family, and was consistent with their individual contributions to in vivo BSA degradation (Table 1). One explanation for this observed effect on fecundity is that each of these serine proteases may degrade blood meal proteins by cleaving at preferred sites, such that decreased expression of any one of the proteases, retards blood meal digestion and deprives the mosquito of necessary protein-derived nutrients. Therefore, even though carboxypeptidases and aminopeptidases are able to degrade polypeptides from the terminal ends as exopeptidases, the efficiency of exopeptidase-mediated degradation may require the involvement of all three late phase endopeptidases to generate a sufficient number of termini in a fixed amount of time. This would be analogous to a mosquito blood meal that was completely digested, but smaller in volume than a normal meal, which would lead to fewer protein-derived nutrients.
To test this idea, we mimicked the effect of a smaller blood meal by diluting whole blood with feeding buffer. As seen in figure 7, oocyte maturation in dsRNA-injected mosquitoes was qualitatively similar to uninjected mosquitoes that were fed a diluted blood meal. Specifically, under both conditions, fewer oocytes were present in these ovaries compared to normal ovaries, but of those oocytes that were present, they appeared to be mature and of normal size. This observation is supported by the finding that the ~30% reduction in oocyte numbers in the dsRNA-injected mosquitoes (figure 7A), was similar to the ~30% reduction in the number of oviposited eggs, the majority of which produced viable larvae (Table 2). The alternative outcome would have been that nutrient-deprived ovaries contained the same number of oocytes as normal ovaries, however, most of the oocytes would have been immature and smaller in size. Since this was not the case, the simplest interpretation of our results is that loss of serine protease function deprives the mosquito of necessary protein-derived nutrients, and the female mosquito compensates for this by producing fewer viable eggs.
Substrate specificity differences between AaLT, AaSPVI, and AaSPVII, would not explain why injecting a mixture of all three dsRNA species, compared to injecting individual dsRNA species, was neither additive nor synergistic with regard to in vivo BSA degradation (figure 6) or egg production (Table 2). Perhaps substrate cleavage is selective but not specific, such that primary sites are cleaved first, with secondary sites being cleaved more often as serine protease levels increase during the late phase. For example, then an initial decrease in substrate-specific cleavage events due to loss of a particular serine protease, could eventually be compensated for by increased cleavage at secondary sites by other serine proteases. Biochemical studies are currently under way to test this idea using purified recombinant AaLT, AaSPVI, and AaSPVII proteins to determine the catalytic efficiency and substrate specificity of each enzyme.
We used RNAi knockdown methods to functionally characterize several candidate proteins that have the potential to be enzyme inhibitor targets in a metabolism-based vector control strategy. While knocking down expression was not found to be lethal in either unfed or fed mosquitoes, we did observe a significant decrease in fecundity that could provide a population control mechanism. One way to visualize how this might work is to compare the number of eggs produced by individual mosquitoes in the first and second gonotrophic cycles. Using the same data plotted in figure 9 for Fluc and mix (AaSPVI, AaSPVII, AaLT) dsRNA-injected mosquitoes, figure 10 shows a two-way plot of egg numbers for each individual mosquito analyzed in this fecundity experiment. It can be seen that mosquitoes injected with the mix dsRNA produced on average fewer eggs in both gonotrophic cycles as did the mosquitoes injected with Fluc dsRNA. At the two extremes, there was a 63% reduction in total egg production over two consecutive gonotrophic cycles when comparing the two highest egg producers amongst the Fluc dsRNA-injected mosquitoes (254 total eggs/ mosquito), to the two lowest egg producers within the group of mix dsRNA-injected mosquitoes (94 total eggs/ mosquito). It is possible therefore, that chronic exposure to a small molecule serine protease inhibitor could lead to a substantial decrease in fecundity over the lifetime of a female mosquito as a result of nutrient deprivation. This inhibitory effect on blood meal metabolism could potentially be enhanced by blocking the activity of midgut exopeptidases in addition to serine proteases, a strategy we are currently testing.
Figure 10.
Two-way plot of fecundity data from figure 9 for mosquitoes that were injected with Fluc or mix (AaSPVI, AaSPVII, AaLT) dsRNA as function of the number of eggs oviposited in the first and second gonotrophic cycles. Each icon represents a single mosquito. Boxes highlight the two highest and two lowest egg producing mosquitoes in the experiment. The mean number of total eggs produced over consecutive gonotrophic cycles for the two mosquitoes in each box is shown.
In summary, we have optimized dsRNA injection methods to improve knockdown efficiencies in the mosquito midgut as a means to facilitate a systematic molecular genetic analysis of serine protease function in blood meal metabolism. These studies show that AaET is indeed the major early phase trypsin-like serine protease in the midgut of blood fed Ae. aegypti mosquitoes, and that AaSPVI has a similar role during the late phase of digestion. Two other highly expressed late phase serine proteases, AaLT and AaSPVII, were found to be required for complete in vivo BSA digestion and maximal fecundity, although their exact function in the digestive process is unknown. Future studies are aimed at elucidating the enzymatic properties of AaLT, AaSPVI, and AaSPVII and determining if small molecule inhibitors of these enzymes would be useful as mosquito-selective control agents.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mary Hernandez for rearing mosquitoes, Dr. Vincent Guerriero for the HspBP1 antibody, and Brianna Kolody for assistance during the initial phases of this project. This work was supported by NIH Grant R01AI031951 to RLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barillas-Mury C, Graf R, Hagedorn HH, Wells MA. cDNA and deduced amino acid sequence of the blood meal-induced trypsin from the mosquito, Aedes aegypti. Insect Biochemistry. 1991;21:825–831. [Google Scholar]
- Barillas-Mury C, Wells MA. Cloning and sequencing of the blood meal-induced late trypsin gene from the mosquito Aedes aegypti and characterization of the upstream regulatory region. Insect Mol Biol. 1993;2:7–12. doi: 10.1111/j.1365-2583.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Barillas-Mury CV, Noriega FG, Wells MA. Early trypsin activity is part of the signal transduction system that activates transcription of the late trypsin gene in the midgut of the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995;25:241–246. doi: 10.1016/0965-1748(94)00061-l. [DOI] [PubMed] [Google Scholar]
- Bian G, Raikhel AS, Zhu J. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem Mol Biol. 2008;38:190–200. doi: 10.1016/j.ibmb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley PF, Rudin W. The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J Parasitol. 1992;78:430–440. [PubMed] [Google Scholar]
- Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Foy BD, Olson KE. The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J Trop Med Hyg. 2008;79:267–274. [PMC free article] [PubMed] [Google Scholar]
- Brandon MC, Pennington JE, Isoe J, Zamora J, Schillinger AS, Miesfeld RL. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2008;38:916–922. doi: 10.1016/j.ibmb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddehe M. Mexico fights rise in dengue fever. Lancet. 2009;374:602. doi: 10.1016/s0140-6736(09)61509-9. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Moskalyk LA, Donelly-Doman M, Vlaskova M, Noriega FG, Walker VK, Jacobs-Lorena M. Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol Biol. 2000;9:33–38. doi: 10.1046/j.1365-2583.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- Erlanger BF, Kokowsky N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Felix CR, Betschart B, Billingsley PF, Freyvogel TA. Post -feeding induction of trypsin in the midgut of Aedes aegypti is separable into two cellular phases. Insect Bioch. 1991;21:197–203. [Google Scholar]
- Isoe J, Zamora J, Miesfeld RL. Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem Mol Biol. 2009;39:68–73. doi: 10.1016/j.ibmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Hall M, Noriega FG, Wells M. cDNA cloning and pattern of expression of an adult, female-specific chymotrypsin from Aedes aegypti midgut. Insect Biochem Mol Biol. 1997;27:283–289. doi: 10.1016/s0965-1748(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Kalhok SE, Tabak LM, Prosser DE, Brook W, Downe AE, White BN. Isolation, sequencing and characterization of two cDNA clones coding for trypsin-like enzymes from the midgut of Aedes aegypti. Insect Mol Biol. 1993;2:71–79. doi: 10.1111/j.1365-2583.1993.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Pennington JE, Stonehouse AR, Mobula MM, Wells MA. Reevaluation of the role of early trypsin activity in the transcriptional activation of the late trypsin gene in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2006;36:336–343. doi: 10.1016/j.ibmb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Marquardt WC. Biology of Disease Vectors. 2nd ed. Oxford: Elsevier; 2005. [Google Scholar]
- Nene V, Wortman JR, Lawson D, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, Pennington JE, Barillas-Mury C, Wang XY, Wells MA. Aedes aegypti midgut early trypsin is post-transcriptionally regulated by blood feeding. Insect Mol Biol. 1996a;5:25–29. doi: 10.1111/j.1365-2583.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wang XY, Pennington JE, Barillas-Mury CV, Wells MA. Early trypsin, a female-specific midgut protease in Aedes aegypti: isolation, amino terminal sequence determination, and cloning and sequencing of the gene. Insect Biochem Mol Biol. 1996b;26:119–126. doi: 10.1016/0965-1748(95)00068-2. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. J Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Edgar KA, Bechet R, Wells MA. Midgut exopeptidase activities in Aedes aegypti are induced by blood feeding. J Insect Physiol. 2002;48:205–212. doi: 10.1016/s0022-1910(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Peters T, Jr, Feldhoff RC. Fragments of bovine serum albumin produced by limited proteolysis. Isolation and characterization of tryptic fragments. Biochemistry. 1975;14:3384–3391. doi: 10.1021/bi00686a015. [DOI] [PubMed] [Google Scholar]
- Raynes DA, Guerriero V. Isolation and characterization of isoforms of HspBP1, inhibitors of Hsp70. Biochim Biophys Acta. 2000;1490:203–207. doi: 10.1016/s0167-4781(99)00238-9. [DOI] [PubMed] [Google Scholar]
- Scaraffia PY, Tan G, Isoe J, Wysocki VH, Wells MA, Miesfeld RL. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2008;105:518–523. doi: 10.1073/pnas.0708098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Flowers M, Friedrich K, Horton J, Pennington J, Wells MA. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J Insect Physiol. 2004;50:337–349. doi: 10.1016/j.jinsphys.2004.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.