Abstract
The striatum is thought to play a central role in learning how to choose acts that lead to reward and avoid punishment. Dopamine-dependent modification of striatal synapses in the action selection circuitry has long been thought to be a key step toward this type of learning. The development of new genetic and optical tools have pushed this field forward in the last couple of years, demanding a re-evaluation of models of how experience controls dopamine-dependent synaptic plasticity and how disease states like Parkinson’s disease affect the striatal circuitry.
Introduction
The largest of the basal ganglia nuclei, the dorsal striatum integrates information about sensory and motor state conveyed by cortical and thalamic neurons, facilitating the selection of actions that achieve desirable outcomes, like reward, and avoid undesirable ones. Current models of how this happens have been built upon the notion that reward prediction errors signaled by mesencephalic dopaminergic neurons innervating the striatum provide a means by which experience shapes the strength of corticostriatal synapses of principal medium spiny neurons and, in so doing, action selection [1–3]. One of the most compelling pieces of evidence for this view comes from inability of Parkinson’s disease patients, who have lost their striatal dopaminergic innervation, to translate thought into action [4].
Although there is strong support for the basic tenets of these models, precisely how dopamine modulates the strength of corticostriatal synapses has been the subject of continuing debate. One of the experimental obstacles that has slowed physiological study is the cellular heterogeneity of the striatum and the seemingly random anatomical distribution of cell types within it. The principal neurons of the striatum are medium spiny neurons (MSNs), constituting roughly 90% of all striatal neurons in most mammals [5]. MSNs can be divided into at least two groups based upon their dopamine receptor expression and axonal projection site: striatopallidal MSNs send their principal axonal arbor to the globus pallidus and express high levels of the D2 dopamine receptor whereas striatonigral MSNs send their principal axonal arbor to the substantia nigra and express high levels of the D1 dopamine receptor [6]. In physiological studies performed either in vitro or in vivo, these two types of MSNs have been virtually impossible to tell apart, clouding the interpretation of plasticity studies exploring the role of dopamine. The recent development of bacteria artificial chromosome (BAC) transgenic mice in which the expression of D1 or D2 receptors is reported by expression of red or green fluorescent protein [7] has eliminated this problem and led to a flurry of discoveries about striatal synaptic plasticity – providing the primary motivation for this review.
Long-term depression at glutamatergic synapses on MSNs
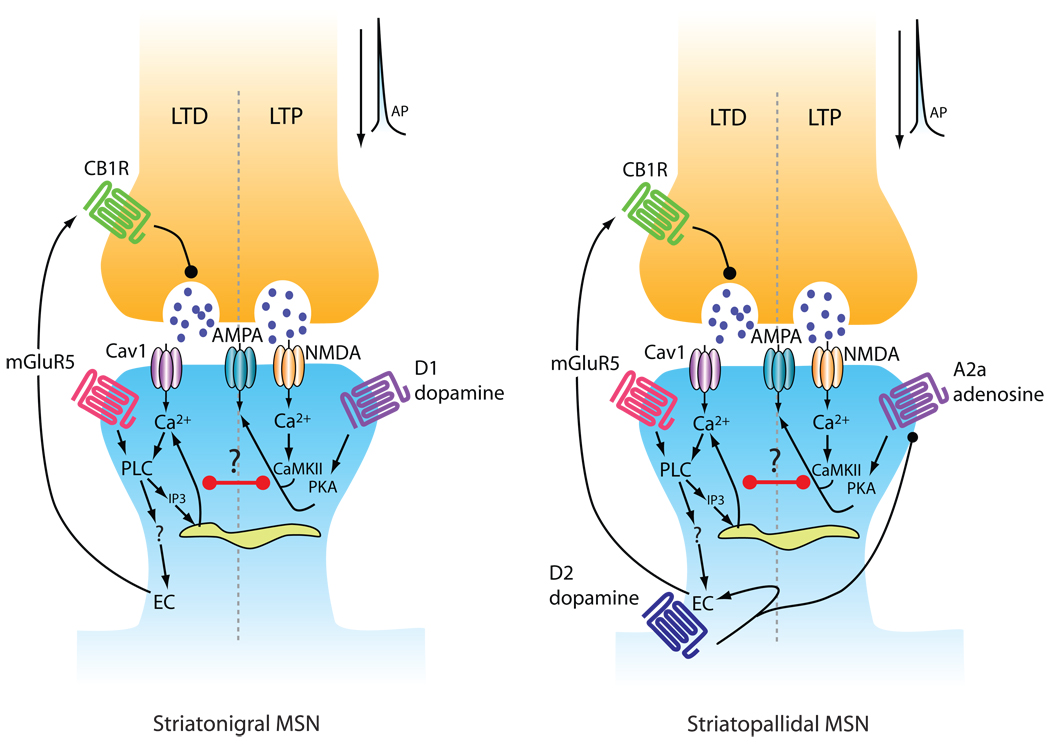
Long-term depression (LTD) at MSN glutamatergic synapses is the easiest form of synaptic plasticity to see in the dorsal striatum and, as a consequence, has been studied the most thoroughly. Unlike the situation at many other synapses, striatal LTD induction requires pairing of postsynaptic depolarization with moderate to high frequency (not low frequency) afferent stimulation at physiological temperatures [8]. Typically for the induction to be successful, postsynaptic L-type Ca2+ channels and Gq-linked mGluR5 receptors need to be co-activated [8,9]. Both L-type Ca2+ channels and mGluR5 receptors are appropriately positioned at glutamatergic synapses on MSN spines [10–13]. What is less clear is the nature of the interaction between these two membrane proteins in the process of induction. A clue has come from recent work showing that prolonging the opening of L-type channels with an allosteric modulator eliminates the need to stimulate mGluR5 receptors [14], pointing to shared regulation of dendritic Ca2+ concentration, elevation of which is required for LTD induction. However, there is an asymmetry, as increasing mGluR5 activation by bath application of agonists does not eliminate the need for L-type Ca2+ channel opening [8,15]. This might reflect a requirement for Ca2+-induced Ca2+ release (CICR) from intracellular stores in LTD induction. In many cell types, CICR depends upon Ca2+ influx through L-type channels [16]. Activation of mGluR5 and the production of inositol-1,4,5-triphosphate (IP3) could serve to prime these dendritic Ca2+ stores, boosting CICR evoked by activity-dependent Ca2+ entry through L-type Ca2+ channels and thus promoting LTD induction [17–19].
Although the induction of LTD is postsynaptic, its expression is presynaptic. The activity-induced elevation in dendritic Ca2+ concentration triggers the production of an endocannabinoid (EC) that diffuses to presynaptically located CB1 receptors. The combination of presynaptic CB1 receptor activation, spiking and altered gene expression in the presynaptic cell leads to a long-term reduction in glutamate release [20]. Having both pre- and postsynaptic induction criteria confers synaptic specificity on LTD expression [21]. The molecular identity of the metabolic pathway leading to EC production in MSNs is still uncertain. This is important for a variety of reasons, not the least of which is knowledge of the critical signaling event responsible for triggering plasticity. There are two abundant striatal ECs: anandamide and 2-arachidonylglycerol (2-AG). Although previous studies have underscored the neural regulation of anandamide synthesis in the striatum [22], collateral support for it as the obligate signaling molecule has been scant; but recent work has provided additional support for a role of anandamide, rather than 2-AG, in striatal synaptic plasticity [23]. A dependence on anandamide would help to explain the ability of Ca2+ alone to induce EC-dependent LTD, as phospholipase D (one of key enzymes in the anandamide cascade) is activated by high intracellular Ca2+ [24] .
One still unresolved question about the induction of striatal LTD is whether activation of D2 receptors is necessary. Activation of D2 receptors is a potent stimulus for anandamide production [22]. However, recent work showing the sufficiency of L-type channel opening in EC-dependent LTD [14], makes it clear that D2 receptors play a modulatory – not obligatory – role. The real issue is the role of D2 receptors in LTD induction using synaptic stimulation. Attempts to address this question using BAC mice have consistently found that in D2 receptor expressing striatopallidal MSNs, D2 receptor activation seems to be necessary [25–27]. This could be due to the need to suppress A2a adenosine receptor signaling that prevents efficient endocannabinoid synthesis (see below) [28]. The issue is whether EC-dependent LTD is inducible in the other major population of MSNs that do not express D2 receptors – the D1 receptor dominated striatonigral MSNs. Kreitzer and Malenka [25] reported that LTD was not inducible in these MSNs using a minimal local stimulation. This result was confirmed subsequently [26]. However, using macroelectrode stimulation, EC-dependent LTD is readily inducible in identified D1 MSNs [27], consistent with the high probability of MSN induction seen in previous work [29]. Thus, the stimulation paradigm seems critical to LTD induction in D1 MSNs. Why? The problem with these induction protocols is that the type of axon and cell activated by the electrical stimulus is poorly controlled. With intrastriatal stimulation or with nominal white matter stimulation in coronal brain slices, glutamatergic afferent fibers, dopaminergic fibers and fibers intrinsic to the striatum are all activated, producing a mixture of neuromodulators that makes the interpretation of results less than straightforward. In Kreitzer and Malenka’s case, minimal local stimulation of both dopaminergic and glutamatergic fibers appears to be critical to the LTD induction failure, as blocking D1 receptors unmasked a robust EC-dependent LTD in D1 MSNs [26]. This kind of complication also appears to be responsible for the apparent D2 receptor dependence of LTD induction in D1 MSNs using macroelectrodes that more effectively activate cholinergic interneuron axons [27].
The neuromodulator mixture created by non-specific electrical stimulation could also be a factor in slice studies implicating nitric oxide (NO) signaling in LTD induction. First, it must be acknowledged that this form of LTD might not be EC-dependent, in spite of the fact that its induction occludes conventional HFS stimulation induced LTD [30]. Because activation of striatal interneurons containing nitric oxide synthase (NOS) depends upon NMDA and D1/D5 dopamine receptors [31], this form of LTD should be sensitive to antagonism of either. Although a form of NMDA receptor dependent LTD has been reported in ventral striatum, this isn’t the case in dorsal striatum. Moreover, LTD is not commonly found to be dependent upon D1/D5 receptors in the dorsal striatum.
The lack of specificity in activating inputs to MSNs during the induction of plasticity also raises questions about the type of glutamatergic synapse being affected by EC-dependent LTD. Studies using nominal white matter or cortical stimulation in a coronal brain slices typically assume that the glutamatergic fibers being stimulated are of cortical origin, but very few of these fibers are left intact in this preparation [32]. The thalamic glutamatergic innervation of MSNs is similar in magnitude to that of the cerebral cortex, perhaps constituting as much as 40% of the total glutamatergic input to MSNs, terminating on both shafts and spines [33]. As a consequence, it isn’t really known whether EC-dependent LTD is present at corticostriatal or thalamostriatal synapses or both. The localization of CB1 receptors on corticostriatal terminals, but not thalamostriatal terminals [34] is consistent with the hypothesis that LTD is a corticostriatal phenomenon, but more definitive studies are needed. Cutting brain slices in planes that preserve cortical and/or thalamic connectivity is one way to sort this out [32,35,36]. But these approaches have limitations given the highly convergent nature of the glutamatergic input to MSNs [33]. Optogenetic approaches offer a powerful alternative strategy [37] that would allow glutamatergic inputs from various cortical and thalamic regions to be dissected.
Long-term potentiation at glutamatergic synapses on MSNs
Less is known about the mechanisms controlling induction and expression of long-term potentiation (LTP) at glutamatergic synapses than LTD. Most of the work describing LTP at glutamatergic synapses has been done with sharp electrodes (either in vivo or in vitro), not with patch clamp electrodes in brain slices that afford greater experimental control and definition of the cellular and molecular determinants of induction. However, there have been a number of studies using these approaches in the last few years that have shed new light on LTP mechanisms.
Previous studies have argued that LTP induced in MSNs by pairing HFS of glutamatergic inputs and postsynaptic depolarization depends upon co-activation of D1 dopamine and NMDA receptors [29]. The involvement of NMDA receptors in LTP induction is not controversial. What is controversial is the involvement of D1 receptors. Robust expression of these receptors is only found in striatonigral MSNs, roughly half of the MSN population, making it difficult to understand how LTP induction could be universally dependent upon them unless some rather complicated, indirect mechanism was involved. Again, the advent of BAC transgenic mice has provided a tool to sort this issue out. Using perforated patch recording to preserve the intracellular milieu controlling the induction of synaptic plasticity, our group found that the induction of LTP at glutamatergic synapses was dependent upon D1 dopamine receptors only in striatonigral MSNs, not in D2 receptor expressing striatopallidal MSNs [26,38]. In these MSNs, LTP induction required activation of A2a adenosine receptors. These receptors are robustly expressed in striatopallidal MSNs and have a very similar intracellular signaling linkage to that of D1 receptors; that is, they positively couple to adenylyl cyclase and protein kinase A (PKA). Acting through PKA, D1 and A2a receptor activation leads to the phosphorylation of DARPP-32 and a variety of other signaling molecules, including MAPKs, linked to synaptic plasticity [39].
The nature of the cooperativity between NMDA receptors and D1/A2a receptor signaling in the induction of LTP remains to be resolved. Certainly, postsynaptic Ca2+ will figure prominently in this equation, but it is hard to map the simple model derived from the study of hippocampal synapses onto the striatum, given the apparent necessity of high intracellular Ca2+ concentration for the production of ECs and LTD induction. The site of Ca2+ entry will undoubtedly prove to be important, as this will contain information about the timing and magnitude of pre- and postsynaptic activity. For example, Ca2+ entry through NMDA receptors occurs only when glutamate is released at a time when there is sufficient postsynaptic depolarization to relieve Mg2+ block; scaffolding signaling molecules, like CaMKII, near the cytoplasmic mouth of these receptors could lead to their selective activation during conditions favoring LTP induction, and not during conditions favoring LTD induction, in spite of a very similar spatially averaged Ca2+ signal.
Neuromodulator signaling should also be a factor governing the effects of an activity-induced elevation in intracellular Ca2+ concentration. A concrete example of the role of neuromodulators in regulating the sign of synaptic plasticity can be found in our recent study of spike-timing dependent plasticity (STDP). In both types of MSN, STDP plasticity was Hebbian in the sense that when synaptic activation was followed by postsynaptic spiking, LTP was induced; whereas when the order of stimulation was reversed, LTD was induced. This rule for STDP in MSNs was also reported by Pawlak and Kerr [40]. The LTP and LTD induced in these protocols had all the features of plasticity induced by more conventional protocols, as outlined above, arguing that the core mechanisms were the same. However, the Hebbian character of the plasticity was malleable. For example, the sign and timing dependence of plasticity depended upon dopamine signaling. In striatopallidal MSNs, D2 receptor signaling was necessary for STDP LTD induction when postsynaptic spiking preceded synaptic stimulation; when D2 receptors were blocked and A2a receptors were stimulated, this pairing led to LTP induction. In contrast, in striatonigral MSNs, pairing postsynaptic spiking with a trailing presynaptic volley only produced LTD in the absence of D1 receptor stimulation, suggesting that PKA signaling could abrogate LTD induction. Reversing the order of stimulation gave LTP only when D1 receptors were stimulated and yielded LTD otherwise, arguing that PKA signaling not only could shut down LTD induction, but was also necessary for LTP induction. Conceptually similar results have been reported in other cell types [41,42], leading to the notion that LTD and LTP induction are governed by ‘opponent processes’ that interact at synaptic sites to determine the sign of synaptic plasticity. Altered activation of these processes could be responsible for ‘anti-Hebbian’ plasticity reported in the striatum [43]. How these opponent processes interact with one another and the cellular mechanisms underlying changes in synaptic strength remains to be determined (Fig. 1). Molecules like RCS (Regulator of Calmodulin Signaling), whose affinity for calmodulin and negative regulation of Ca2+ signaling is dramatically elevated by PKA phosphorylation, could be mediators of the opponent interaction [44].
Figure 1.
The nature of this interaction also has implications for the distal reward problem [45]. The change in dopamine release produced by the consequences of action selection occurs later in time than the pre- and postsynaptic activity that produced the action. In theoretical treatments of this issue, there are two strategies for dealing with this temporal delay or distal reward. One way is to have temporally coincident pre- and post-synaptic activity create an eligibility trace that subsequently can be acted on by an outcome-dependent signal, in this case dopamine. However, if dopamine receptor signaling changes the impact of patterned synaptic stimulation on intracellular signaling cascades controlling the induction of plasticity, it is difficult to see how this could work. An alternative approach is to have the outcome event trigger a fictive replay of the action selection. That is, the action leading to a particular outcome is remembered and in the process of remembering the key corticostriatal circuits underlying the action selection are re-activated, allowing dopamine to correctly modify synaptic strength. A recent study has provided evidence for the sustained activity in corticostriatal circuits that would be required for this type of mechanism [46].
What type of striatal activity normally triggers the induction of synaptic plasticity?
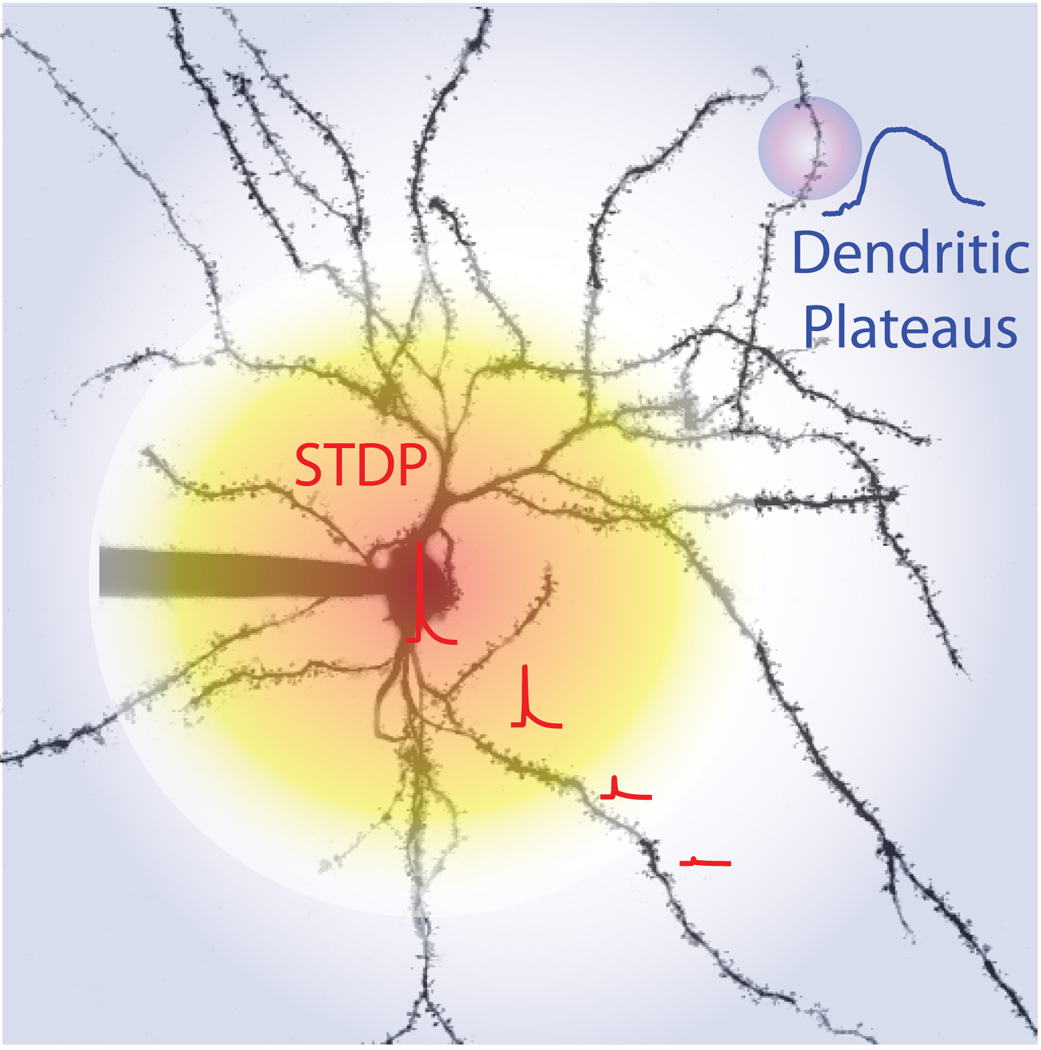
Although most of the induction protocols for synaptic plasticity that have been used to study striatal plasticity are decidedly unphysiological, involving sustained, strong depolarization and/or high frequency synaptic stimulation that induces dendritic depolarization, they do make the necessity of postsynaptic depolarization clear. In a physiological setting, what types of depolarization are likely to gate induction? One possibility is that spikes generated in the axon initial segment (AIS) propagate into dendritic regions where synapses are formed. Recent work has shown that STDP is present in MSNs [26,40,43]. But there are reasons to believe that this type of plasticity is relevant for only a subset of the synapses formed on MSNs. MSN dendrites are several hundred microns long, thin and modestly branched. Their initial 20–30 microns are largely devoid of spines and glutamatergic synapses. Glutamatergic synapse and spine density peaks near 50 microns from the soma and then modestly declines with distance [33]. Because of their geometry and ion channel expression, AIS generated spikes rapidly decline in amplitude as they invade MSN dendrites (as judged by their ability to open voltage-dependent Ca2+ channels), producing only a modest depolarization 80–100 microns from the soma. This is less than half the way to the dendritic tips [47], arguing that a large portion of the synaptic surface area is not normally accessible to somatic feedback about the outcome of aggregate synaptic activity. High frequency, repetitive somatic spiking improves dendritic invasion, but distal (>100 microns) synapses remain relatively inaccessible (Fig.2).
Figure 2.
What controls the induction of plasticity in the more distal dendritic regions, if not back-propagating action potentials? The situation in MSNs might be very similar to that found in deep layer pyramidal neurons where somatically generated bAPs do not invade the apical dendritic tuft [48]. In this region, convergent synaptic stimulation is capable of producing a local Ca2+ spike or plateau potential that produces a strong enough depolarization to open L-type Ca2+ channels, to unblock NMDA receptors and promote plasticity. In vivo, convergent synaptic inputs to MSNs can trigger plateau potentials called up-states [49]. Although transitions from the resting down-state to the up-state have all the hallmarks of an active, regenerative process (e.g., stereotyped transition kinetics, a narrow range of up-state potentials), transitions are very difficult to manipulate with a sharp electrode impaling the somatic region [49]. This suggests that the site of up-state generation is in distal dendritic regions that cannot be easily manipulated. If this were the case, distal dendrites should have ionic conductances that could support a plateau. Ca2+ imaging using 2PLSM has shown that there is robust expression of both low threshold Cav3 and Cav1 channels in MSN dendrites [10,11,13], a result that has been confirmed using cell-type specific gene profiling [11] (unpublished observations). The rich investment of MSN dendrites with strongly rectifying Kir2 K+ channels also creates a favorable biophysical condition for plateau potential generation.
The question is how the plateaus or up-states are normally generated. Based upon the sparse connectivity between individual cortical axons and MSNs [33,50], modeling studies have concluded that several hundred pyramidal neurons need to be near simultaneously active for a sufficient amount of current to be injected into dendrites for an up-state to be generated [33,51]. These studies have assumed that MSN dendrites are passive. However, if dendrites are not passive but active, then the convergence requirements could be dramatically different. Although glutamate uncaging experiments at proximal spines have not revealed regenerative processes [13], the situation could be different at more distal locations. If this is the case, spatial convergence of glutamatergic inputs onto a distal dendrite could induce a local plateau potential capable of pulling the rest of the cell into the up-state, fundamentally altering the impact of synaptic input on other dendrites. This is a way in which spatially convergent excitatory input to one dendrite could gate synaptic input to another. The lack of temporal correlation between up-state transitions and EPSP-driven spike generation is consistent with a scenario like this one [52]. If this were how MSNs operated, it would fundamentally change our models of striatal information processing. The problem is how to test it. Because glutamatergic connections are sparse, it is virtually impossible to reliably stimulate a collection of synapses onto a particular MSN dendrite with an electrode in a brain slice. Optogenetic techniques might provide a feasible alternative strategy. Another strategy would be to employ two photon laser uncaging (2PLU) of glutamate at visualized synaptic sites [10]. These tools are becoming more widely available and should allow the regenerative capacity of MSN dendrites to be tested soon. If it turns out to be the case that up-states are locally generated in dendrites, then it also becomes feasible to characterize their role in the induction of synaptic plasticity. Up-states could be sufficient, as in the apical tuft of pyramidal neurons, or they could simply be necessary by promoting back-propagation of spikes into the distal dendrites [53].
Homeostatic plasticity in striatal circuits in Parkinson’s disease models
Sorting out how dopamine regulates synaptic plasticity in striatal MSNs has obvious implications for disease states that are triggered by alterations in the function of dopaminergic neurons. Second in prominence among dopamine-dependent disorders only to drug abuse, Parkinson’s disease is a common neurodegenerative disorder whose motor symptoms are attributable largely to the loss of dopaminergic neurons innervating the dorsal striatum. In the prevailing model, the excitability of the two major populations of MSNs shift in opposite directions following dopamine depleting lesions, creating an ‘imbalance’ in the regulation of the motor thalamus favoring suppression of movement [54]. In particular, D2 receptor expressing striatopallidal MSNs spike more, whereas D1 receptor expressing striatonigral MSNs spike less in the PD state. The mechanisms underlying this shift were not known at the time the model was formulated, but have widely been assumed to reflect changes in intrinsic excitability that accompanied loss of inhibitory D2 receptor signaling and excitatory D1 receptor signaling. Indeed, studies by our group and others have found electrophysiological support for this view [55,56].
What about synaptic remodeling? Several studies have suggested that in the absence of dopamine, synaptic plasticity is lost, essentially ‘freezing’ the striatal circuit in its pre-depleted state [25,29]. However, recent studies of defined MSN populations have shown that although dopamine is necessary for plasticity to be bidirectional and Hebbian, it is not necessary for the induction of plasticity per se [26]. Following DA depletion, pairing presynaptic and postsynaptic activity – regardless of which came first – induced LTP in D2 MSNs and LTD in D1 MSNs. This result adds a new dimension to the prevailing model by showing that activity dependent changes in synaptic strength parallel those of intrinsic excitability following DA depletion. Work in vivo examining the responsiveness of antidromically identified MSNs to cortical stimulation following unilateral lesions of the striatal dopaminergic innervation is consistent with this broader model [55].
But this poses a problem. Neurons are homeostatic; sustained perturbations in synaptic or intrinsic properties that make neurons spike more or less than their set-point engage homeostatic mechanisms that attempt to bring activity back to the desired level [57,58]. One of the most common mechanisms of homeostatic plasticity is to alter synaptic strength or to scale synapses. In striatopallidal MSNs, the elevation in activity following dopamine depletion triggers a dramatic down-regulation of glutamatergic synapses formed on spines [11]. This can be viewed as a form of homeostatic plasticity. Like scaling seen in other cell types, the synaptic modification depends upon Ca2+ entry through voltage-dependent L-type channels that presumably report activity levels. There are other recently described network adaptations relevant to homeostatic plasticity in PD models. For example, although feed-forward inhibition through fast spiking GABAergic interneurons does not appear to be directly altered, low-threshold GABAergic interneurons do elevate their input to at least a subset of MSNs in PD models [59,60]. Recurrent collateral inhibition between MSNs, which is normally strongest between D2 MSNs, is almost abolished following dopamine depletion [61]. These adaptations in conjunction with enhanced striatopallidal MSN excitability are likely to contribute to the transmission of beta band activity from the cortex through the striatum to the globus pallidus [62]. That said, a major gap in the existing literature is a description of the intrinsic changes in MSN excitability following prolonged DA depletion. All of the work with identified cell types has relied upon short term (~< 1 week) DA depletions [e.g., 11,25], but there clearly are slower adaptations that take 3–4 weeks to stabilize. Given the robust differences in the anatomy and intrinsic physiology of striatonigral and striatopallidal MSNs that exists in the normal striatum [63]. It is easy to conjecture that these resting differences are due to differential regulation of basal excitability by DA. If that were true, losing DA could trigger homeostatic processes that make MSNs much more alike.
Concluding Remarks
In the last few years, our understanding of the mechanisms controlling synaptic plasticity in the corticostriatal circuits underlying action selection has significantly deepened. Dopamine remains an important player in the induction of plasticity at corticostriatal synapses on principal MSNs, but it is not the only player and its effects are dictated by the type of dopamine receptor expressed. In large measure, this advance has been made possible by the development of BAC transgenic mice that make the cellular heterogeneity of the striatum tractable. How the relatively sparse but functionally important interneuron populations contribute to plasticity remains to be clearly defined. The development transgenic mice expressing Cre in select neuronal populations (and the growing stable of mice with floxed genes) should propel this effort forward and allow a molecular dissection of these mechanisms in coming years. The growing application of optical approaches, like 2PLSM and 2PLU, also promises to yield insights into synaptic integration and plasticity not achievable with any other approach. Coupling these new tools with optogenetic strategies for activating microcircuits relevant to action selection should prove to be a watershed for basal ganglia and motor systems research. These approaches should allow us to gain a better grasp of basal ganglia pathophysiology in disease states – like Parkinson’s disease, Huntington’s disease and drug abuse – and in so doing develop a new generation of therapeutics.
Acknowledgements
This work was supported by NS34696 to DJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. This paper discusses how phasic DA release in response to positive “reward prediction errors” (RPE) could promote the selection of actions associated with reward. It also discusses how transient drops in DA release in response to negative RPE could lead to the suppression of actions associated with aversive outcomes.
- 2.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 3.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 4.Dujardin K, Laurent B. Dysfunction of the human memory systems: role of the dopaminergic transmission. Curr. Opin. Neurol. 2003;16 Suppl 2:S11–S16. doi: 10.1097/00019052-200312002-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- 6.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 7.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 8.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- 10.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 12.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci. 2007;27:8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007. In this study the authors utilized 2PLU and 2PLSM to uncage gluatamate on indivivdual MSN spines under experimentally manipulated conditions. Using this technique, this group elegantly describes rules governing postsynaptic calcium summation, which will shed light on the mechanisms involved in STDP induction.
- 14. Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. In this study, the authors showed that corticostriatal LTD can be induced in acute brainslices by enhancing the contribution of L-type voltage activated Ca2+ channels. Under these conditions LTD induction is not occluded by mGluR1/5 antagonists. Such compensation suggests that L-type Ca2+ channels and mGluR1/5 may both contribute to a shared Ca2+ pool required for the generation of LTD.
- 15.Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 18. Taufiq UrR, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. The authors performed nuclear patch clamp recordings in DT40 cells to determine the effects of IP3 and Ca2+ on intracellular Ca2+ release. The authors showed that IP3 causes IP3 receptors to cluster. Furthermore, elevated Ca2+ concentration increases the synchrony and open probability of clustered IP3 receptors. This is an important concept in explaining how increased IP3 production (via mGluR5 activation) may ‘prime’ intracellular Ca2+ stores and enhance CICR in LTD induction. Such a mechanism may help explain the synergism between L-type Ca2+ channels and mGluR1/5.
- 19.Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 20.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 21.Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 23.Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenowitz SD, Best AR, Regehr WG. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J Neurosci. 2006;26:6841–6850. doi: 10.1523/JNEUROSCI.1280-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. This study shows that striatopallidal LTD is lost in parkinsonian animal models and suggests it can be rescued by enhanced endocannabinoid signaling.
- 26. Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. This is the first demonstration that DA controls the induction of LTP and LTD in a receptor and cell-type specific manner. In a DA depleted state, mimicking Parkinson’s disease, synaptic plasticity was still inducible in both cell types, but was no longer bidirectional or Hebbian, suggesting that DA acts to coordinate the induction of corticostriatal plasticity in complementary, receptor-dependent ways.
- 27.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci. 1999;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ondracek JM, Dec A, Hoque KE, Lim SA, Rasouli G, Indorkar RP, Linardakis J, Klika B, Mukherji SJ, Burnazi M, et al. Feed-forward excitation of striatal neuron activity by frontal cortical activation of nitric oxide signaling in vivo. Eur J Neurosci. 2008;27:1739–1754. doi: 10.1111/j.1460-9568.2008.06157.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- 33.Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. vol 5. Oxford UP: 2004. pp. 361–414. [Google Scholar]
- 34.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. Journal of Neuroscience. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J Neurosci Methods. 2007;159:224–235. doi: 10.1016/j.jneumeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 38.Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. These two studies (41,42) show that STDP is controlled by two interactive processes. One is timing dependent process; the other is neuromodulator mediated signaling. Interaction between these neuromodulator signaling modulates the opponent processes responsible for the outcomes of STDP.
- 43.Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J. Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 45.Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychol Rev. 1981;88:135–170. [PubMed] [Google Scholar]
- 46.Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. This study used 2PLSM and BAC transgenic mice to examine the dendritic excitability of identified MSN populations. The authors demonstrate that somatically generated back propagating action potentials degrade with distance from the soma, and do so more rapidly in striatonigral than striatopallidal MSNs. The data from this study ultimately raise questions about how far from the soma STDP may be efficiently evoked in MSNs.
- 48.Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- 49.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- 52.Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- 53.Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 55.Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 58.Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- 59.Dehorter N, Guigoni C, Lopez C, Hirsch J, Eusebio A, Ben-Ari Y, Hammond C. Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons. J Neurosci. 2009;29:7776–7787. doi: 10.1523/JNEUROSCI.1527-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. Brain oscillations, medium spiny neurons, and dopamine. Cell Mol Neurobiol. 2002;22:611–632. doi: 10.1023/a:1021840504342. [DOI] [PubMed] [Google Scholar]
- 63.Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]