Abstract
The MYC oncogene, which is frequently deregulated in human cancers, encodes a master transcription factor c-Myc (herein termed Myc) that integrates cell proliferation with metabolism through its regulation of thousands of genes including microRNAs (miRNAs). In addition to its known function in regulating the cell cycle and glucose metabolism, recent studies document a role for Myc in stimulating glutamine catabolism, in part through the repression of microRNAsmiR-23a and miR-23b. These observations suggest an additional level of complexity in tumor metabolism, which includes the commensal metabolic relationship between hypoxic and non-hypoxic regions of tumors as well as the surrounding stroma. Thus, a re-evaluation of cancer metabolism considering glutamine catabolism with a better understanding of the tumor histological complexity is needed before cancer metabolism can be effectively targeted in therapy.
For more than 80 years, the Warburg effect, which describes the propensity for cancer cells and tissues to take up glucose avidly and convert it almost exclusively to lactate (aerobic glycolysis), has been the central tenet of cancer cell metabolism (1, 2). The importance of aerobic glycolysis is underscored clinically by the distinct phenotype of high glucose uptake documented by positron emission tomography (PET) scanning of human cancers with radiolabeled 2-deoxyglucose, and molecularly by the de-regulation of oncogenes and tumor suppressors that result in cell autonomous changes promoting the conversion of glucose to lactate. However, not all cancers are PET-positive, and not all models of neoplastic transformation are associated with increased aerobic glycolysis; in fact, a few have increased mitochondrial function. Along with previous studies documenting that Myc induces mitochondrial biogenesis, the recent findings that glutamine catabolism is stimulated by Myc forces us to re-think the prevailing models of cancer metabolism, particularly if these alterations are to be exploited for therapeutic purposes.
The Warburg effect describes the high flux of glucose through glycolysis, which converts hexoses to trioses – a source of glycerol for lipid synthesis, ending with pyruvate that is converted to lactate. This results in a high output of lactate even with adequate oxygen levels (Figure 1A). Glucose may also be catabolized through the pentose phosphate pathway to generate NADPH for redox homeostasis and ribose for nucleotide biosynthesis. In the presence of oxygen, pyruvate can be converted by pyruvate dehydrogenase (PDH) to acetyl-CoA for further oxidation through the tricarboxylic acid (TCA) cycle. The conversion of pyruvate to acetyl-CoA is blocked when pyruvate dehydrogenase kinase 1 (PDK1) is up-regulated. The PDK1 kinase is induced under hypoxic conditions by the hypoxia inducible transcription factor HIF-1 and functions to phosphorylate and inactivate PDH. Less well celebrated as an energy source and anabolic source of carbon and nitrogen is glutamine (Figure 1A), the amino acid with the highest circulating concentration in human blood. Glutamine is taken up by cells and may be utilized as an amino acid for protein synthesis, although it is primarily converted to glutamate by glutaminase. Glutamate is then converted to α-ketoglutarate, an oxidative substrate for the TCA cycle. Glutamine can also be converted to pyruvate and then to lactate through malate, a TCA cycle metabolite of α-ketoglutarate, in a less well understood process termed glutaminolysis (Figure 1A). Thus, glutamine can serve as an important source of cellular energy and anabolic carbon and nitrogen.
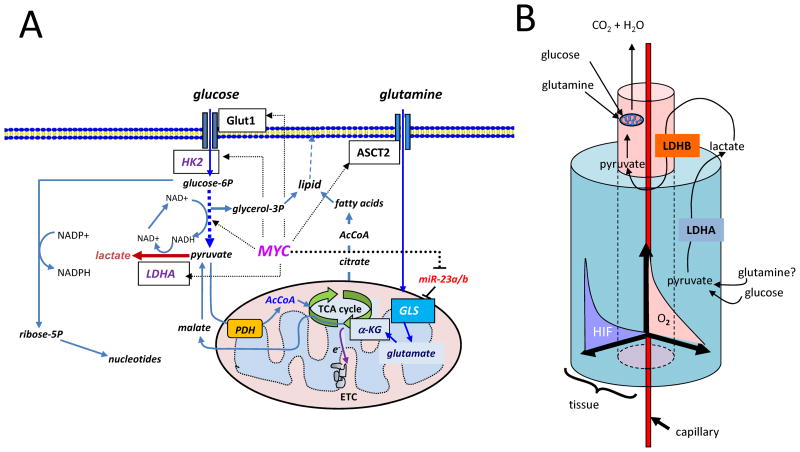
Figure 1.
1A) Diagram depicting glucose and glutamine metabolic pathways and targets (boxed) therein regulated by Myc (dashed arrows). Glucose is transported by Glut1 and phosphorylated by hexokinase 2 (HK2) with subsequent conversion to trioses, producing NADH and ATP, culminating in pyruvate. Intermediate trioses yield glycerol-3-phosphate as a backbone for lipids. Pyruvate can be converted to lactate by lactate dehydrogenase A (LDHA), which is a target of Myc and HIF-1. In the presence of oxygen, pyruvate could be further converted to acetyl-CoA (AcCoA) that is further oxidized in the mitochondria through the tricarboxylic acid (TCA) cycle, which donates high energy electrons (e−) to the electron transport chain (ETC) for the production of ATP and pyrimidine biosynthesis. Citrate transported into cytoplasm from the TCA cycle provides substrate for cytoplasmic acetyl-CoA production, necessary for fatty acid synthesis, which together with glycerol-3-phosphate generate lipids. Glucose-6-phosphate (glucose-6P) can alternatively be catabolized to ribose through the pentose phosphate shunt, which also generates NADPH for redox homeostasis. Glutamine is shown transported into the cell through ASCT2 and converted to glutamate by glutaminase (GLS), which is under the control of MYC through microRNA miR-23a/b. Glutamate is further catabolized to α-ketoglutarate (α-KG) for further oxidation in the TCA cycle. Malate generated from α-ketoglutarate can exit the TCA cycle into the cytoplasm for conversion to pyruvate. 1B) Cartoon depicting a 3-D cutout of a tumor tissue block with a central capillary feeding an inner kernel of cells with oxygen and nutrients. This kernel uses oxidative phosphorylation with glucose and glutamine serving as substrates. As cell proliferate and are pushed away from the blood vessel, an oxygen gradient (O2) is created with a concomitant increase in HIF-1 levels in the peripheral cuff of hypoxic cells, which utilizes glycolysis and perhaps glutaminolysis (conversion of glutamine to lactate). Note that lactate produced by LDHA in the hypoxic cuff is converted to pyruvate by LDHB in the central kernel of cells for oxidation in the mitochondrion (see Figure 1A).
For the past two decades, many studies of oncogenic alterations of metabolism have suggested that cell autonomous changes due to the activation of oncogenes or loss of tumor suppressors are key drivers for the high conversion of glucose to lactate. These include early studies that documented the association of Src- and Ras-transformation with increased glucose transporter expression and the direct transactivation of the LDHA (lactate dehydrogenase A) gene by the MYC oncogene (Figure 1A). Later studies linked Ras, VHL, and mutations of isocitrate dehydrogenase 1 (IDH1), succinate dehydrogenase (SDH), and fumarate hydratase (FH) to the activation ofHIF-1, even in normoxia, which in turn induces glycolytic enzyme gene expression (3–6). Furthermore, the Akt oncogene was shown to stimulate glycolysis post-transcriptionally, and the p53 tumor suppressor emerged as another regulator of mitochondrial function and glycolysis, such that loss of p53 is associated with enhanced glycolysis (3). Collectively, these observations could be neatly packaged to support the notion that activation of oncogenes and loss of tumor suppressors result in the induction of cell autonomous aerobic glycolysis, independent of hypoxia, or the Warburg effect. However, there are a number of observations that are not encompassed by our current model of cancer metabolism.
First, the former model of cancer metabolism is largely based on studies from in vitro tissue culture conditions, which do not recapitulate the hypoxic conditions present within a naturally occurring tumor (7–11) (Figure 1B). Tumor cell adaptation and tolerance of hypoxia are critical features of a robust cancer cell. Compared to most normal tissues, which are well-oxygenated, tumor tissues are generally hypoxic with some more oxygenated areas around poorly-formed tumor blood vessels (9–11). While constitutive cell autonomous changes favoring aerobic glycolysis could be advantageous to cancer cells under certain conditions, the presence of tumor hypoxia indicates that adaptive changes are also important, when HIF-1 is not constitutively activated. For example, the activation of HIF-1 in hypoxic conditions not only induces an adaptive metabolic program, but also triggers angiogenesis (11). In tumors with constitutive activation of HIF-1 downstream of altered oncogenes or tumor suppressors, the adaptive response to hypoxia may not be as relevant -but how constitutive HIF-1 expression, which inhibits normal cell proliferation, permits continued neoplastic cell proliferation remains poorly understood.
Secondly, the commensal relationships amongst hypoxic and non-hypoxic cells in the tumor tissue are important for tumor neo-vascularization and maintenance, in addition to the cell autonomous genetic changes that initiate the neoplasm. The importance of heterogeneity within a tumor bed and symbiosis between tumor cells was recently underscored by the documentation of hypoxic tumor cells producing lactate that can be re-cycled and re-utilized as pyruvate for oxidative phosphorylation by the more oxygenated tumor cells (Figure 1B)(12). Another study suggests a symbiotic metabolic relation between tumor and stromal cells. Compared to human lung cancer cells, which express high levels of HIF-1, LDHA and PDK1, the accompanying tumor associated stromal fibroblasts have decreased PDK1 and increased PDH. This suggests a commensal relation between tumor and stroma, in which stromal cells may not only undergo oxidative phosphorylation but also recycle lactate released from tumor cells (13). The microenvironmental heterogeneity of tumor cells, in the backdrop of findings that Myc can stimulate mitochondrial biogenesis, oxygen consumption, and glycolysis, suggests that MYC drives both aerobic glycolysis and oxidative phosphorylation when oxygen is ample in tumor cells located immediately around a blood vessel (14, 15). When oxygen is limited in cells located distal to the blood vessel, deregulated Myc collaborates with HIF-1 to attenuate mitochondrial respiration, but not necessarily other mitochondrial biosynthetic functions, and to increase glycolysis for adaptation to the tumor microenvironment.
In retrospect, the ability of Myc to induce mitochondrial biogenesis in proliferating cells while inhibiting mitochondrial respiration should not be surprising, because mitochondria not only provide a means for efficient production of ATP in the presence of oxygen, but they also serve as a factory for many other building blocks of a growing cell. These building blocks include pyrimidines, whose synthesis is strictly linked to the electron transport chain via the activity of dihydro-orotate dehydrogenase, the carbon backbone for amino acids, as well as citrate which is extruded into the cytoplasm and converted to acetyl-CoA for lipid biosynthesis (Figure 1A)(14–17). The stimulation of glucose uptake and metabolism by Myc, on the other hand, provide carbon backbone for critical cellular processes, such as ribose for nucleotide biosynthesis and NADPH through the pentose phosphate pathway for redox homeostasis, triglycerides and ATP through glycolysis (1).
The fact that Myc induces mitochondrial biogenesis led us to determine whether Myc could also affect the composition of the mitochondria and alter their function. To this end, we performed high-resolution 2-D gel electrophoresis of purified mitochondria from human B lymphocytes with low Myc or high Myc expression (18). Among the seven proteins that were identified by mass spectrometry as being highly induced by Myc was mitochondrial glutaminase (encoded by GLS). This enzyme catalyzes the conversion of glutamine to glutamate, which can then be converted to α-ketoglutarate as a substrate for the TCA cycle. Because of this observation, we examined genes involved in glutamine catabolism and found that ASCT2 (or SLC5A1) and SLC7A1, both involved in glutamine transport, behave as direct Myc target genes (Figure 1A). Given that glutamine influx could be increased by Myc, it is notable that glutamine is not only an energetic and anabolic substrate, but it also provides nitrogen for amino acid and nucleic acid biosynthesis and precursors for glutathione synthesis for redox homeostasis (Figure 1A). The Thompson laboratory independently discovered that Myc stimulates glutamine metabolism (19). As for GLS, however, we found that while the protein levels robustly responded to Myc, its mRNA levels did not vary significantly with changes in Myc levels in a human B cell line. We therefore hypothesized that Myc regulates GLS at the post-transcriptional level. We then documented that Myc directly represses expression of miR-23a and miR-23b, which target the 3′-UTR seed sequence in the GLS transcript to down-regulate its translation (Figure 1A). It is notable that GLS transcript levels might also be affected by miRNAs, particularly since GLS mRNA levels vary with Myc in fibroblasts (19). These observations, along with early observations that glutamine deprivation of Myc-transformed human cells triggered apoptosis (20), indicate that Myc stimulation of glutamine catabolism is part-and-parcel of its central role in integrating cell proliferation with metabolism.
If Myc stimulates glutamine oxidation and mitochondrial biogenesis as well as glycolysis, then how does aerobic glycolysis and oxidative phosphorylation participate in tumorigenesis and tumor maintenance? We found that the inducible-MYC human B cell lymphoma model consumes oxygen and glutamine in the presence of oxygen (8, 18) When oxygen is deprived, however, Myc collaborates with HIF-1 to increase glycolytic enzyme gene expression and attenuate the conversion of pyruvate to acetyl-CoA by PDH through the induction its negative-regulatory kinase PDK1 (3, 8). While there are extant data supporting the existence of the conversion of glutamine to lactate via glutaminolysis which uses half of the TCA cycle, it is not clear what role glutaminolysis plays in hypoxic cells (1). Nonetheless, the ability of Myc to stimulate oxidative phosphorylation and glycolysis simultaneously suggests that it offers an advantage to cancer cells in the tumor tissue microenvironment which consists of both hypoxic regions and more oxygenated regions around blood vessels (Figure 1B). In this regard, our re-thinking about cancer metabolism must consider the cellular heterogeneity within a tumor, such that Myc confers a growth advantage to tumor cells with adequate oxygen both by increasing mitochondrial biogenesis and glutamine metabolism for use in ATP production, anabolic carbon and nitrogen sources, and redox homeostasis, and by increasing glucose flux which provides anabolic carbons for ribose and fatty acid biosynthesis, and produces NADPH. Cells that consume oxygen and proliferate around the tumor blood vessels are pushed into hypoxic regions, which are caused by oxygen consumption by the remaining non-hypoxic cells around blood vessels. The hypoxic cells then induce HIF-1 and adapt or die, unless they already have constitutive expression of HIF-1 which should provide a growth advantage (Figure 1B). The proposed model, hence, suggests that the ability of Myc to collaborate with HIF-1 confers a metabolic advantage by inducing high fluxes of glucose through glycolysis, which must be accompanied by a replenishable nitrogen source of substrate for continued nucleotide biosynthesis. In some cases, hypoxic cells cease to proliferate and allow for the ensuing angiogenesis triggered by both HIF-1 and Myc to replenish nutrients and oxygen. Once the new fuel lines are available, the cells could resume proliferating with total disregard to normal external cues. In this fashion, Myc and HIF-1 are both critical tumor maintenance factors, whose target genes could be exploited for therapeutic purposes. LDHA, a transcription target gene common to Myc and HIF-1, is necessary for the transformation phenotype in vitro and tumor maintenance in vivo and is hence an attractive therapeutic target. However, the rethinking of cancer metabolism in the context of the tumor tissue suggests that combinations of multiple agents affecting either glycolysis, glutamine, or both maybe necessary for the effective targeting of tumor metabolism for cancer therapy (1, 8).
Acknowledgments
I thank R. Arceci, L. Gardner, and L. Resar for comments. Our work is supported by NIH grants CA51497 and CA57341, the Leukemia Lymphoma Society and AACR SU2C initiative.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 6.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2:266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 10.Dewhirst MW. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor-1 targeting. Cancer Res. 2007;67:854–855. doi: 10.1158/0008-5472.CAN-06-4744. [DOI] [PubMed] [Google Scholar]
- 11.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005;7:1–6. doi: 10.1593/neo.04373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PLoS One. 2008;3:e1798. doi: 10.1371/journal.pone.0001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrish F, Isern N, Sadilek M, Jeffrey M, Hockenbery DM. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28:2485–2491. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrish F, Neretti N, Sedivy JM, Hockenbery DM. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle. 2008;7:1054–1066. doi: 10.4161/cc.7.8.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]