Abstract
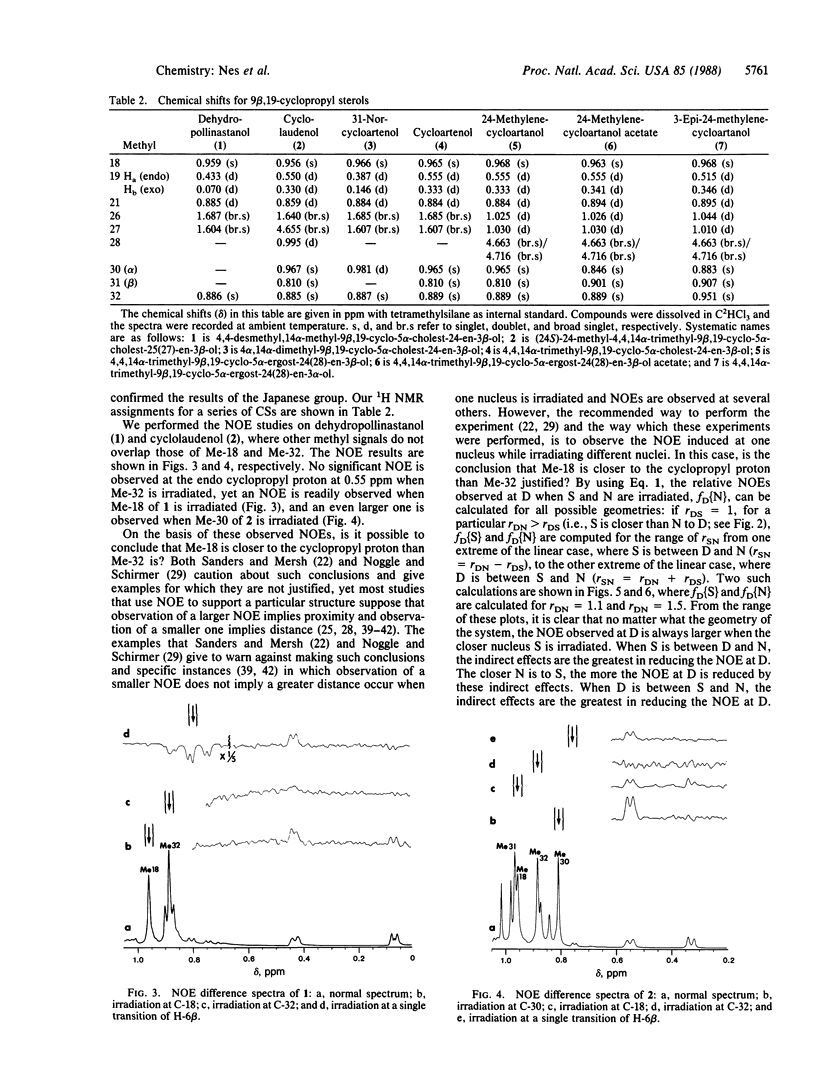
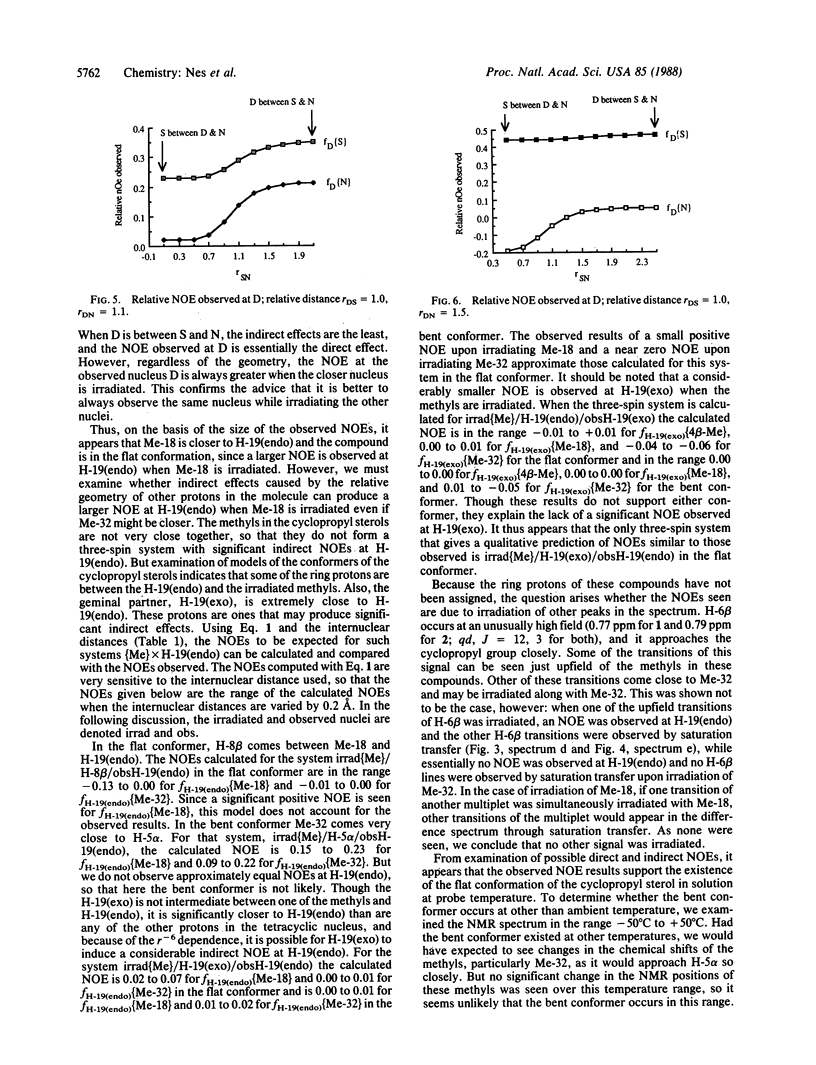
Nuclear Overhauser difference spectroscopy and variable temperature studies of the 9β,19-cyclopropyl sterols 24,25-dehydropollinastanol (4,4-desmethyl-5α-cycloart-24-en-3β-ol) and cyclolaudenol [(24S)-24-methyl-5α-cycloart-25(27)-en-3β-ol] have shown the solution conformation of the B/C rings to be twist-chair/twist-boat rather than boat/chair as suggested in the literature. This is very similar to the known crystal structure conformation of 9β,19-cyclopropyl sterols. The effect of these conformations on the molecular shape is highly significant: the first conformation orients into a pseudoplanar or flat shape analogous to lanosterol, whereas the latter conformation exhibits a bent shape. The results are interpreted to imply that, for conformational reasons, cyclopropyl sterols can be expected to maintain the pseudoplanar shape in membrane bilayers.
Keywords: periplanar structure; cycloartenol; 24,25-dehydropollinastanol; membranes; sterols
Full text
PDF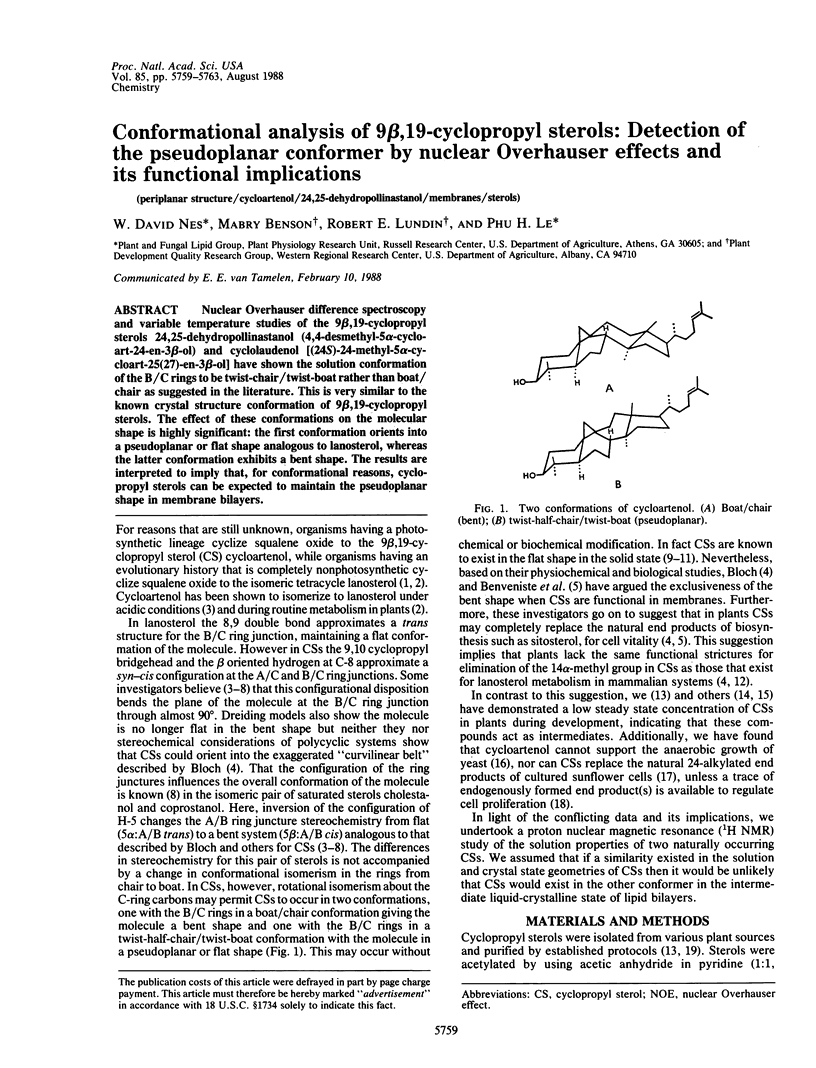


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bladocha M., Benveniste P. Manipulation by tridemorph, a systemic fungicide, of the sterol composition of maize leaves and roots. Plant Physiol. 1983 Apr;71(4):756–762. doi: 10.1104/pp.71.4.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Dahl C. E. Effect of sterol structure on acyl chain ordering in phosphatidylcholine vesicles: a deuterium nuclear magnetic resonance and electron spin resonance study. Biochemistry. 1981 Dec 8;20(25):7158–7161. doi: 10.1021/bi00528a016. [DOI] [PubMed] [Google Scholar]
- Ma QC, Cheng XX, Liu ZH, Liu YY, Watanabe T. First-order Born and first-order distorted-wave Born cross sections for micro++( micro-p)-->( micro+ micro-)+p. Phys Rev A Gen Phys. 1985 Nov;32(5):2645–2649. doi: 10.1103/physreva.32.2645. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Wong R. Y., Benson M., Landrey J. R., Nes W. R. Rotational isomerism about the 17(20)-bond of steroids and euphoids as shown by the crystal structures of euphol and tirucallol. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5896–5900. doi: 10.1073/pnas.81.18.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Popják G., Meenan A., Nes W. D. Effects of 2,3-iminosqualene on cultured cells. Proc R Soc Lond B Biol Sci. 1987 Dec 22;232(1268):273–287. doi: 10.1098/rspb.1987.0074. [DOI] [PubMed] [Google Scholar]
- Rees H. H., Goad L. J., Goodwin T. W. Studies in phytosterol biosynthesis. Mechanism of biosynthesis of cycloartenol. Biochem J. 1968 Apr;107(3):417–426. doi: 10.1042/bj1070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Rubinstein I., Goad L. J. The sterols of the echinoderm Asterias rubens. Biochem J. 1973 Nov;135(3):443–455. doi: 10.1042/bj1350443. [DOI] [PMC free article] [PubMed] [Google Scholar]



