Abstract
The dachshund (dac) gene was initially described as a mutant phenotype in flies featuring extremely short legs relative to their body length. Functioning as a dominant suppressor of the ellipse mutation, a hypermorphic allele of the Epidermal Growth Factor Receptor (EGFR), the dac gene plays a key role in metazoan development, regulating ocular, limb, brain, and gonadal development. In the Drosophila eye, dac is a key component of the Retinal Determination Gene Network (RDGN) governing the normal initiation of the morphogenetic furrow and thereby eye development. Recent studies have demonstrated an important role for human DACHSHUND in tumorigenesis, in particular, breast, prostate and ovarian cancer. The molecular mechanisms by which DACH1 regulates differentiation and tumorigenesis are discussed herein.
dachshund in Gonadal Development
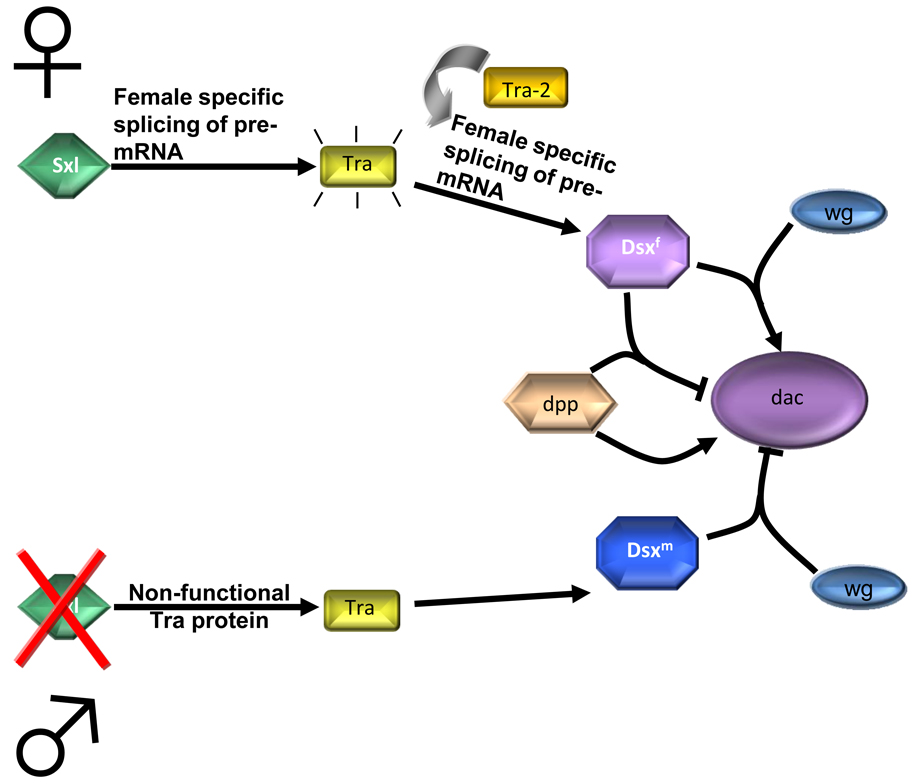
The Retinal Determination Gene Network, RDGN, plays an important role in Drosophila gonadal development [1–3]. In Drosophila, the male and female genital tracts undergo patterning gene expression in homologous regions in both sexes. In this regard, wg is expressed along the anterior-posterior (AP) border, flanked by a broad stripe of dpp expression [4]. In contrast, dac is expressed in a sex-specific manner. dac function is important for development of both male and female genitalia. Male dac mutant flies have abnormal external male genital structures known as claspers, whereas female dac mutant flies have defective ovarian duct formation [4]. In the male genital disc, wg represses dac expression while dpp signaling induces it [4, 5]. In contrast, wg activates dac expression while dpp represses dac expression in the female genital disc (Figure 1). The Eya protein also plays an important role in Drosophila gonad development [2, 6]. eya is expressed in the somatic gonadal precursor and required for the maintenance of somatic gonadal precursor (SGP) cell fate. The function of dac as a part of the RDGN in sex determination in flies indicates the importance of this gene in development not only in Arthropods but also in mammalian models, as we will discuss later in this review. dac has also been shown to play a crucial role in Drosophila eye development (Box 1).
Figure 1. Involvement of dac in Drosophila sex determination.
(i) The main regulatory gene Sex lethal (Sxl) is activated only in females. It directs the female-specific splicing of transformer (tra) pre-mRNA. Together with the Tra-2, Tra regulates female specific splicing of dsx pre-mRNA, which results in functional Dsxf protein. (ii) In males, Sxl protein is absent, so Tra, after being spliced by a default, is nonfunctional. This results in default splicing of dsx, and Dsxm protein synthesis. As depicted, wg and dpp can either promote or inhibit dac function in males or females.
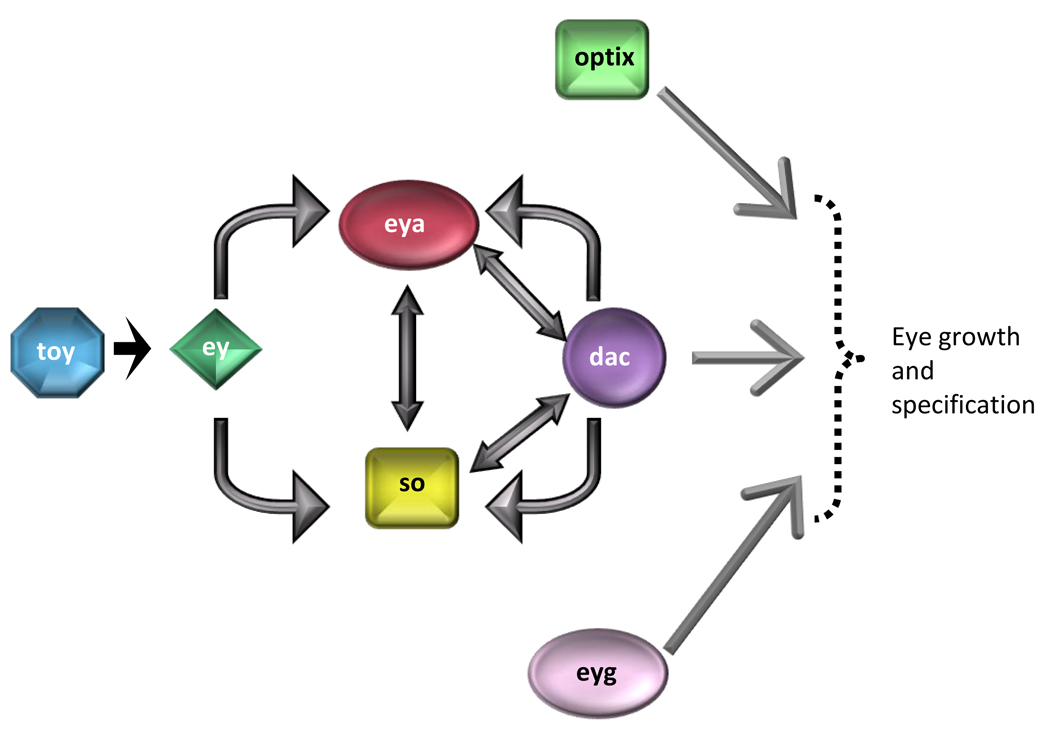
Box 1. The Retinal Determination Gene Network (RDGN)
The Drosophila dac gene was originally isolated as a dominant suppressor of the EGF receptor ellipse mutation [18], a dominant hyperactive form of EGFR that causes a roughened eye phenotype. Retinal morphogenesis in dac mutant eye discs is also defective, presenting with a drastically reduced number of photoreceptors [18]. dachshund induces ectopic retinal development in a variety of tissues including the thorax, legs, and head [65]. The ectopic eyes induced by dac resemble normal adult eyes, although smaller and highly disorganized and the defect in photoreceptor development in dac mutants has been attributed to defective initiation of the morphogenetic furrow.
The RDGN, which governs tissue specification fate in diverse organisms, comprises seven genes that encode either transcription factors or co-factors. The RDGN is required for normal development of the eye, ear, central nervous system, muscle and gonads. The seven key genes required for specification in the compound Drosophila eye include twin of eyeless (toy), eyeless (ey), eyes absent (eya), sine oculis (so), dachshund (dac), eye gone (eyg), and optix (opt) (Figure I). Loss of function mutations in each of these genes results in failure of proper eye development, whereas ectopic expression of each gene is sufficient to induce eye development [66–68].
Analysis of the components of the RDGN has implicated dac in cell fate specification. Six6−/− mutant mice present hypoplastic pituitary glands with a variable penetrance and retinal hypoplasia with decreased ganglion cell layer cell number. Mammalian 2-hybrid experiments suggest that Six6 interacts with DACH1, while molecular mapping studies reveal co-precipitation of DACH1 with NCoR, HDAC3, and Sin3a/b [7]. Investigation of the mechanisms responsible for hypoproliferation in Six6−/− pituitary cells and retinal epithelial cells implicated the cyclin dependent kinase inhibitor p27KIP1 [8]. Six6 in association with DACH1 represses p27KIP1 promoter activity, suggesting that loss of Six6 increases p27KIP1 contributing to cellular hypoproliferation. These molecular interactions are consistent with studies by Ikeda et al., which suggest that Eya interacts with Six6 in mammalian 2-hybrid, but does not interact with DACH1 [9]. DACH1, however, is capable of transactivating in the presence of an Eya fusion protein, suggesting that CREB binding protein (CBP) mediates the interaction between Eya and DACH1. CBP is a known mediator for different transcription factors with histone acetyltransferase (HAT) activity. The complex nature of DACH1 and its ability to interact with both HATs like CBP, and histone deacetylases like HDAC3, could explain the vast array of functions this protein can have in different cancers. For example, while DACH1 has antiproliferative effects in breast and prostate cancers, DACH1 is also known to inhibit TGF-β signaling in ovarian cancer [30]. The function of DACH1 in cancer will be described in more detail later in this review.
The Drosophila dachshund Gene
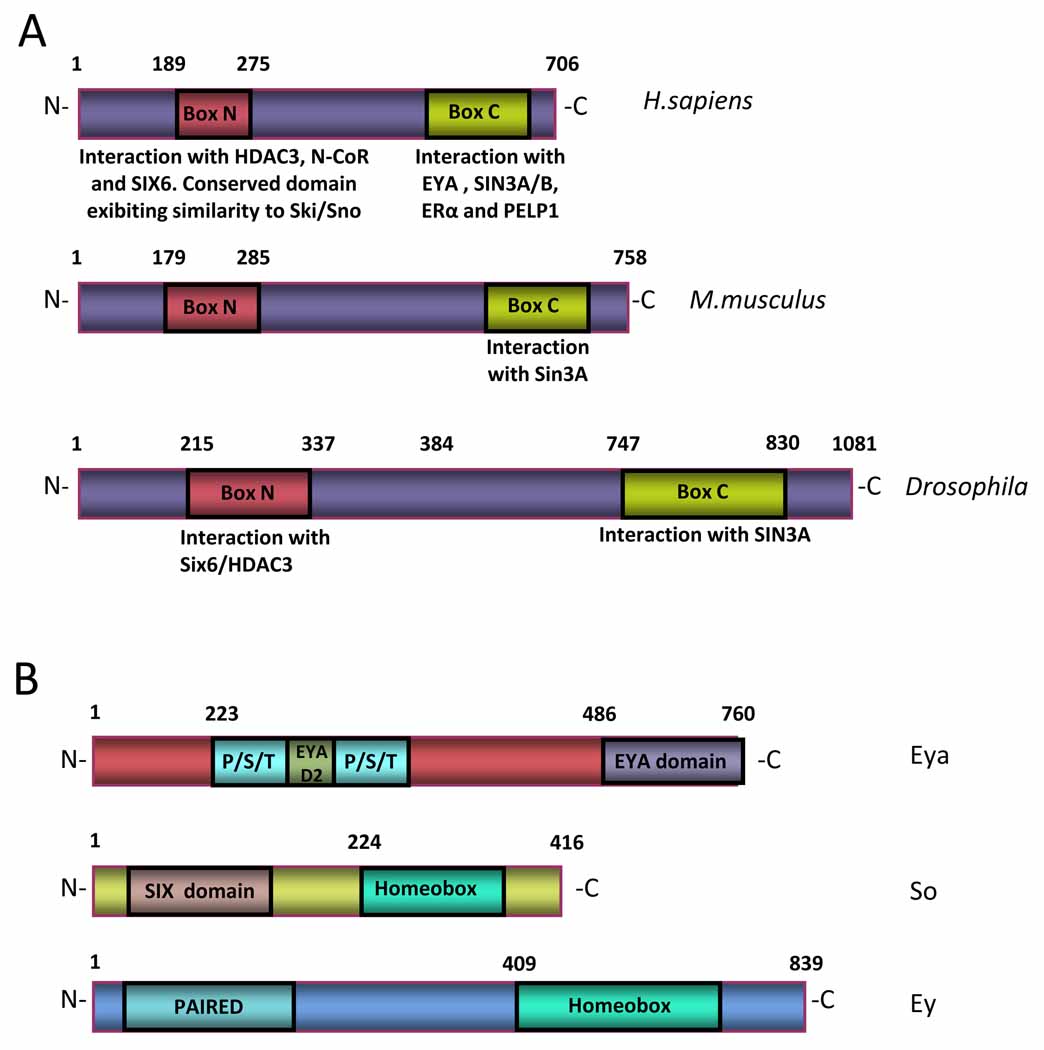
The dachshund (dac) gene encodes a well-conserved nuclear protein related to the Sno/Ski family of co-repressors. In Drosophila, the dac genomic locus spans approximately 20 kilobases (kb), encompassing 12 exons that encode a protein of 1081 amino acids (Figure 2a). A human homologue of Drosophila dac, DACH1, localizes to chromosome 13q21 [10]. The DACH1 protein is predominantly nuclear and exhibits two domains: DachBox-N and DachBox-C, both of which are highly conserved from Drosophila to humans. The human DachBox-N shares approximately 35% amino acid identity to the Ski/Sno proteins and is also known as the DACH Ski/Sno (DS) domain. Its crystal structure reveals a winged helix Forkhead subgroup structure of the helix-turn-helix family. DACH1 binds NCoR, HDAC3, and Six6 through its N-terminus [11] and even though no sequence-specific DNA binding activity has been identified, DACH1 is capable of binding naked DNA [9]. A carboxy terminal domain, or DachBox-C, is predicted to consist of an α-helical coiled-coil structure through which DACH1 protein associates with Ubc9, a ubiquitin conjugating enzyme [12], and Eya through its Eya domain in cooperation with Sin3A/B (Figure 2a). The DACH1 protein is detected as a 97 kilodalton band, considerably larger than the 73 kilodalton size predicted from the cDNA. It has been hypothesized that this difference in size is likely due to post-translational modification and intrinsic properties of the protein structure such as the α-helical domains [13].
Figure 2. Dachshund proteins are conserved among species.
(a) Dachshund proteins have two domains that are highly conserved among species. Located on the N-terminus, DachBox-N contains a DNA-binding domain. Through this domain Dachshund proteins interact with HDAC3, NCoR and SIX6. DachBox-N domain also exhibits a high similarity to Ski/Sno family of co-repressors. (b) Required for the specification of the compound Drosophila eye, eyeless (ey), eyes absent (eya), sine oculis (so) are part of the Retinal Determination Gene Network (RDGN). dac and eya act together as transcriptional factors in Drosophila, binding to DNA.
Genetic Analysis of Dach Gene Function in Mice
One mouse homolog of the Drosophila dachshund gene, Dach1, has been cloned, and its expression pattern has been characterized [14–18]. Dach1 knockout mice (Dach1−/−) die shortly after birth with no gross histological abnormalities of eyes, limbs, or brain [19, 20]. These studies suggest functional redundancy with Dach2 [19], since homozygous deletion of Dach2 results in mice with no detectable abnormalities [21], whereas Dach1/Dach2 double mutant mice grossly resemble the phenotype of Dach1 homozygotes, dying at birth with no obvious abnormalities of the eye, limbs, or brain. However, Dach1 and Dach2 are redundantly required for normal female reproductive tract development [22]. Defects were associated with the Müllerian duct, but not the Wolffian ducts. Indeed, Dach1 and Dach2 mutants resulted in abnormal expression of Wnt7a and Lim1, known target genes involved in regulation of normal Müllerian duct development.
The RDGN Complex in Hormone-Response Cancers
Recent studies provide compelling evidence that RDGN complex components regulate tumorigenesis. DACH1 expression is altered in human breast, prostate, ovarian and endometrial cancer [23–25]. The expression pattern of complex components has been identified in a number of tumors, including human breast cancer. Analysis of more than 2200 patients show that reduced DACH1 levels are associated with poor prognosis [26]. Patients with reduced DACH1 levels died more than three years earlier than those with normal DACH1 levels. DACH1 expression is reduced in prostate and uterine cancer, correlating with tumor progression and invasiveness [24, 27]. The RDGN network protein Six1 transcriptionally activates multiple pro-tumorigenic genes [28]. Many members of the Six family, including Six1, Six3, and Six6, regulate proliferation in events that precede differentiation [8, 29–32], and their overexpression is detected in various tumors. For example, Six6 is overexpressed in mammalian tumors [33], SIX3 in extraskeletal myxoid chondosarcomas [34], and SIX1 in human breast cancer, Wilms’s Tumor and myxoid chondrosarcomas [35–37]. The Six1 gene, which regulates the G2 phase of the cell cycle, induces expression and activity of the cyclin D1 gene [26, 28]. The SIX family of homeobox genes are overexpressed in breast cancer [37], and analysis of 214 human infiltrating ductal breast carcinomas confirmed that approximately 5% showed SIX1 amplification or overrepresentation [38]. Transduction of human breast cancer cells with SIX1 induces cyclin A expression and cellular proliferation, suggesting that cyclin A may be a downstream target of SIX1-induced proliferation [37].
DACH1 regulation of Androgen Receptor Activity
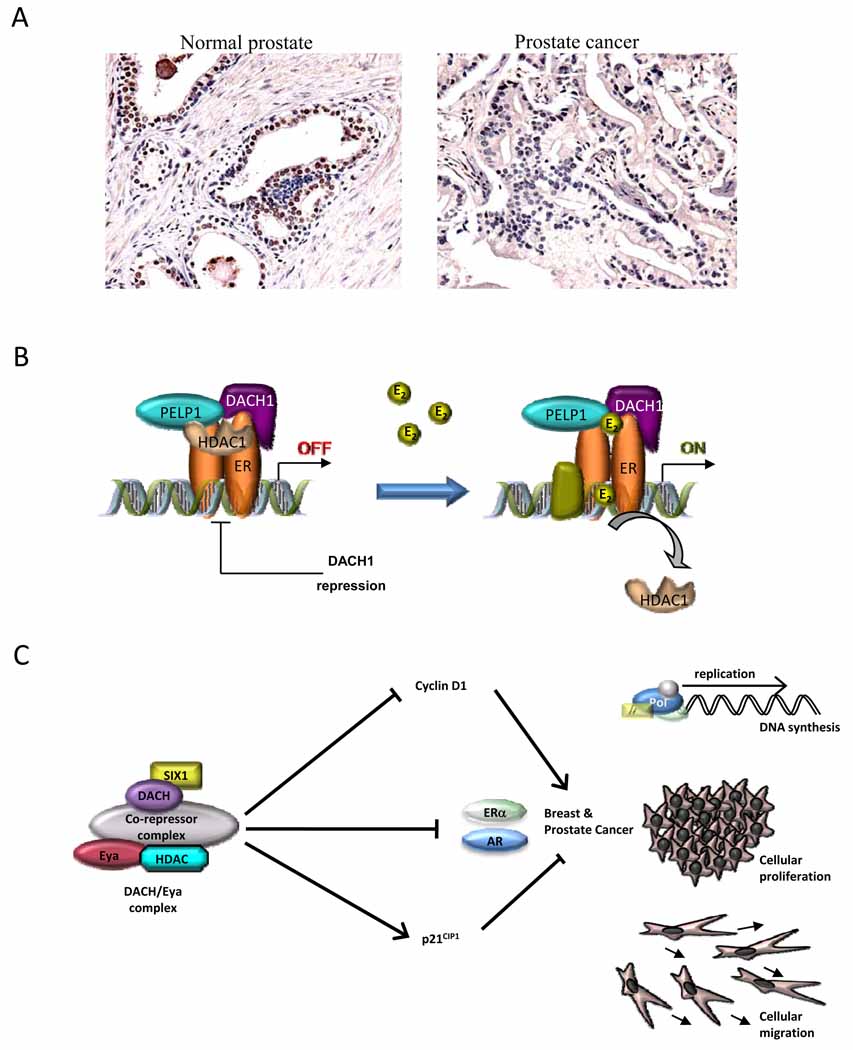
Recent studies provided compelling evidence for the important role of DACH in regulating nuclear receptor signaling and function [23, 24]. The DACH1 gene is expressed in normal human prostate epithelial cells with reduced expression in human prostate cancer [24] (Figure 3a). DACH1 expression inhibited prostate cancer cellular DNA synthesis and growth in colony forming assays. DACH1 physically associates with the androgen receptor (AR) inhibiting AR activity via a conserved DS domain. The DS domain recruits NCoR and HDAC1 in the context of local chromatin to an androgen-responsive gene promoter and inhibits transactivation of the AR; point mutation of the AR acetylation site abrogated repression. Of interest, a series of AR mutations within the ligand binding domain, known to occur in androgen ablation therapy-resistant prostate cancer, were sensitive to repression by DACH1.
Figure 3. DACH1 mediated inhibition of ERα and AR function.
(a) Immunohistochemical staining for DACH1 in human prostate cancer samples. Relative intensity of immunostaining for DACH1 in normal prostate and prostate cancer samples, shown also as % cells staining for DACH1 [24]. (b) Schematic representation of how DACH1 inhibits ERα function in breast cancer. DACH1 competes with the ERα coactivator PELP1 in the context of local chromatin of ERE [23]. (c) Schematic representation of hypothetical mechanism by which DACH1 inhibits cellular proliferation in hormone response cancer cells. Through interaction with transcriptional co-factors and a co-repressor complex, DACH1 inhibits cyclin D1, inhibits proliferative function of the ERα and AR and the abundant expression of p21CIP and p27KIP1. Through these actions, DACH1 represses breast and prostate cancer proliferation. DACH1 also re-organizes oncogene-mediated disruption of cell polarity and reverts disruption.
The androgen receptor is known to be repressed by the NAD-dependent histone deacetylase Sirt1 [39]. Indeed, Sirt1 deacetylates the AR with similar kinetics as that described for p53 and p300 [39, 40]. As inhibition of class III HDAC activity enhanced liganded AR activity and expression of the AR, it has been suggested that endogenous Sirt1 plays an important biological role in repressing AR signaling [41]. DACH1 repression of AR was reversed by either sirtinol or nicotinamide, consistent with the role of class III HDACs in DACH1 repression [24], suggesting DACH1 repression of AR may be at least, in part, dependent upon endogenous Sirt1 function. In addition, DACH1 repression of AR was reduced by Trichostatin A (TSA) indicating an additional role for type I and II HDAC activity [24].
Careful ChIP analysis characterized the interactions of DACH1 with endogenous AREs. DACH1 was recruited to the proximal and distal AREs of the PSA gene, and DACH1 augmented recruitment of the co-repressor NCoR. DACH1 recruitment to the PSA AREs was reduced by DHT and enhanced by the DHT antagonist Casodex (bicalutamide). Like Casodex, DACH1 expression enhanced Sirt1 recruitment to an endogenous ARE [24]. NCoR seems to be required for the transcriptional repression by androgen antagonist, and Casodex (bicalutamide) may function as an antagonist in the absence of this co-repressor [42, 43]. Recent studies demonstrate that DACH expression is associated with the recruitment of NCoR in the absence of androgen antagonist and may therefore play a role in the therapeutic response to Casodex.
The inhibition of DHT-dependent cellular growth, DNA synthesis and proliferation by DACH1, together with evidence that DACH inhibits the transcriptional activity of DHT-dependent AR function, suggests the two activities may be functionally linked. Alternatively, DACH1 may inhibit cellular proliferation via additional targets to the AR including c-jun or cyclin D1 [26, 44].
DACH in estrogen receptor (ER) signaling
In view of the clinical observation that DACH1 expression was lost in poor prognosis breast cancer [44] and that DACH1 and ERα expression were inversely correlated in human breast cancer [23], molecular analyses were conducted to determine whether DACH1 interacts with the ERα. DACH1 physically associates with ERα and inhibits ligand-dependent and basal ERα activity [23] (Figure 3b). Estradiol-induced DNA synthesis and cellular proliferation was inhibited by DACH1 physically associating with ERα, and as with ligand-dependent AR activity, requires the conserved DS domain of DACH1.
A blinded proteomic analysis was conducted in order to identify DACH1 interactive proteins that may contribute to ERα signaling. Using DACH1 as bait, MS/MS and peptide sequencing identified a key DACH-binding protein as proline, glutamic acid and leucine rich protein 1 (PELP1) [23]. PELP1, initially cloned as an ERα co-activator [45], encodes a proline-rich protein with several interactive motifs including nuclear receptor (NR), interacting boxes (LXXLL), and WW, PDZ, SH2, SH3, and FHA domains. PELP1 interaction with the AF2 domain of ERα occurs via LXXLL motif 4 and 5 [45–47]. In recent studies, PELP1 associated with DACH1 in intranuclear/extranucleolar sites and reverted DACH1-mediated ERα repression. A series of PELP mutants were used to identify a minimal interaction domain with DACH1. Amino acids 400–600 of PELP1 were required for interaction with DACH1. DACH1 expression inhibited PELP1-dependent co-activation of ERα in the presence of ligand. DACH1 purified in bacteria competed against the physical association of PELP1 with ERα in vitro. This finding was consistent with a model in which DACH1 may inhibit ERα activity via competition for limiting amounts of the PELP co-activator. Furthermore, shRNA to PELP1 demonstrated an important role for PELP1 in estrogen signaling. Specifically estradiol-mediated reduction in HDAC1 occupancy, at an ERE in the context of local chromatin, was reduced by PELP1 shRNA. Collectively these studies suggest DACH1 plays an important role in ERα activity by titrating the relative abundance of co-activators and co-repressors with HDAC activity, to target estrogen responsive element in the context of local chromatin.
The relative balance of DACH1 and PELP1 in breast cancer cells thus appears to modulate ERα signaling [23]. PELP1 expression is increased in human breast cancer compared with normal human breast epithelium, and breast tumors often co-express PELP1 and ERα [45, 48]. The loss of DACH1 expression during tumor progression and/or relocalization of DACH1 subcellular distribution may contribute to tumor progression through enhancing ERα activity.
DACH1 expression is regulated by a variety of growth and differentiation signals both in vivo and in vitro. DACH1 colocalizes with p27KIP1 and p57KIP2 expression in chondrocytes while its protein levels can be upregulated by FGF1 in murine limb buds and repressed by BMP4 [49]. BMP2 represses DACH1 abundance in the presence of dihydrotestosterone (DHT) in the LNCaP human prostate cancer cell line [50]. In prostate cancer, loss of DACH1 expression is associated with prostate tumor progression and may be due to enhanced AR activity and thereby increased cellular proliferation. Together these studies are of interest in demonstrating a component of the RDGN in regulating cellular growth and signaling via hormone nuclear receptors (Figure 3c).
Studies in ovarian cancer identified DACH1 as an up-regulated gene in advanced stage ovarian cancer associated with TGFβ resistance [25]. Semi-quantitative RT-PCR analysis of micro-dissected ovarian cancer specimens confirmed that DACH1 is increased in ovarian cancer. Consistent with prior findings in other cell types [7], DACH1 inhibited TGFβ signaling in immortalized, normal, ovarian epithelial cells [22]. Collectively, these studies are consistent with a model in which DACH1 may promote resistance to TGFβ signaling in ovarian cancer, and raise the possibility that targeted therapeutics to block DACH1 function locally within the ovarian tumor may provide a novel therapeutic option for ovarian cancer. These findings suggest the importance of regulating DACH1 abundance locally, as opposing functions to inhibit tumor growth (breast, prostate) or promote growth (ovary) have been suggested.
DACH1 is a Cell Fate Determination Factor that Inhibits Cyclin D1 and Breast Tumor Growth
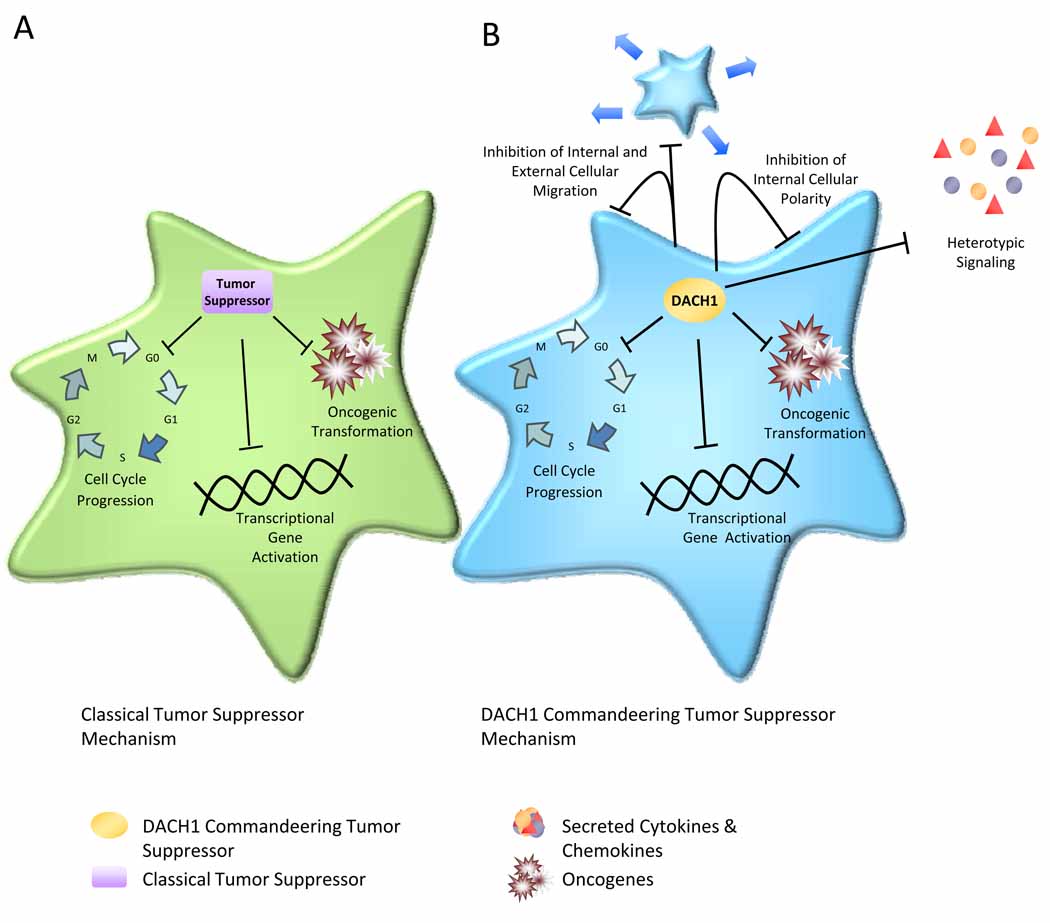
In view that reduced DACH1 expression correlates with poor prognosis in human breast cancer, molecular genetic studies were conducted to determine the mechanism by which DACH1 regulates cellular proliferation [26]. Forced re-expression of DACH1 in the MDA-MB-231 breast cancer cell line represses cyclin D1 and induces abundant expression of p21CIP1 and p27KIP1 without affecting the expression of cyclin A (Figure 3c). In addition, DACH1 inhibits ErbB2-induced DNA synthesis. Similarly, when immortal MCF10A cells are transduced with either ErbB2, Ras or Myc oncogenes, DACH1 expression reverts oncogene-mediated disruption of breast cell polarity. MCF10A cells are an immortalized human breast epithelial cell line, which in the presence of complex matrices, form three-dimensional structures referred to as acini. MCF10A cells undergo specific molecular changes when transformed with either c-Myc, Ras or ErbB2, where acini undergo striking oncogene-specific morphological changes [51, 52]. The multi-cellular spheroids consisting of an unpolarized epithelium induced by Ras or ErbB2 are reverted by DACH1, resulting in a normal, polarized epithelium accompanied by lumen formation. This peculiar property of DACH1 to revert oncogene-dependent disruption of polarity suggests that DACH1 has an organizing function on cellular polarity (Figure 4).
Figure 4. Commandeering Tumor Suppressor function of DACH1.
(a) The traditional tumor suppressor inhibits cell cycle to block cellular proliferation. (b) The Dachshund gene reverts oncogene-induced disruption of cellular polarity via altering expression and secretion of specific cytokines and chemokines (IL8). This function is transferable via the supernatant of DACH1 cellular conditioned medium. This function, via a heterotypic signal differs from the traditional intracelluar tumor suppressor function via p53/p21 and is referred to, herein as a “commandeering” function. DACH1 inhibits cellular proliferation via cyclin D1 repression but also inhibits migration, reverts oncogene-induced disruption of polarity, and inhibits metastasis via inhibition of IL8 secretion.
DACH1 inhibits MCF-7 cell DNA synthesis, the rate-limiting component of which is the abundance of cyclin D1. DACH1 was identified by ChIP assays in the context of local chromatin of the human cyclin D1 promoter, whose transcriptional repression by endogenous DACH1 was demonstrated in experiments using DACH1 siRNA [26]. DACH1 thus associates with the endogenous cyclin D1 promoter through AP-1 and CRE sites within the context of local chromatin associated with DACH1/HDAC1/NCoR complexes. Immunoprecipitation analysis demonstrates that DACH1 physically associates with both c-Jun and CREB [26, 44]. Further studies have shown that the DachBox-N of DACH1 is required for the physical association with c-Jun and CREB. Thus DACH1 is likely to regulate transcription through a physical association with AP-1 binding proteins, consistent with prior microarray analysis in which DACH1 repressed a variety of AP-1 responsive genes [7]. Cyclin D1 plays an important role in hormone-responsive cancers and is known to bind ERα and the AR. The transcriptional repression of Cyclin D1 by DACH1 provides another level of complexity, or perhaps “fine tuning” how DACH1 regulates hormone signaling. As cyclin D1 is induced by Six1 and repressed by DACH1, the RDGN regulates the cell-cycle machinery through cyclin D1, a common rate-limiting downstream step of DNA synthesis common to breast cancer epithelial cells and many other hormone responsive cell types [53].
DACH1 Inhibits c-Jun-mediated in hormone responsive cells
Using genome-wide microarray analysis in breast cancer cells, DACH1 expression was shown to repress a variety of AP-1-responsive genes [7]. For example, DACH1 inhibits c-Jun mediated contact-independent growth [44], and recent studies showed that DACH1 physically associates with c-Jun and represses its function by binding to the δ domain [26] (Figure 4b). c-Jun contributes to contact independent growth of breast cancer cells and is induced by estrogen. Estrogen-dependent signaling occurs through a non-canonical pathway, via AP-1 sites in a subset of target genes. siRNA analysis identified DACH1 as an endogenous protein attenuating c-Jun activity [44]. A variety of c-Jun target genes are repressed by DACH1 in breast cancer cells. Use of c-Junfl/fl cells reveals a requirement for c-Jun in DACH1-mediated inhibition of DNA synthesis and repression of c-Jun through the conserved δ domain, as c-Jun δ domain deletion mutants are resistant to DACH1 repression [44]. Given the importance of c-Jun as a downstream target for a variety of oncogenic signals in hormone-responsive cancers, the demonstration that DACH1 associates with, and constrains the activity of, c-Jun places DACH1 in a pivotal position in regulating a variety of AP-1 signaling functions.
DACH1 and the RDGN Network In Cellular Migration and Invasion
Recent studies using Dach1 knockout mice demonstrated a role for endogenous Dachshund in repressing mammalian cell migration [54]. Immunohistochemical studies of tumors including breast cancer, show that reduced DACH1 expression correlates with the invasive tumor phenotype [31].
DACH1 transduction of mammary epithelial cells regulated secretion of cytokines and chemokines, implicating DACH1 in heterotypic signaling [54]. Whether the DachBox-N domain of the DACH1 protein contains DNA sequence specific binding capacity remains to be formally determined. DACH1 transduction of epithelial cells induced secreted factors which inhibited the metastatic phenotype of nearby cells. Such findings have led to the proposal that DACH1 may function as a “commandeering factor” to inhibit the malignant phenotype of nearby cells. The mechanisms by which DACH1 commandeers and restrains the oncogenic phenotype through heterotypic signaling of nearby cells remains to be determined.
DACH1 inhibits breast cancer cellular migration and metastasis in vivo through inhibition of IL8 secretion. Immunoneutralizing antibody and antisense experiments in vivo demonstrated that DACH1 is a potent inhibitor of breast cancer spread to the lungs of mice [54]. Previous studies implicated DACH1 in regulating migration via the RhoA/ROCK/LIM kinase cytoskeletal signaling pathway [44]. Molecular analysis of the mechanisms by which DACH1 inhibits AP-1 activity demonstrates that DACH1 inhibits c-Fos expression. Mutational analysis identified the SRF1 binding site within the c-Fos promoter as the target of DACH1 repression. Serum-induced c-Fos promoter activity is reduced by point mutations in both TCF and SRF binding sites, while the SRF site of the c-Fos promoter is induced by RhoA, independently of MAP kinases [55, 56]. Induction of SRF by RhoA involves ROCK and phosphorylation of LIM kinase [57], and its activation responds to a decrease in the cellular pool of monomeric actin and stabilization of polymerized actin (F-actin). The finding that DACH1 inhibits gene transcription through SRF [44] raises the possibility that DACH1 may regulate the RhoA/ROCK/LIM kinase cytoskeletal signaling pathway. Such speculation is supported by genome-wide molecular analysis in which DACH1 regulates expression of cytoskeletal proteins regulating cellular migration in breast cancer cells [7]. Collectively these studies demonstrate a pivotal role for DACH1 in attenuating the migratory and metastatic phenotype of breast cancer cells. Further studies will be required to determine if this is a general principal in other hormone-responsive tumors.
SIX1 overexpression identified in myxoid chondosarcomas is linked to the metastatic process [58]. Six1 induces Ezrin expression, while ezrin siRNA reverses the invasive effects of SIX1 in a rhabdoid sarcoma cell line. Ezrin is encoded by the Vilt gene and through association with CD4, an intercellular adhesion molecule, regulates cellular morphology. In addition, Ezrin overexpression has been implicated in the metastatic phenotypes of mammary and pancreatic adenocarcinoma, myosarcoma, and rhabdoid sarcoma [59]. DACH1 inhibits oncogene-induced polarity [31], which is characterized by altered distribution of the ezrin-radixin-moesin (ERM) complex [60]. Thus, the role of DACH1 in regulating Ezrin expression will be of interest. It is known that cyclin D1, which is repressed by DACH1, can enhance cellular migration [61–63] and regulate the abundance and activity of RhoA, which plays a key role in the estrogen-mediated metastatic potential of rhabdoid sarcoma [58, 64]. Six1 has been shown to induce invasiveness as well as cyclin D1 levels [26, 28]. It will be of interest to examine the role of cyclin D1 in Six1-dependent invasion.
Conclusion
Recent studies demonstrated altered DACH1 expression in hormone responsive cancers (breast, ovary, prostate and uterus) [23, 24, 26] and that DACH1 regulates hormone-dependent signaling [23, 24]. Immunohistochemical studies demonstrated the colocalization of DACH1 with ERα in breast cancer and DACH1 with AR in normal prostate [23, 24]. DACH1 inhibits ligand-dependent transactivation of the ERα and AR in cultured cells. DACH1 has been identified using ChIP assays in the context of local chromatin. DACH1 co-repression appears to involve both NAD and TSA-dependent histone deacetylase activity. Loss of DACH1 expression and/or relocalization within the cell occurs in tumorous breast and prostate cells. As DACH1 encodes a key component of the RDGN governing cell fate in several species, the intersection of the RDGN with hormone-receptor signaling may be important in normal development and tumorigenesis. As DACH1 inhibits oncogene-dependent function in cell culture, future studies will be required to determine whether DACH1 functions as an endogenous tumor suppressor in vivo.
Several key questions remain unanswered. First, at this time only a small subset of nuclear receptors have been examined for functional interactions with DACH1. What additional nuclear receptors interact with DACH1? What other hormone pathways are regulated by DACH1? Second, it will be important to determine the additional cell types in which DACH1 may regulate hormone signaling. In addition to breast, prostate and ovary, for example, pituitary, thyroid, endocrine pancreas and several other hormone-responsive cell types express DACH1; however, the function of DACH1 in these tissues remains undetermined. Third, DACH1 transcriptional repression is in part NAD-dependent. The biological and biochemical significance of this property and its potential role in NAD-dependent processes remains to be determined. Fourth, it will be important to characterize the biochemical components of the DACH1 multiprotein complex. The RDGN in Drosophila is regulated in a phosphatase-dependent manner. At this time, it is unknown whether other components of the RDGN modify or regulate hormone signaling activity of the DACH1 complex. Fifth, the x-ray crystal structure of DACH1 raises the possibility that DACH1 directly binds DNA, potentially in a DNA sequence-specific manner. Although no published data exist at this time to support this speculation, analysis appears warranted. Finally, the DACH1 gene encodes multiple distinct isoforms. Characterization of these isoforms, their expression, subcellular distribution and function may provide important insights into this novel mechanism regulating hormonal signaling.
Figure I. RDGN hierarchy.
Toy leads to ey expression, which leads to expression of so, eya and dac. eya, dac and so which then promotes expression of ey and also interacts with other members of RDGN
Acknowledgements
We thank Atenssa L. Cheek for assistance in preparing this manuscript. Support for this work was provided by R01CA70896, R01CA75503, R01CA86072, R01CA86071 (R.G.P.) and the Susan Komen Breast Cancer Foundation (BCTR0504227, C.W.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core grant P30CA56036 (R.G.P.). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust (R.G.P.), grants from Pennsylvania Department of Health (R.G.P. and C.W.) and supported by the Margaret Q. Landenberger Research Foundation (K.W.). The Department specifically disclaims responsibility for an analysis, interpretations or conclusions. There are no conflicts of interest associated with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai J, Montell D. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development. 2002;129:5377–5388. doi: 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- 2.Fabrizio JJ, et al. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol. 2003;258:117–128. doi: 10.1016/s0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 3.Bonini NM, et al. Multiple roles of the eyes absent gene in Drosophila. Dev Biol. 1998;196:42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- 4.Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development. 2001;128:1643–1656. doi: 10.1242/dev.128.9.1643. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez L, et al. Sex determination genes control the development of the Drosophila genital disc, modulating the response to Hedgehog, Wingless and Decapentaplegic signals. Development. 2001;128:1033–1043. doi: 10.1242/dev.128.7.1033. [DOI] [PubMed] [Google Scholar]
- 6.Boyle M, et al. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–982. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- 7.Wu K, et al. DACH1 inhibits transforming growth factor-beta signaling through binding Smad4. J Biol Chem. 2003;278:51673–51684. doi: 10.1074/jbc.M310021200. [DOI] [PubMed] [Google Scholar]
- 8.Li X, et al. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, et al. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol. 2002;22:6759–6766. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozmik Z, Cvekl A. Localization of the human homologue of the Drosophila dachshund gene (DACH) to chromosome 13q21. Genomics. 1999;59:110–111. doi: 10.1006/geno.1999.5797. [DOI] [PubMed] [Google Scholar]
- 11.Kim SS, et al. Structure of the retinal determination protein Dachshund reveals a DNA binding motif. Structure. 2002;10:787–795. doi: 10.1016/s0969-2126(02)00769-4. [DOI] [PubMed] [Google Scholar]
- 12.Machon O, et al. Yeast two-hybrid system identifies the ubiquitin-conjugating enzyme mUbc9 as a potential partner of mouse Dac. Mechanisms of Development. 2000;97:3–12. doi: 10.1016/s0925-4773(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 13.Ayres JA, et al. DACH: genomic characterization, evaluation as a candidate for postaxial polydactyly type A2, and developmental expression pattern of the mouse homologue. Genomics. 2001;77:18–26. doi: 10.1006/geno.2001.6618. [DOI] [PubMed] [Google Scholar]
- 14.Caubit X, et al. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev Dyn. 1999;214:66–80. doi: 10.1002/(SICI)1097-0177(199901)214:1<66::AID-DVDY7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Davis RJ, et al. Mouse Dach, a homologue of Drosophila dachshund, is expressed in the developing retina, brain and limbs. Dev Genes Evol. 1999;209:526–536. doi: 10.1007/s004270050285. [DOI] [PubMed] [Google Scholar]
- 16.Hammond KL, et al. Mammalian and drosophila dachshund genes are related to the ski proto-oncogene and are expressed in eye and limb. Mech. Dev. 1998;74:121–131. doi: 10.1016/s0925-4773(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 17.Kozmik Z, et al. Molecular cloning and expression of the human and mouse homologues of the drosophila dachshund gene. Genes Evol. 1999;209:537–545. doi: 10.1007/s004270050286. [DOI] [PubMed] [Google Scholar]
- 18.Mardon G, et al. Dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 19.Davis RJ, et al. Dach1 mutant mice bear no gross abnormalities in eye, limb, and brain development and exhibit postnatal lethality. Mol. Cell. Biol. 2001;21:1484–1490. doi: 10.1128/MCB.21.5.1484-1490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backman M, et al. Targeted disruption of mouse Dach1 results in postnatal lethality. Dev Dyn. 2003;226:139–144. doi: 10.1002/dvdy.10210. [DOI] [PubMed] [Google Scholar]
- 21.Davis RJ, et al. Mouse Dach2 mutants do not exhibit gross defects in eye development or brain function. Genesis. 2006;44:84–92. doi: 10.1002/gene.20188. [DOI] [PubMed] [Google Scholar]
- 22.Davis RJ, et al. Mouse Dach1 and Dach2 are redundantly required for Mullerian duct development. Genesis. 2008;46:205–213. doi: 10.1002/dvg.20385. [DOI] [PubMed] [Google Scholar]
- 23.Popov VM, et al. The Cell Fate Determination Factor DACH1 is expressed in Estrogen Receptor α (ERα) positive breast cancer and represses ERα signaling. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-3992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu K, et al. The cell fate determination factor dachshund inhibits androgen receptor signaling and prostate cancer cellular growth. Cancer Res. 2009;69:3347–3355. doi: 10.1158/0008-5472.CAN-08-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunde JS, et al. Expression Profiling Identifies Altered Expression of Genes That Contribute to the Inhibition of Transforming Growth Factor-{beta} Signaling in Ovarian Cancer. Cancer Res. 2006;66:8404–8412. doi: 10.1158/0008-5472.CAN-06-0683. [DOI] [PubMed] [Google Scholar]
- 26.Wu K, et al. DACH1 is a cell fate determination factor that inhibits Cyclin D1 and breast tumor growth. Mol Cell Biol. 2006;26:7116–7129. doi: 10.1128/MCB.00268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nan F, et al. Altered expression of DACH1 and cyclin D1 in endometrial cancer. Cancer Biol Ther. 2009;8 doi: 10.4161/cbt.8.16.8963. in press. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, et al. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66:1982–1989. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- 29.Zuber ME, et al. Giant eyes in Xenopus laevis by overexpression of XOptx2. Cell. 1999;98:341–352. doi: 10.1016/s0092-8674(00)81963-7. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, et al. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozaki H, et al. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- 32.Laclef C, et al. Altered myogenesis in Six1-deficient mice. Development. 2003;130:2239–2252. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- 33.Winchester C, et al. Expression of a homeobox gene (SIX5) in borderline ovarian tumours. J Clin Pathol. 2000;53:212–217. doi: 10.1136/jcp.53.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laflamme C, et al. The homeotic protein Six3 is a coactivator of the nuclear receptor NOR-1 and a corepressor of the fusion protein EWS/NOR-1 in human extraskeletal myxoid chondrosarcomas. Cancer Res. 2003;63:449–454. [PubMed] [Google Scholar]
- 35.Li CM, et al. Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. The American journal of pathology. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan J, et al. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci U S A. 1999;96:13264–13269. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coletta RD, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichenberger KJ, et al. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Res. 2005;65:2668–2675. doi: 10.1158/0008-5472.CAN-04-4286. [DOI] [PubMed] [Google Scholar]
- 39.Fu M, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouras T, et al. SIRT1 deacetylation and repression of P300 involves lysine residues 1020/1024 within the cell-cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 41.Whittle JR, et al. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–364. doi: 10.1016/j.tem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, et al. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 43.Liao G, et al. Regulation of androgen receptor activity by the nuclear receptor co-repressor SMRT. J Biol Chem. 2003;278:5052–5061. doi: 10.1074/jbc.M206374200. [DOI] [PubMed] [Google Scholar]
- 44.Wu K, et al. The Cell Fate Determination Factor DACH1 Inhibits c-Jun Induced Contact-Independent Growth. Mol Biol Cell. 2007;18:755–767. doi: 10.1091/mbc.E06-09-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vadlamudi RK, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem. 2001;276:38272–38279. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 46.Mishra SK, et al. Upstream determinants of estrogen receptor-alpha regulation of metastatic tumor antigen 3 pathway. J Biol Chem. 2004;279:32709–32715. doi: 10.1074/jbc.M402942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra SK, et al. Cloning and functional characterization of PELP1/MNAR promoter. Gene. 2004;330:115–122. doi: 10.1016/j.gene.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Vadlamudi RK, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horner A, et al. Fibroblast growth factor signaling regulates Dach1 expression during skeletal development. Dev Dyn. 2002;225:35–45. doi: 10.1002/dvdy.10132. [DOI] [PubMed] [Google Scholar]
- 50.Kumagai T, et al. Alteration of gene expression in response to bone morphogenetic protein-2 in androgen-dependent human prostate cancer LNCaP cells. Int J Mol Med. 2006;17:285–291. [PubMed] [Google Scholar]
- 51.Schulze A, et al. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soule HD, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 53.Fu M, et al. Minireview: Cyclin D1: Normal and Abnormal Functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 54.Wu K, et al. Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8. Proc Natl Acad Sci U S A. 2008;105:6924–6929. doi: 10.1073/pnas.0802085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sotiropoulos A, et al. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 56.Miralles F, et al. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 57.Hill CS, et al. The Rho family of Rho A, Rac 1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 58.Yu Y, et al. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 59.Curto M, McClatchey AI. Ezrin…tastatic detERMinant? Cancer Cell. 2004;5:113–114. doi: 10.1016/s1535-6108(04)00031-5. [DOI] [PubMed] [Google Scholar]
- 60.Ju X, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci U S A. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, et al. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Z, et al. Cyclin D1 induction of cellular migration requires p27KIP1. Cancer Res. 2006;66:9986–9994. doi: 10.1158/0008-5472.CAN-06-1596. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, et al. Cyclin D1 functions in cell migration. Cell Cycle. 2006;5:2440–2442. doi: 10.4161/cc.5.21.3428. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen W, Mardon G. Ectopic eye development in Drosophila induced by directed dachshund expression. Development. 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- 66.Heberlein U, Treisman JE. Early retinal development in Drosophila. Results Probl Cell Differ. 2000;31:37–50. doi: 10.1007/978-3-540-46826-4_3. [DOI] [PubMed] [Google Scholar]
- 67.Seimiya M, Gehring WJ. The Drosophila homeobox gene optix is capable of inducing ectopic eyes by an eyeless-independent mechanism. Development. 2000;127:1879–1886. doi: 10.1242/dev.127.9.1879. [DOI] [PubMed] [Google Scholar]
- 68.Pappu KS, Mardon G. Genetic control of retinal specification and determination in Drosophila. Int J Dev Biol. 2004;48:913–924. doi: 10.1387/ijdb.041875kp. [DOI] [PubMed] [Google Scholar]