Abstract
Efforts to identify differential or core cognitive deficits in schizophrenia have been made for several decades, with limited success. Part of the difficulty in establishing a cognitive profile in schizophrenia is the considerable inter-patient heterogeneity in the level of cognitive impairment associated with this condition. Thus, it may be useful to examine the presence of relative cognitive weaknesses on an intra-patient level. In the present study we examined the rates of significant intra-person differences between crystallized verbal ability versus five other cognitive abilities among 127 persons with schizophrenia or schizoaffective disorder and 127 demographically matched normal comparison (NC) subjects. We found that the rates of significant discrepancies above the NC group base-rates was significantly greater in reference to those discrepancies involving visual memory relative to those associated with auditory memory, working memory, processing speed, and perceptual organization. The findings conflict with prior suggestions that working memory or auditory episodic memory are differential or core deficits in schizophrenia, and highlight the importance of considering visual memory in characterizing the cognitive effects of this condition.
Keywords: psychosis, neuropsychology, neurocognitive, heterogeneity, idiographic
1. INTRODUCTION
Much neuropsychological research on schizophrenia over the last three decades has been motivated by the hope that discovery of specific neurocognitive profiles may elucidate the underlying neuropathology of the disorder or foster development of effective rehabilitation programs (Palmer, Dawes, & Heaton, 2009). The answer to one basic question remains unclear: are there differential or “core” cognitive deficits in schizophrenia? A variety of cognitive domains have been proposed as the “core” deficit in schizophrenia: attention (Barr, 2001; Elvevag & Goldberg, 2000; Hilti et al., 2008), working memory (Elvevag & Goldberg, 2000; Forbes et al., 2009; Goldman-Rakic, 1994; Lee & Park, 2005; Mitropoulou et al., 2005; Silver et al., 2003), processing speed (Rodriguez-Sanchez et al., 2007), episodic memory (Aleman et al., 1999; Gur et al., 2000; Palmer et al., 1997; Saykin et al., 1991; Whyte et al., 2005), and executive function (Wobrock et al., 2008; Zec, 1995).
Meta-analyses indicate episodic memory tests have the largest or near largest effect sizes in schizophrenia patients versus normal comparison (NC) subjects (Fioravanti et al., 2005; Heinrichs & Zakzanis, 1998; Mesholam-Gately et al., 2009), supporting the century-long focus on temporal/hippocampal and frontal–subcortical regions as prime suspects in the neurogenesis of schizophrenia (Goldstein, 1939; Kraepelin, 1913). However, it is difficult to draw definitive conclusions due to heterogeneity in effect sizes between studies (Fioravanti et al., 2005). Part of the difficulty establishing differential cognitive impairment in schizophrenia rests in the marked inter-patient heterogeneity in level and pattern of cognitive impairment. On average, schizophrenia is associated with mild-to-moderate cognitive impairment, but 20–30% of patients have normal range neurocognition (Palmer et al., 2009). Thus, it may be useful to examine the presence of relative cognitive weaknesses on an intra-patient level.
The present study examined within-person cognitive differences among 127 patients with schizophrenia and 127 NC subjects. Cognition was measured with the Index scores from the 6-factor model for Wechsler Adult Intelligence Scale – Third Edition (WAIS-III)/Wechsler Memory Scale – Third Edition (WMS-III) (The Psychological Corporation [TPC], 1997; Tulsky et al., 2003).
Crystallized knowledge is relatively unaffected by schizophrenia and many other neurocognitive disorders (Allen et al., 1998; Dickinson & Coursey, 2002; Iverson et al., 2006), and has the strongest correlation with Full Scale IQ (TPC, 1997), so the Verbal Comprehension Index (VCI) was used as a marker of general cognitive ability. We hypothesized that VCI scores would be higher than each of the other five cognitive abilities for a significantly larger proportion of people with schizophrenia relative to the proportion among NC subjects. Given the effect sizes for episodic memory in means comparisons (Heinrichs & Zakzanis, 1998), and long-held suspicion of the frontal and temporal regions in neurogenesis of schizophrenia (Palmer et al., 2009), as well as cogent models of working memory as a core deficit underlying many facets of schizophrenia (Goldman-Rakic, 1994), we hypothesized that discrepancies with VCI would be particularly common among schizophrenia patients when evaluated in reference to episodic memory and working memory.
2. METHODS
2.1 Participants
Participants included 127 persons with schizophrenia or schizoaffective disorder and 127 NC subjects. Patient data were collected as part of baseline evaluations for a study of medication adherence among middle-aged and older patients. NC data were derived from the WAIS-III/WMS-III standardization sample.
Inclusion criteria for patients were: i) DSM-IV-TR diagnosis of schizophrenia or schizoaffective disorder determined with the Structured Clinical Interview for the DSM-IV-TR (First et al., 2002); ii) age > 40; iii) outpatient status; iv) current antipsychotic medication; and v) written consent for participation. Exclusion criteria were known diagnosis of dementia, or Mini Mental State Exam total ≤ 20 (Folstein, Folstein, & McHugh, 1975). Recruitment sources included the University of California, San Diego, Veterans Affairs San Diego Healthcare System outpatient psychiatry services, and San Diego area assisted living facilities and physicians.
NC subjects were screened to exclude those with uncorrected sensory impairments and other conditions that might affect cognitive functioning or test performance (TPC, 1997). NC data were provided by the test publisher using one-to-one matching (as closely as possible) based on age, education, sex, and ethnicity.
2.2 Measures
2.2.1 Demographics
Age, education, sex, and ethnicity were determined by self-report.
2.2.2 Clinical characteristics
Patients’ medications were determined via interview or record review (10% received conventional neuroleptics, 69% second generation antipsychotics, and 21% a combination; 51% were also on anticholinergic medication). Patients’ severity of psychopathology was assessed with the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler).
2.2.3 Neurocognitive assessment
Cognitive functioning was evaluated with the WAIS-III/WMS-III battery 6-factor Index scores (Tulsky et al., 2003; Tulsky & Price, 2003), including:
Verbal Comprehension (VCI): Vocabulary, Information, Similarities
Perceptual Organization (POI): Block Design, Picture Completion, Matrix Reasoning
Processing Speed (PSI): Digit Symbol, Symbol Search
Working Memory (WMI): Spatial Span, Letter Number Sequencing
Auditory Memory (AMI): Logical Memory I (immediate recall) and II (delayed recall), Verbal Paired Associates I (immediate recall) and II (delayed recall)
Visual Memory (VMI): Family Pictures Recall I (immediate recall) and II (delayed recall), Visual Reproductions I (immediate recall) and II (delayed recall).
Raw scores were converted to Index scores (normative mean = 100, SD = 15; higher scores represent better performance) using the published norms (Tulsky et al., 2003; Wechsler, 1997a; b).
2.3 Analyses
Demographic and Index scores among patients versus NC subjects were compared with t-tests or Pearson Chi-square, as appropriate.
Cut-scores defining “significant” (p<.05) discrepancy between VCI and other Index scores were calculated with the “simple difference” method (Wechsler, 1997a):
wherein z = 1.96; the Standard Error of Measurement (SEM) for each Index score was determined from published norms for the overall standardization sample (TPC, 1997; Tulsky et al., 2003). We tallied the proportion of subjects for whom the VCI was better than, worse than, or not reliably different from the compared Index score.
We examined the degree of concordance in specific discrepancy score patterns within each patient-NC matched pair. For each of the five discrepancy scores, within each subject, we determined whether the comparator index score was significantly lower than his/her VCI. If the result was the same in the patient and NC in a pair, that pair was assigned a score of 0. If the comparison indicated VCI>comparator index score in the patient but not the NC subject, that pair was assigned a score of +1. IfVCI>comparator in the NC subject but not the patient, that pair was assigned a score of −1. The null hypothesis of no diagnostic effect (number of +1s/[number +1s plus number −1s] = 50%) was evaluated with binomial tests. Friedman’s test was used to determine whether the pattern of differential proportions of significant discrepancies differed among the five types of discrepancy scores. Wilcoxon Signed Ranks Test was used to identify which discrepancy scores types were associated with the largest differences among patients versus NC subjects.
As Baddeley’s (2007) model of working memory includes separate channels for visual-spatial versus auditory information, we also calculated the rates of significant discrepancies between VCI and the WMI visual (Spatial Span) and auditory (Letter Number Sequencing) subtests. Also, verbal processes may affect the Family Pictures subtest (Chapin, Busch, Naugle, & Najm, 2009; Dulay et al., 2002), so we conducted similar analyses using the immediate and delayed recall scores from the VMI Family Pictures and Visual Reproductions subtests.
We also calculated Spearman’s rho correlations between the magnitude of the five primary discrepancy scores with severity of symptoms (PANSS), chlorpromazine equivalent daily antipsychotic dose (Jeste & Wyatt, 1982; Woods, 2003) and benztropine equivalent dose (de Leon, Canuso, White, & Simpson, 1994). Significance was defined as p <.05, two-tailed, for all tests.
3. RESULTS
3.1 Participant characteristics
Demographic characteristics, clinical ratings, and cognitive scores are summarized in Table 1. Patients had mild levels of psychopathology. Patients had worse cognitive performance than NC subjects all six Index scores (t-values > 7.70, p-values <. 001).
Table 1.
Comparison of demographic, clinical, and cognitive characteristics of healthy comparison subjects versus schizophrenia patients.
| Normal Comparison Subjects (N = 127) | Schizophrenia (N = 127) | t or X2 | df | p-level | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) | 52.1 (8.5) | 52.8 (7.1) | 0.72 | 245.0 | .471 |
| Education | 5.31 | 4 | .257 | ||
| ≤ 8 years | 5.5% | 4.9% | |||
| 9 to 11 years | 14.2% | 22.8% | |||
| 12 years | 42.5% | 43.9% | |||
| 13 to 15 years | 20.5% | 18.7% | |||
| ≥ 16 years | 17.3% | 9.8% | |||
| Gender (% men) | 57.5% | 64.6% | 1.34 | 1 | .247 |
| Ethnic Background | 0.19 | 2 | .909 | ||
| (non-Hispanic) Caucasian | 81.1% | 80.3% | |||
| African American | 11.0% | 12.6% | |||
| Other | 7.9% | 7.1% | |||
| Clinical characteristics | |||||
| Psychiatric diagnosis | |||||
| % Schizophrenia | n/a | 66.2 | |||
| % Schizoaffective Disorder | n/a | 33.8 | |||
| Severity of psychopathology (PANSS subscale scores) | |||||
| Positive subscale total | n/a | 14.4 (6.0) | |||
| Negative subscale total | n/a | 13.4 (4.7) | |||
| General subscale total | n/a | 27.4 (7.7) | |||
| Cognitive performance | |||||
| Verbal Comprehension Index | 100.2 (13.7) | 86.4 (14.9) | 7.71 | 252.0 | <.001 |
| Perceptual Organization Index | 99.2 (13.3) | 79.1 (11.6) | 12.81 | 250.0 | <.001 |
| Processing Speed Index | 99.3 (13.6) | 73.7 (10.1) | 17.00 | 232.2 | <.001 |
| Working Memory Index | 100.3 (13.8) | 79.3 (15.8) | 11.21 | 249.0 | <.001 |
| Auditory Memory Index | 100.3 (14.7) | 78.0 (15.3) | 11.87 | 252 | <.001 |
| Visual Memory Index | 99.6 (14.8) | 67.7 (13.2) | 18.02 | 248 | <.001 |
Note: Values represent means (and SDs) for continuous variables or proportions (percent) for categorical variables. Differences between groups are calculated via t-tests for continuous variables and chi-squares for categorical variables.
Key to abbreviations: n/a = not applicable; PANSS = Positive and Negative Syndrome Scale
3.2 VCI-comparator Index discrepancies
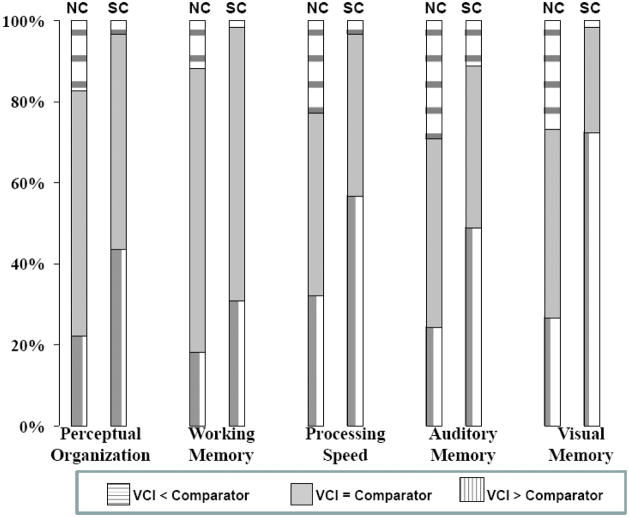
Frequencies of significant differences between VCI and each of the five comparator Index scores are illustrated in Figure 1 by diagnostic group. Among matched patient-NC pairs discordant regarding whether VCI> comparator Index score, the member with VCI>comparator Index was significantly more likely to be from the patient group for each discrepancy score type (exact binomial tests, all p-values <.015) (see Table 2). Significant variance was present among the five discrepancy score types with regard to the magnitude of these differences in proportion, X2 (4, N=118) = 28.30, p <.001. The highest discordance rates were found for VCI>VMI, and the next widest discordance rates were those for VCI>PSI. When the data involving the VMI were excluded, the overall difference between discrepancy score types in the magnitude of these proportions was not statistically significant, X2 (3, N=120) = 6.40, p = .094. When data for PSI were excluded, but those for VMI were retained, the overall differences between discrepancy score types in the magnitude of these proportions remained statistically significant (X2 (3, N=122) = 29.06, p <.001). The proportional differences associated with the VMI were significantly greater than those associated with each of the other discrepancy score types (all zs > 2.65, all ps <.007). The proportional differences associated with PSI were significantly greater than those associated with WMI (z=2.24, p = .030), but no other pairwise comparisons were statistically significant (all zs < 1.51, ps >.074)
Figure 1.
Table 2.
Pattern of discordant results within the patient-NC matched pairs in regard to whether each comparator index score is significantly lower than the individual’s verbal comprehension index score.
| Pattern of Discordance |
||||
|---|---|---|---|---|
| Comparator Index | Number of Discordant SC-NC pairs | SC VCI > Comparator & NC VCI ≤ Comparator | SC VCI ≤ Comparator & NC VCI > Comparator | p |
| Perceptual Organization | 55 | 41 (75%) | 14 (25%) | .001 |
| Working Memory | 43 | 30 (70%) | 13 (30%) | .014 |
| Processing Speed | 58 | 47 (81%) | 11 (19%) | <.001 |
| Auditory Memory | 66 | 49 (74%) | 17 (26%) | <.001 |
| Visual Memory | 68 | 64 (94%) | 4 (6%) | <.001 |
Note: “Discordant pairs” are those in which the patient and NC in a given pair had discordant results in regard to whether his or her comparator score was significantly worse than his or her VCI score. The p-values reflect binomial test against the null hypothesis that, among such discordant pairs, the pair member whose comparator score is lower than VCI is equally likely to be from the patient versus NC group (i.e., the null hypothesis is that two proportions both equal 50%).
Key to abbreviations: schizophrenia = SC; normal comparison subject = NC, Verbal Comprehension Index = VCI
3.3. Selected subtests
There were no significant diagnostic effects among patient-NC matched pairs discordant regarding whether VCI > Spatial Span or Letter Number Sequencing (exact binomial tests, Spatial Span p = .652 and Letter Number Sequencing p = .272) (see Table 3). On the other hand, the proportional differences associated with the full WMI were significantly different from those associated with Spatial Span (z = 3.40, p <.001), but not from those associated with the Letter Number Sequencing (z = 1.29, p = .207). [In 70% of discordant pairs for WMI, it was the patient member who evidenced VCI >WMI, whereas for Spatial Span only 45% of the discordant pairs had the patient with VCI>Spatial Span.] Among pairs discordant with regard to whether VCI>Visual Reproductions I or II, or Family Pictures I or II, the pair member showing the lower subscale component was significantly more likely to be from the patient group for three of the four VMI component scores (exact binomial tests, p-values <.001) (Table 3). The one exception to the latter pattern was VCI>Visual Reproductions II (delayed recall), for which there were no significant diagnostic effects.
Table 3.
Selected subtest comparisons.
| Pattern of Discordance |
||||
|---|---|---|---|---|
| Comparator Score | Number of Discordant SC-NC pairs | SC VCI > Comparator & NC VCI ≤ Comparator | SC VCI ≤ Comparator & NC VCI > Comparator | p |
| Working Memory component scores | ||||
| Spatial Span | 44 | 20 (45%) | 24 (55%) | .652 |
| Letter Number Sequencing | 53 | 31 (58%) | 22 (42%) | .272 |
| Visual Memory component scores | ||||
| Family Pictures Immediate | 57 | 45 (79%) | 12 (21%) | <.001 |
| Family Pictures Delayed | 52 | 43 (83%) | 9 (17%) | <.001 |
| Visual Reproductions Immediate | 58 | 51 (88%) | 7 (12%) | <.001 |
| Visual Reproductions Delayed | 31 | 15 (48%) | 16 (52%) | 1.00 |
Note: “Discordant pairs” are those in which the patient and NC in a given pair had discordant results in regard to whether his or her comparator score was significantly worse than his or her VCI score. The p-values reflect binomial test against the null hypothesis that, among such discordant pairs, the pair member whose comparator score is lower than VCI is equally likely to be from the patient versus NC group (i.e., the null hypothesis is that two proportions both equal 50%).
Key to abbreviations: schizophrenia = SC; normal comparison subject = NC, Verbal Comprehension Index = VCI
3.4. Association with symptoms and medications
There were no significant correlations between antipsychotic dose or anticholinergic dose and the magnitude of the five cognitive discrepancy scores (absolute value of all rhos < .09, all p-values > .485). Severity of negative symptoms was inversely correlated with the discrepancy between VCI and POI (rho = −.244, p = .006), VCI and PSI (rho = −.211, p = . 021), and VCI and WMI (rho = −.193, p = .032). In particular, more severe negative symptoms were associated with smaller VCI-comparator discrepancies. It might be noted, however, that severity of negative symptoms was also correlated with lower VCI scores (rho = −.349, p <.001), which may explain the diminished magnitude of VCI-comparator discrepancies. There were no other statistically significant correlations between severity of psychopathologic symptoms and the magnitude of VCI-comparator discrepancies.
4. DISCUSSION
As hypothesized, crystallized knowledge was significantly higher than each of the other five cognitive abilities for a larger proportion of people with schizophrenia than for NC subjects. The hypotheses concerning episodic memory and working memory were only partially confirmed; visual episodic memory was associated with higher rates of significant discrepancies than other ability areas, but the rates associated with auditory episodic memory and working memory were not significantly greater than those associated with other cognitive domains. In fact, there was more within-person impairment in processing speed than in working memory among persons with schizophrenia.
Limitations of this study include restriction of the sample to middle-aged and older persons, so the generalizability to persons under age 40 is not known. But, age and duration of illness have no discernable effects on level or pattern of cognitive impairment in schizophrenia (Kurtz, 2005), so there is no reason to expect a different pattern with young adult patients. Also, matching patients and NC subjects on education may obscure some schizophrenia-related cognitive deficits (Kremen et al., 1996), but the reliability of differences (and therefore the size of the confidence interval defining a significant difference) may also be affected by education. Thus, if we had not used an education-matched NC sample, group comparisons of the frequency of within-person differences in cognitive performance could have been distorted. Another limitation is that our test battery lacked an executive function Index, so the possibility of differential executive impairment was not examined. On the other hand, meta-analyses have not found executive function tests to yield differentially large effect sizes in terms of patient versus NC group mean comparisons (Dickinson, Ramsey, & Gold, 2007).
Although we hypothesized that schizophrenia patients would show particular impairment in episodic memory and working memory, we did not anticipate this effect to be limited to visual episodic memory alone. Meta-analyses indicate that, if anything, the effect sizes may be higher for auditory versus visual memory tests (Aleman et al., 1999; Dickinson et al., 2007; Heinrichs & Zakzanis, 1998; Mesholam-Gately et al., 2009). It is possible that the VMI was contaminated by the verbal processes which may be involved in the Family Pictures subtest, but the diagnostic effect was present for the immediate recall scores from both Visual Reproductions and Family Pictures, as well as Family Pictures delayed recall. The lack of a significant effect for the delayed recall Visual Reproductions task is difficult to interpret, as immediate and delayed recall memory scores tend to be highly correlated in schizophrenia and other non-amnestic populations (Aleman et al., 1999; Millis, Malina, Bowers, & Ricker, 1999; Price, Tulsky, Millis, & Weiss, 2002). Skelley et al. (2008) found that schizophrenia patients had large effect size decrements, relative to NC subjects, on visual as well as auditory memory tasks, but healthy siblings of schizophrenia patients showed decrements only on the auditory memory tasks. Disentangling factors associated with phenotypic expression of schizophrenia from the genetic risk factors has obvious merit, so further investigation of visual memory deficits across the premorbid, peri-onset, and chronic course of schizophrenia are warranted.
Despite the above limitations, some aspects of the present findings remain noteworthy. Although diagnostic status of schizophrenia was associated with worse working memory, there was no indication of a differential impairment in working memory relative to other cognitive abilities. Moreover, the diagnostic effects on the visual and spatial working memory subtests were non-robust, as the diagnostic effects were observed only when examined in terms of the full WMI (not its component subtests.) After VMI, the ability showing the next strongest diagnostic effects was processing speed. The latter is notable given Dickinson et al.’s (2007) meta-analytic report that the mean effect sizes decrements of schizophrenia patients relative to NC subjects on working memory tasks ranged from d = 0.61 to d = 1.18, whereas the effect sizes for psychomotor/processing speed was d = 1.57. Indeed, we found that the diagnostic effect for PSI was significantly higher than that for WMI. A given deficit may be more fundamental or “core” without being noticeably differentially impaired (depending on its position within a longer causal chain or sequence). However, if working memory (or any other ability area) is to be viewed as a credible “core deficit,” it is necessary to develop testable causal-chain models predicting the relationship of that deficit to other manifestations of the disorder.
Given the largely negative findings regarding differential impairment, one may ask whether the present analyses lacked statistical power to detect differential deficits. It is worth reiterating that our use of discrepancy score analyses is intended as a complementary (rather than inherently superior to a means comparisons) approach to the question of differential impairment, albeit one that parallels that frequently used in clinical test interpretation. However, aside from the open question regarding specific impairment in visual memory, the overall pattern is consistent with those using single common factor analyses reported by Dickinson and colleagues (Dickinson, Iannone, Wilk, & Gold, 2004; Dickinson, Ragland, Gold, & Gur, 2008).
Based on the findings from recent studies focused on mean comparisons (Dickinson et al., 2007; Mesholam-Gately et al., 2009) including findings from our own group (Gladsjo et al., 2004), a tenable alternative hypothesis would have been for differential within-patient impairment in processing speed. However, we focused on episodic memory and working memory because of the long-standing interest in frontal-temporal regions in the genesis of schizophrenia (reviewed in Palmer et al., 2009). Working memory is also of special interest because of Goldman-Rakic’s (1994) model relating working memory deficits to other features of schizophrenia. Even if disproven, the latter model provides an exemplary illustration of the type of process model, grounded in the clinical and neuroscience literature, needed to move neurocognitive research in schizophrenia away from observational studies and toward model building and testing. The present findings, in concert with those reported in reference to group means comparisons, may provide impetus to develop process models of the nature and impact of visual memory and processing speed deficits in schizophrenia (cf. Salthouse, 1996). Also, the difficulty in identifying a meaningful neurocognitive pattern in schizophrenia reflects, in part, the extremely heterogeneous nature of this disorder. Integration of the intra-person examination of performance patterns as illustrated in the present study, together with cluster analyses of those patterns (cf. Dawes et al., 2008), and in concert with studies based on other measures of brain structure and function, may ultimately reveal more meaningful neurobiological subtypes.
Acknowledgments
This work was supported, in part, by NIH grants MH062849, MH64722, T32MH019934, 5P30MH066248, and the Department of Veterans Affairs. Standardization data from the Wechsler Adult Intelligence Scale® – Third Edition. Copyright © 1997 by NCS Pearson, Inc. Used with permission. All rights reserved. Standardization data from the Wechsler Memory Scale® – Third Edition. Copyright © 1997 by NCS Pearson, Inc. Used with permission. All rights reserved. We also thank Drs. Sharokh Golshan and Helena Kraemer for feedback and advice on the statistical analyses included in this report and Ms. Rebecca Daly for assistance in creating the combined dataset for analysis.
Role of funding source. This work was supported, in part, by NIH grants MH062849, MH64722, T32MH019934, 5P30MH066248, and the Department of Veterans Affairs. The NIMH and Department of Veterans Affairs had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Standardization data from the Wechsler Adult Intelligence Scale® – Third Edition. Copyright © 1997 by NCS Pearson, Inc. Used with permission. All rights reserved. Standardization data from the Wechsler Memory Scale® – Third Edition. Copyright © 1997 by NCS Pearson, Inc. Used with permission. All rights reserved. NCS Pearson, Inc. reviewed this report prior to submission for publication but was not otherwise involved in the design of the present study, interpretation of the results, or preparation or revision of this report.
Footnotes
Conflict of Interest. All of the authors declare that they have no conflicts of interest.
Contributors
Dr. Palmer designed the study, planned the analyses, conducted some of the literature searches and review, and some of the data analyses, interpreted the findings, and prepared the first draft of the manuscript for publication.
Dr. Savla conducted some of the literature searches, assisted in creation of the data base, and initial data analyses, as well as assisting in the data interpretation and manuscript preparation. Mr. Fellows was actively involved in planning and conducting the data analysis, interpretation of findings, and writing the analysis sections of the methods section of the manuscript.
Dr. Twamley was involved in data interpretation and manuscript preparation and revision.
Dr. Jeste was involved in data interpretation and manuscript preparation and revision.
Dr. Lacro was Principal Investigator of the primary parent study through which the patient data was collected, designed the cognitive test battery with input from Dr. Palmer, and assisted in manuscript preparation/revision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: A meta-analysis. American Journal of Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen DN, Huegel SG, Seaton BE, Goldstein G, Gurklis JA, Jr, van Kammen DP. Confirmatory factor analysis of the WAIS-R in patients with schizophrenia. Schizophrenia Research. 1998;34(1–2):87–94. doi: 10.1016/s0920-9964(98)00090-5. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory, thought, and action. New York: Oxford University Press; 2007. [Google Scholar]
- Barr WB. Schizophrenia and attention deficit disorder: Two complex disorders of attention. Annals of the New York Academy of Sciences. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- Chapin JS, Busch RM, Naugle RI, Najm IM. The Family Pictures subtest of the WMS-III: relationship to verbal and visual memory following temporal lobectomy for intractable epilepsy. Journal of Clinical and Experimental Neuropsychology. 2009;31(4):498–504. doi: 10.1080/13803390802317575. [DOI] [PubMed] [Google Scholar]
- Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, et al. Variable patterns of neuropsychological performance in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):613–626. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Canuso C, White AO, Simpson GM. A pilot effort to determine benztropine equivalents of anticholinergic medications. Hospital and Community Psychiatry. 1994;45(6):606–607. doi: 10.1176/ps.45.6.606. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Coursey RD. Independence and overlap among neurocognitive correlates of community functioning in schizophrenia. Schizophrenia Research. 2002;56(1–2):161–170. doi: 10.1016/s0920-9964(01)00229-8. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Iannone VN, Wilk CM, Gold JM. General and specific cognitive deficits in schizophrenia. Biological Psychiatry. 2004;55(8):826–833. doi: 10.1016/j.biopsych.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biological Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: A meta-analytic comparison of Digit Symbol Coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Schefft BK, Testa SM, Fargo JD, Privitera M, Yeh HS. What does the family pictures subtest of the Wechsler Memory Scale-III measure? Insight gained from patients evaluated for epilepsy surgery. Clin Neuropsychol. 2002;16(4):452–462. doi: 10.1076/clin.16.4.452.13915. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14(1):1–21. [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology Review. 2005;15(2):73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: A meta-analysis. Psychological Medicine. 2009;39(6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, McAdams LA, Palmer BW, Moore DJ, Jeste DV, Heaton RK. A six-factor model of cognition in schizophrenia and related psychotic disorders: Relationships with clinical symptoms and functional capacity. Schizophrenia Bulletin. 2004;30(4):739–754. doi: 10.1093/oxfordjournals.schbul.a007127. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldstein K. The significance of special mental tests for diagnosis and prognosis in schizophrenia. American Journal of Psychiatry. 1939;96(3):575–588. [Google Scholar]
- Gur RC, Moelter ST, Ragland JD. Learning and memory in schizophrenia. In: Sharma T, Harvey PD, editors. Cognition in schizophrenia: Impairments, importance and treatment strategies. New York: Oxford University Press; 2000. pp. 73–91. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hilti LM, Hilti CC, Seifritz E, Cattapan-Ludewig K. Sustained attentional performance of healthy first-degree relatives of schizophrenia patients. Schizophrenia Research. 2008;98(Supplement 1):22–23. [Google Scholar]
- Iverson GL, Lange RT, Viljoen H, Brink J. WAIS-III General Ability Index in neuropsychiatry and forensic psychiatry inpatient samples. Archives of Clinical Neuropsychology. 2006;21(1):77–82. doi: 10.1016/j.acn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wyatt RJ. Understanding and treating tardive dyskinesia. New York: Guilford Press; 1982. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia [Translated in 1919 from the eighth German edition of the ‘Text-book of psychiatry,’ vol. iii, part ii., section on Endogenous Dementias published in 1913] New York: Krieger; 197119191913. [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT. The “3 Rs” and neuropsychological function in schizophrenia: An empirical test of the matching fallacy. Neuropsychology. 1996;10(1):22–31. doi: 10.1016/0165-1781(94)02652-1. [DOI] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: An update. Schizophrenia Research. 2005;74(1):15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Millis SR, Malina AC, Bowers DA, Ricker JH. Confirmatory factor analysis of the Wechsler Memory Scale-III. Journal of Clinical and Experimental Neuropsychology. 1999;21(1):87–93. doi: 10.1076/jcen.21.1.87.937. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: Importance of working memory. American Journal of Psychiatry. 2005;162(10):1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychology Review. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Price L, Tulsky D, Millis S, Weiss L. Redefining the factor structure of the Wechsler Memory Scale-III: confirmatory factor analysis with cross-validation. Journal of Clinical and Experimental Neuropsychology. 2002;24(5):574–585. doi: 10.1076/jcen.24.5.574.1013. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Crespo-Facorro B, Gonzalez-Blanch C, Perez-Iglesias R, Vazquez-Barquero JL the PGS. Cognitive dysfunction in first-episode psychosis: The processing speed hypothesis. The British Journal of Psychiatry. 2007;191(51):s107–110. doi: 10.1192/bjp.191.51.s107. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160(10):1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Skelley SL, Goldberg TE, Egan MF, Weinberger DR, Gold JM. Verbal and visual memory: Characterizing the clinical and intermediate phenotype in schizophrenia. Schizophrenia Research. 2008 doi: 10.1016/j.schres.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. WAIS-III/WMS-III Technical Manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Tulsky DS, Ivnik RJ, Price LR, Wilkins C. Assessment of cognitive functioning with the WAIS-III and WMS-III: Development of a six-factor model. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, editors. Clinical interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2003. pp. 147–179. [Google Scholar]
- Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: Development and cross-validation of a six-factor model of cognitive functioning. Psychological Assessment. 2003;15(2):149–162. doi: 10.1037/1040-3590.15.2.149. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring manual. San Antonio, TX: The Psychological Corporation; 1997a. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) [Google Scholar]
- Wechsler D. Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997b. Wechsler Memory Scale - Third Edition (WMS-III) [Google Scholar]
- Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: A systematic review and meta-analysis. Schizophrenia Research. 2005;78(1):13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Ecker UK, Scherk H, Schneider-Axmann T, Falkai P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World Journal of Biological Psychiatry. 2008:1–10. doi: 10.1080/15622970701849986. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zec RF. Neuropsychology of schizophrenia according to Kraepelin: Disorders of volition and executive functioning. European Archives of Psychiatry and Clinical Neuroscience. 1995;245(4–5):216–223. doi: 10.1007/BF02191800. [DOI] [PubMed] [Google Scholar]