Abstract
OBJECTIVES
We examined correlations between blood pressure and dementia-related pathologic brain changes in a community-based autopsy sample.
DESIGN
Prospective cohort study.
SETTING
A large health maintenance organization in Seattle, Washington.
PARTICIPANTS
A cohort of 250 participants who were ≥ 65 years old and cognitively normal at time of enrollment in the Adult Changes in Thought (ACT) study, and who underwent autopsy.
MEASUREMENTS
Blood pressure and history of antihypertensive treatment were taken at enrollment. A linear regression model was used to examine the relationship between blood pressure (systolic and diastolic blood pressure) at enrollment and pathologic changes in cerebrum (cystic macroscopic infarcts, microinfarcts, neuritic plaques, neurofibrillary tangles, and cortical Lewy bodies).
RESULTS
The presence of more than 2 microinfarcts, but not any other pathologic change, was independently associated with systolic (SBP) in younger participants (age 65–80, n=137), but not in older participants (age >80, n=91). The relative risk (RR) for >2 microinfarcts with each 10 mmHg increase in SBP was 1.15 (95% confidence interval (CI): 1.00, 1.33) in the younger participants, adjusted for age-at-entry, gender, and time to death. This RR was particularly strong in younger participants not taking antihypertensive medications (RR: 1.48; 95% CI: 1.21, 1.81); significant associations were not observed in participants treated for hypertension. Findings for diastolic blood pressure were negative.
CONCLUSION
The association between elevated SBP and cerebrovascular damage in untreated older adults (age 65–80) suggests that adequate hypertension treatment may reduce dementia risk by minimizing microvascular injury to cerebrum.
Keywords: dementia, blood pressure, hypertension, neuropathology, autopsy
INTRODUCTION
Understanding modifiable risk factors for dementia, including vascular dementia, Alzheimer’s disease (AD), and Lewy body dementia, is an important step toward finding preventions and treatments for these devastating illnesses. One of these modifiable risk factors is hypertension.1–5 We previously reported findings on the association between hypertension and clinical dementia from the Adult Changes in Thought (ACT) study, an ongoing prospective, longitudinal, community-based study of brain aging and incident dementia.6 We found that participants aged 65 to 74 with elevated systolic blood pressure (SBP) at enrollment had an increased risk for clinical dementia compared to participants with normal blood pressure, suggesting adequate blood pressure treatment may reduce dementia risk.
Several pathologic changes in the brain, including both cerebrovascular damage and neurodegenerative changes, contribute to clinical expression of dementia.7–10 A previous autopsy study of the ACT sample showed that cerebral microinfarcts, neurofibrillary tangles, and neocortical Lewy bodies are strongly and independently associated with cognitive impairment in this cohort.11 These and other pathologic changes, including neuritic plaques, macroscopic infarcts such as lacunes or territorial infarcts, and atherosclerosis commonly coexist and interact with each other.12, 13 Which one of these pathologic changes is more highly associated with blood pressure is less well understood. Previous studies have primarily examined the association of risk factors, such as hypertension, with specific pathologic changes, such as the hallmark changes of AD or cerebrovascular damage separately, making direct comparison amongst different pathologic processes difficult.14–16 In this study, we assessed common pathologic changes in the aging brain, including changes already independently associated with dementia (neurofibrillary tangles, cortical Lewy bodies, and microinfarcts) as well as others (neuritic plaques, cystic macroscopic infarcts), to determine what type of brain injury is most closely associated with elevated blood pressure.11–13 We also examined whether anti-hypertensive treatment is associated with apparent protection.
METHODS
Participants
The ACT study was initiated in 1994 at which time it recruited 2,581 cognitively normal individuals aged 65 years and older from Group Health Cooperative (GHC) of Puget Sound, a large health maintenance organization based in Seattle, Washington. In the years 2000–2002, an additional 811 cognitively normal participants were recruited from GHC as an expansion cohort. From 2005 to June 1, 2007, an additional 238 participants were recruited as the ongoing Continuous Enrollment cohort. As of June 1, 2007, of the total 3630 participants enrolled in ACT, 419 participants had withdrawn from the study and 1167 had died (30 of whom had also withdrawn from the study before they died). Of those who died, 250 underwent brain autopsy with neuropathologic assessment and are included in this analysis.
Detailed ACT study design and methods have been described.17 Briefly, each participant underwent a baseline cognitive examination and was then followed up biennially for cognitive decline as detected by a score less than 86 on the Cognitive Abilities Screening Instrument (CASI).18 Participants with cognitive decline underwent a complete dementia evaluation with a neuropsychological test battery and a physical/neurological examination by a geriatrician or neurologist. Relevant laboratory results and brain CT or MRI scans were obtained, after which diagnoses were assigned at a consensus diagnosis conference using Diagnostic and Statistical Manual of Mental Disorders-IV edition (DSM-IV)19 and National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) criteria for AD.20 The protocol for this study was approved by the University of Washington (UW) and GHC human subjects review committees. All participants provided written informed consent prior to enrollment.
Blood Pressure Measurement and History of Antihypertensive Treatment
A trained nurse or technician measured blood pressure using standardized techniques at enrollment and at each biennial follow-up examination. After participants sat at rest for at least 5 minutes, the SBP was taken as the point of first hearing the Korotkoff sound and diastolic blood pressure (DBP) as the point for disappearance of the Korotkoff sound. Two blood pressure measurements were taken 5 minutes apart, and the average of the two readings was used for analysis. At baseline and each biennial visit, a detailed medical history and medication use were obtained by a standardized questionnaire. Specifically, the question of “Are you taking prescribed medication for blood pressure now?” (yes/no) was used to assess antihypertensive treatment at the time of evaluation.
Assessment of Neuropathologic Outcomes
Neuropathologic examinations were preformed in the UW Division of Neuropathology and the UW AD Research Center (ADRC) Neuropathology Core. Neuropathologic assessment of autopsy brains was performed as previously described.11, 21 In brief, all neuropathologic evaluations were performed blinded to the clinical diagnosis and status of risk factors. Brains were immersion-fixed in formalin for at least 2 weeks prior to dissection. Following fixation, all brains were evaluated for any gross lesions, including the number of macroscopic infarcts, which include lacunar and territorial infarcts. We limited our evaluation of macroinfarcts to remote cystic infarcts (estimated > 1 year old), as acute and subacute infarcts were thought unlikely to have contributed to longstanding cognitive decline. Cerebral microinfarcts, infarcts too small to observe grossly but identified by microscopy, were evaluated in bilateral sections of frontal lobe, temporal lobe, parietal lobe, occipital lobe, caudate nucleus, putamen, internal capsule, and thalamus exactly according to a protocol previously published by us11, 21 and fashioned after a method proposed by the Honolulu Asia Aging Study.10 Greater than 2 microinfarcts is strongly and independently associated with dementia in ACT participants.10, 11Neuritic plaques, which are associated with AD, were scored according to the criteria of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), resulting in a graded scale (0, A, B, or C; with scores B or C associated with a higher likelihood of clinical AD).9, 22 Neurofibrillary tangles, which are also associated with AD, were scored according to the methods of Braak and Braak, resulting in a graded scale (0 to VI).7, 23 Neocortical Lewy bodies were evaluated by immunohistochemical staining for α-synuclein according to an established method24 exactly as previously described.11
Statistical Analysis
All statistical analyses were stratified by baseline age (65 to 80 versus >80). Differences in baseline characteristics in deceased ACT participants by autopsy status were assessed using two sample t-tests for continuous measures and Fisher's exact tests for categorical measures. Summary measures for continuous data are presented as means (± standard deviation (SD)) unless otherwise noted. In the subsample of deceased participants who were autopsied, we sought to determine which of the five pathologic measures which have an association with dementia (presence of 1or more macroscopic infarcts, presence of 2 or more microinfarcts, neuritic plaque score B or C, neurofibrillary tangle Braak stage modeled as a continuous variable, and presence of 1 or more neocortical Lewy bodies) were associated with baseline blood pressure using linear regression models. Baseline SBP or DBP was the dependent variable, and the five brain pathologic assessments were in the model together as independent variables, with additional adjustment for age at baseline, gender and follow-up time. The regression coefficients obtained for each pathologic measure represent the estimated difference in blood pressure when the measured pathology is present versus absent in individuals who have the same values for all other pathologies. For any pathologic measure with a significant association with blood pressure (p ≤ 0.05), we then estimated the relative risk (RR) of pathology presence by a 10 mmHg increase in SBP or a 5 mmHg increase in DBP, using a generalized linear model with a log link, and Gaussian error and robust estimations of the standard errors of the model coefficients.25, 26 The RRs were adjusted for age at baseline, gender and follow-up time. The modifying effect of antihypertensive treatment was examined by adding interaction terms for treatment × SBP or treatment × DBP into the multiple regression model. Separate RRs were estimated for untreated and treated participants. Linearity of the blood pressure-neuropathologic measures association was assessed by constructing loess-smoothed curves of log risk by blood pressure estimated from generalized additive models with Gaussian errors and log links.
Participants who underwent autopsy included all ACT participants who consented to autopsy and whose bodies or brains could be transported to the UW Division of Neuropathology for evaluation. Potential selection bias for autopsy was accounted for by inverse probability weighting in a two-stage approach to adjust the composition of the autopsied group to reflect the characteristics of the deceased group as a whole.27 First, the probability that a participant underwent autopsy was estimated using logistic regression on all participants who died (n=1167) with study characteristics that differed by autopsy status as model covariates. Second, the inverse of these probabilities were used as weights in the linear regression and the generalized linear models described above. A bootstrap was applied to the 2-stage procedure to estimate 95% confidence intervals for the RRs. Analyses were carried out using R 2.6.1 (R Foundation for Statistical Computing, Vienna, Austria)28 and Stata 10.0 (StataCorp LP, College Station, TX).
RESULTS
Participant Characteristics
Two-hundred fifty participants in ACT underwent brain autopsy with neuropathologic assessments while 917 participants died without autopsy. Because we previously found that the relationship between blood pressure at study entry and clinical dementia was age-dependent,6 we examined characteristics in younger participants (age 65 to 80) and older participants (age < 80) separately (Table 1). In younger participants, those who underwent autopsy differed from those who died without autopsy in the following characteristics: age at death, time from study entry to death, gender, ethnicity, years of education, presence of coronary artery disease, hypertension diagnosis, antihypertensive treatment at study entry, and development of dementia. In older participants, those who underwent autopsy differed from those who died without autopsy in the following characteristics: age at death, years from study entry to death, CASI at study entry, and development of dementia.
Table 1.
Characteristics of Autopsied and Non-autopsied Participants
| Age-at-entry 65–80 years | Age-at-entry > 80 years | |||||
|---|---|---|---|---|---|---|
| Autopsied | Died Without Autopsy |
p-value | Autopsied | Died Without Autopsy |
p-value | |
| N | 148 | 605 | 102 | 312 | ||
| Age at study entry, mean (± SD) | 74.4 (4.1) | 73.9 (4.1) | .161 | 85.5 (3.4) | 85.3 (3.8) | .584 |
| Age at death, mean (± SD) | 82.0 (5.1) | 80.2 (5.4) | <.001 | 91.9 (4.2) | 90.2 (4.4) | <.001 |
| Years from study entry to death, mean (± SD) | 7.7 (3.0) | 6.4 (3.4) | <.001 | 6.4 (2.9) | 5.0 (3.2) | <.001 |
| Male (%) | 45 | 55 | .043 | 39 | 37 | .724 |
| Non-Caucasian (%) | 3 | 11 | .001 | 4 | 5 | 1.000 |
| Years of education, mean (± SD) | 14.3 (2.8) | 13.6 (3.0) | .018 | 13.8 (3.3) | 12.9 (3.1) | .015 |
| Cognitive Abilities Screening Instrument score at study entry,* mean (± SD) |
93.2 (5.1) | 92.3 (4.9) | .065 | 91.9 (3.7) | 89.1 (7.4) | <.001 |
| Body Mass Index at study entry, mean (± SD) | 27.0 (4.9) | 27.7 (5.2) | .152 | 25.9 (5.1) | 25.8 (4.3) | .768 |
| Coronary artery disease (%) | 20 | 28 | .038 | 23 | 28 | .304 |
| Cerebrovascular disease (%) | 18 | 17 | .806 | 10 | 17 | .110 |
| Diabetes Mellitus (%) | 18 | 17 | .903 | 6 | 8 | .665 |
| Hypertension (%) | 41 | 50 | .043 | 36 | 40 | .482 |
| Antihypertensive medicine use at study entry (%) | 34 | 46 | .007 | 40 | 44 | .488 |
| Clinical dementia during study (%) | 30 | 21 | .039 | 41 | 27 | .009 |
| Clinical Alzheimer’s disease during study (%) | 15 | 11 | .150 | 18 | 12 | .182 |
Score range: 0 to 100. A score of 86 or above was considered normal for this study.
Blood Pressure and Neuropathologic Changes
Linear regression analysis was performed for participants who had data available for all 5 neuropathologic measures and baseline blood pressure (age 65 to 80, n=137; age >80, n=91; unavailable data specifically included: 2 participants missing microinfarct data, 8 macroinfarct data, 12 Lewy body data, and 1 baseline blood pressure). In younger participants, linear regression analysis showed that of the five brain pathologic changes in the model, only the presence of microinfarcts was independently associated with SBP, where those with greater than two microinfarcts had an estimated mean SBP 11.5 mmHg higher (95% confidence interval [CI]: 1.7, 21.3) than those with two or less microinfarcts (Table 2). The association between SBP and cerebral microinfarcts was not present for older participants (age > 80 years). Of individuals with greater than two microinfarcts, 11.3% had cortical microinfarcts, 11.3% had subcortical microinfarcts, and 77.4% had both. There was no association between DBP and any measure of pathologic change.
Table 2.
Linear Regression of Systolic and Diastolic Blood Pressure, Modeled on Five Brain Pathologic Measures Jointly*
| Age-at-entry 65–80 years (n=137) | Age-at-entry > 80 years (n=91) | |||||
|---|---|---|---|---|---|---|
| Number (%),or mean (± SD) for Braak stage |
Coefficient for SBP (95%CI)† |
Coefficient for DBP (95%CI)† |
Number (%), or mean (SD) for Braak stage |
Coefficient for SBP (95%CI)† |
Coefficient for DBP (95%CI)† |
|
| Microinfarct > 2 | 28 (20) | 11.5 (1.7, 21.3)‡ | −1.7 (−6.3, 3.0) | 22 (24) | 3.7 (−9.5, 16.8) | 2.1 (−2.8, 7.1) |
| Cystic macroinfarct | 38 (28) | −1.3 (−10.1, 7.5) | −0.7 (−4.8, 3.5) | 23 (25) | 4.6 (−8.7, 17.9) | −0.7 (−5.7, 4.2) |
| Braak stage (0–VI) | 2.7 (1.8) | −0.8 (−3.6, 2.0) | −0.4 (−1.7, 0.9) | 3.2 (1.6) | −2.4 (−6.4, 1.5) | −0.2 (−1.6, 1.3) |
| CERAD B or C | 50 (36) | −5.7 (−15.2, 3.9) | 0.7 (−3.8, 5.2) | 37 (41) | 0.8 (−12.2, 13.9) | 1.6 (−3.3, 6.5) |
| Lewy bodies | 36 (26) | 6.8 (−2.1, 15.7) | 1.2 (−3.0, 5.4) | 34 (37) | −2.4 (−13.5, 8.7) | 1.6 (−2.6, 5.8) |
Adjusted for age-at-entry, time to death and gender
Coefficients represent estimated mean differences in blood pressure by the presence versus absence of the pathologic measure, except for Braak Stage where the coefficient represents the slope of an increase in Braak stage per increase in 1 mmHg of blood pressure
p < 0.05.
To assess further the association between SBP and presence of cerebral microinfarcts in the younger age group, we estimated the RR of cerebral microinfarcts with each 10 mmHg increase in SBP for 147 participants with available microinfarct data using the generalized linear model described above, with presence of > 2 cerebral microinfarcts (versus 2 or less cerebral microinfarcts) as the dependent variable and SBP as the independent variable. The association between SBP and risk of > 2 cerebral microinfarcts was mainly linear (confirmed graphically using a generalized additive model), with each 10 mmHg increment of SBP associated with 15% higher risk for microinfarcts after adjusting for age-at-entry, time to death and gender (Table 3).
Table 3.
Relative Risk (95% CI) of Microinfarct > 2 by Blood Pressure Change in Participants Aged 65–80*
| Total (n=147) | No antihypertensive medication use (n=97) |
Antihypertensive medication use (n=50) |
|
|---|---|---|---|
| Systolic Blood Pressure (10 mmHg increase) | 1.15 (1.00, 1.33) | 1.48 (1.21, 1.81) | 0.83 (0.63, 1.10) |
| Diastolic Blood Pressure (5 mmHg increase) | 0.85 (0.64, 1.13) | 1.05 (0.78, 1.41) | 0.84 (0.73, 0.97) |
Adjusted for age at entry, follow-up time and gender.
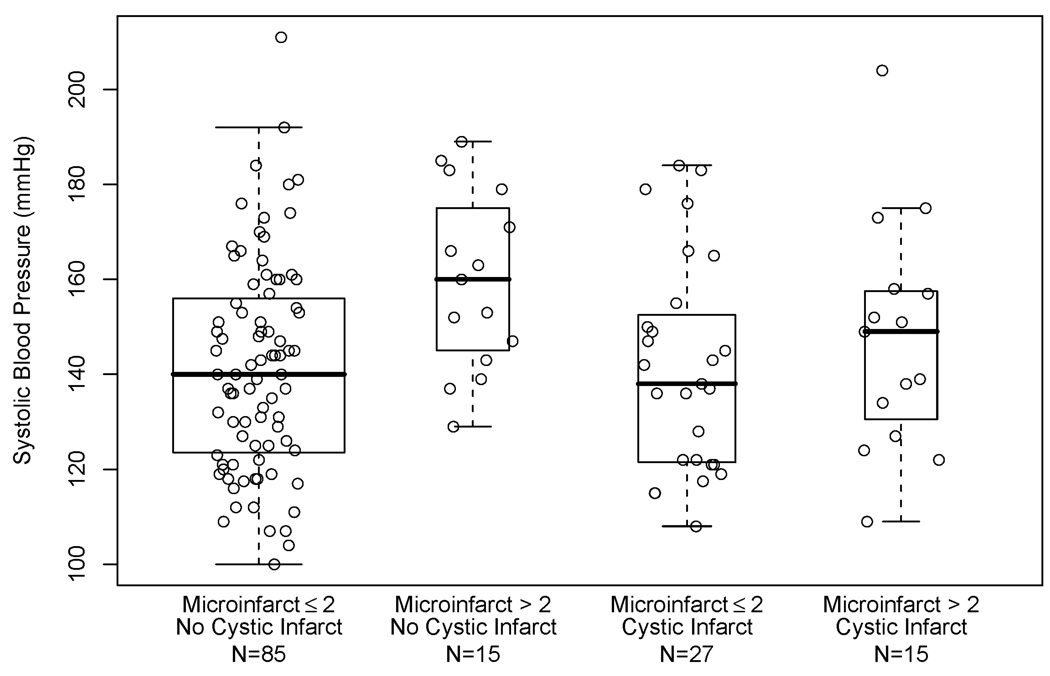
Because cerebral microinfarcts were correlated with cystic macroinfarcts (p < 0.001, Fisher’s exact test), we explored whether colinearity contributed to the lack of association seen between SBP and cystic macroinfarcts in the younger age group in the linear regression model presented in Table 2. Figure 1 shows mean SBP was not related to presence or absence of cystic macroinfarcts. Estimates of the RR of at least one cystic infarct with increasing blood pressure using the generalized linear models described above, demonstrated no evidence of association between cystic macroinfarcts and SBP (RR: 1.03; 95% CI: 0.90, 1.18) or DBP (RR: 0.94; 95% CI: 0.77, 1.14).
Figure 1.
Box plots of systolic blood pressure in participants aged 65 to 80 years, by presence of > 2 microinfarcts and by presence of at least one cystic infarct. Boxes represent 50% of the data with the horizontal bar denoting the median. Data points outside the “whiskers” are considered outliers. A scatterplot of systolic blood pressure by jittered vascular pathology category is overlaid.
Antihypertensive Treatment and Association between SBP and Cerebral Microinfarcts
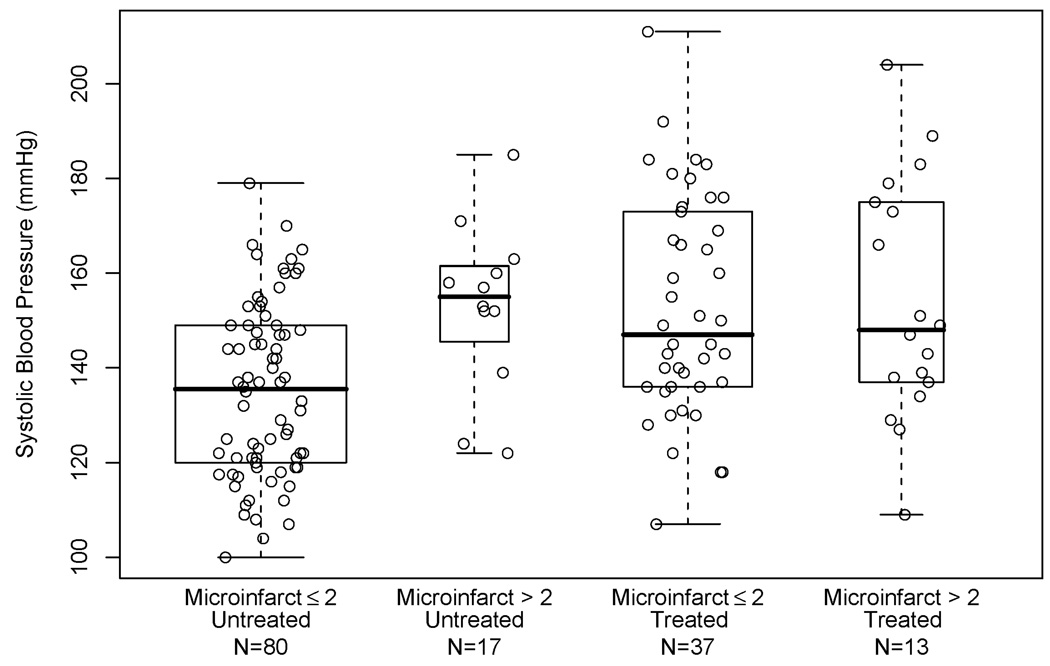
Figure 2 shows SBP in those aged 65 to 80 years old based on presence or absence of microinfarcts and self-reported antihypertensive treatment at study entry (90 participants reported antihypertensive treatment; 50 of these were age 80 or less). Mean SBP was higher in those with > 2 cerebral microinfarcts than those with ≤ 2 cerebral microinfarcts (mean (±SD) 153 (18) versus 135 (18), t-test, p = 0.005) in the untreated group. This difference was not found in the treated group (mean (±SD) 154 (25) versus 153 (23), t-test, p = 0.8). Consistent with this observation, there was an elevated relative risk for > 2 cerebral microinfarcts associated with SBP changes (RR: 1.48 per 10mmHg increase; 95% CI: 1.21, 1.81) in the untreated participants after adjusting for age-at-entry, time to death and gender (Table 3). This elevated RR was not seen in participants who were taking antihypertensive medications. The interaction between SBP and self-reported antihypertensive treatment in the generalized linear model of microinfarcts in younger participants was significant (p<0.001) suggesting that the SBP-microinfarct association in younger participants was modified by antihypertensive treatment.
Figure 2.
Box plots of systolic blood pressure entry in participants aged 65–80 years, by presence of > 2 microinfarcts and by antihypertensive treatment at study entry. Boxes represent 50% of the data with horizontal bar denoting the median. Data points outside the “whiskers” are considered outliers. A scatterplot of systolic blood pressure by jittered treatment/pathology category is overlaid.
Generalization from the Autopsy Sample to all ACT Participants who Died
Because of differences between participants who underwent autopsy and participants who died without autopsy, we reanalyzed our data using inverse probability weighting, as described in the Statistical Analysis section above with emphasis on determining if the microinfarct-SBP association was robust to selection bias. From the results in Table 1, potential selection bias variables were age at death, time from study entry to death, gender, ethnicity, years of education, presence of coronary artery disease, hypertension diagnosis, and clinical dementia. With this adjustment, no cerebrovascular or neurodegenerative change was significantly correlated with blood pressure in the linear regression models of blood pressure on the five neuropathologic measures. In particular, the estimated higher mean difference in SBP in those with cerebral microinfarcts > 2 compared to those with cerebral microinfarcts ≤2 found in participants age 65 to 80 attenuated to 5.1 mmHg (95% CI: −6.4, 15.8). However, there continued to be a significantly elevated relative risk for cerebral microinfarcts associated with SBP in younger participants who were not taking antihypertensive medications after adjusting for selection bias (RR: 1.38; 95% CI: 1.05, 1.94).
DISCUSSION
In this evaluation of blood pressure and neuropathologic correlates of dementia, we found that increased SBP measured at study enrollment (average of 7.7 years prior to death) was associated with higher occurrence of cerebral microinfarcts in younger participants (aged 65 to 80 at enrollment), but not with the presence of cystic macroinfarcts, AD neuropathologic changes (neurofibrillary tangles and neuritic plaques), or neocortical Lewy bodies. This higher risk for microinfarcts associated with increased SBP was particularly strong in those younger participants who did not report taking blood pressure medications. To our knowledge, this is the first study investigating the relationship between blood pressure and the multiple brain pathologic changes which are associated with cognitive impairment in older adults, including measures of both neurodegeneration and cerebrovascular damage.
We, and others, have recently highlighted the importance of cerebral microinfarcts as an independent pathological correlate of age-related cognitive impairment and dementia.10, 11 Microinfarcts are below the current limit of detection by current neuroimaging and are by definition too small to be identified by visual inspection at autopsy; rather, they are revealed by protocol-based regional microscopic examination for small vessel-based damage in the cerebrum. We have shown that a burden of cerebral microinfarcts exceeding two is associated with an approximately 5-fold increase in risk for incident dementia. 11 Although the specific cerebral microinfarcts identified in our screening protocol may not be sufficient by themselves to cause dementia, they may be structural indicators of more widespread cerebral microangiopathy and associated brain injury. The finding of an association between SBP and cerebral microinfarcts is consistent with reports of strong associations between SBP and retinal microvascular abnormalities, such as generalized arteriolar narrowing and arteriovenous nicking, from large population based studies;29, 30 retinal microvascular changes may reflect brain microvascular changes, as they have been associated with an increased risk of stroke, increased grade of white matter MRI changes,31 and cognitive impairment.32
Our study did not find a significant correlation between blood pressure and the number of cystic macroinfarcts on autopsy. Hypertension has been identified as a known risk factor for ischemic stroke in longitudinal epidemiological studies.36–38 For example, the Framingham study showed that the risk of ischemic stroke at age 60 and 70 years was associated with elevated recent and antecedent blood pressure.37 Two other autopsy studies have found conflicting results. Researchers from the Hisayama Study, using clinical and autopsy data, found that hypertension was a significant risk factor for all stroke subtypes except for cardioembolic strokes in men.39, 40 The Honolulu Heart Study also observed that blood pressure was associated with cerebrovascular lesions on autopsy, but this association did not persist after individuals with myocardial infarctions were removed from their analysis; the authors interpreted this finding as suggestive of two subsets of people with cerebral infarction.41 Our study contained a smaller sample size than epidemiological studies and the Hisayama Study, and had a smaller percentage of participants with coronary artery disease than the Honolulu Heart Study. With these differences, it is possible that our sample was underpowered to detect an association between cystic infarcts and blood pressure. Other possible sample characteristics that could explain the lack of association between blood pressure and cystic macroinfarcts in this study might include differences in race (primarily Caucasian in our study versus Asian), gender, the prevalence of hyperlipidemia treatment or aspirin use, or other lifestyle or demographic factors.
The relationship between blood pressure and AD neuropathologic change is less well understood. It has been speculated that blood pressure abnormalities could have a direct effect on the development of AD neuropathologic changes, including the hypothesis that vascular damage can initiate a cascade of events that leads to AD pathology.42–44 Previous autopsy studies have indeed found that hypertension increases risk for AD pathologic changes. For example, researchers in the Honolulu Heart Program/Honolulu-Asia Aging Study (HAAS) found that elevated midlife SBP (≥ 160 mm Hg) was associated with greater numbers of neuritic plaques in the neocortex and hippocampus, and elevated DBP (≥ 95 mm Hg) was associated with greater number of neurofibrillary tangles in the hippocampus. 15 This association was independent of the presence of macroinfarcts, but whether this association was independent of microinfarcts was not investigated. Another difference between our studies is that we have drawn associations with established staging systems for neuritic plaques and neurofibrillary tangles (CERAD and Braak), rather than with the number of these lesions in different regions as done by HAAS investigators. Finally, blood pressure was determined at midlife in HAAS participants rather than in later years as in ACT. Whether there is an independent link between blood pressure and AD pathologic changes in ACT participants needs to be studied in the future as the autopsy sample enlarges.
Our finding of an association between SBP and cerebral microinfarcts in younger participants (age 65 to 80) is consistent with our previous study showing an association between high SBP and greater clinical dementia risk in younger (age <75) but not older participants.6 The current study, however, has a relatively small sample size, so additional studies are needed to more definitively establish age-varying associations between hypertension and dementia neuropathology. The presence of an age dependent association may have implications on clinical practice, as benefits and risks of treating hypertension in the very old versus young older adults are complex issues and greatly debated in the literature.33–35
The strengths of our study are the representative sample of community-dwelling participants from whom its autopsied subjects were drawn, its prospective study design and its relatively large number of brain autopsies. Although our sample is overwhelmingly Caucasian (non-Caucasians <5%), it includes both male and female participants and is more representative of the US population in contrast to other studies, such as HAAS or the Religious Orders Study.45, 46 Finally, we performed a comprehensive evaluation of pathologic changes in brain, which involved examining hallmark changes of neurodegeneration and cerebrovascular damage. Our study also has several limitations. As with all observational studies, our findings suggest association but can not establish causation, and do not determine whether additional cardiovascular risk factors may be involved. There are also sources of potential bias or confounding. A relatively obvious concern relates to the study’s reliance on a select sample of individuals who died and gave permission to be autopsied. Because hypertension relates to death in multiple pathways that are not easily assessed and because the assessment of AD neuropathologic changes can occur only after death, the possibility of confounding is substantial and is not readily adjusted for. Thus, our findings should be extrapolated to living populations with caution. Furthermore, differences in age at death, gender, and ethnicity of autopsied and nonautopsied participants further restrict the generalizability of our findings. When we attempted to adjust for these variables, the overall association between SBP and microinfarcts in those aged 65 to 80 was attenuated and no longer statistically significant. However, an elevated risk of cerebral microinfarcts associated with increased SBP in those participants without antihypertensive treatment remained. One possibility for the reduced strength of association between SBP and cerebral microinfarcts in the autopsied sample is that there were more participants who self-reported hypertension and received antihypertensive treatment in those who died but did not undergo autopsy. Because of the lack of association between SBP and cerebral microinfarcts in those receiving antihypertensive treatment, extrapolating autopsy findings to a population with a higher proportion of treated participants would reduce the association between SBP and microinfarcts. Another concern is that treated versus untreated hypertension was assessed through participants’ self-report that may be affected by recall bias. The recall bias is of relatively less concern in our prospective study design because all participants were free of dementia at the time enrollment. Whether self-reported treatment reflects the effect of blood pressure control and/or the additional protective effect of an individual blood pressure medication or medication class needs to be further investigated.
In summary, the association between SBP and > 2 cerebral microinfarcts in adults aged 65 to 80 in the current study, our previous determination that > 2 cerebral microinfarcts greatly increases the risk of dementia, and our previous observation that elevated SBP increases risk of dementia, provide converging evidence that elevated SBP might cause wide spread microvascular damage to cerebrum, increasing risk of clinical dementia. Dementia risk associated with hypertension as a modifiable risk factor may be reduced with adequate treatment.
ACKNOWLEDGMENTS
We thank all of the faculty and staff who have worked on this study and made this article possible. Specifically, we gratefully acknowledge Mary Lou Thompson, PhD, for providing statistical consultation.
This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington. This work was supported by grants AG020020, AG006781, AG023801, and AG005136 from the National Institute on Aging, the Nancy and Buster Alvord Endowment, and the Department of Veterans Affairs Mental Illness Research, Education, and Clinical Center Special Fellowship in Advanced Psychiatry.
Sponsor’s Role: The funding sources had no role in the design, methodology, data analysis, or preparation of this manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Study concept and design (LY Wang, JA Sonnen, TJ Montine, G Li, EB Larson), acquisition of study participants and data (JD Bowen, W McCormick, EB Larson, JA Sonnen, TJ Montine), analysis and interpretation of data (JB Shofer), preparation of manuscript (LY Wang, JA Sonnen, TJ Montine, G Li), and critical review of the manuscript (LY Wang, EB Larson, JA Sonnen, JB Shofer, W McCormick, JD Bowen, TJ Montine, G Li)
REFERENCES
- 1.Elias MF, Wolf PA, D'Agostino RB, et al. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am J Epidemiol. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 4.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- 5.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Rhew IC, Shofer JB, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: A community-based prospective cohort study. J Am Geriatr Soc. 2007;55:1161–1167. doi: 10.1111/j.1532-5415.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 8.Leverenz JB, McKeith IG. Dementia with Lewy bodies. Med Clin North Am. 2002;86:519–535. doi: 10.1016/s0025-7125(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 9.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: A commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 10.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 11.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 12.Berg L, McKeel DW, Jr., Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 13.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 14.Launer LJ, White LR, Petrovitch H, et al. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 2001;57:1447–1452. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- 15.Petrovitch H, White LR, Izmirilian G, et al. Honolulu-Asia aging Study. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Sparks DL, Scheff SW, Liu H, et al. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- 17.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 18.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 22.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 23.Braak E, Griffing K, Arai K, et al. Neuropathology of Alzheimer's disease: What is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249 Suppl 3:14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 24.Leverenz JB, Hamilton R, Tsuang DW, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 2008;18:220–224. doi: 10.1111/j.1750-3639.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumley T, Kronmal R, Ma S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators, and Algorithms; UW Biostatistics Working Paper Series Working Paper; 2006. [(July 19, 2006)]. [Google Scholar]
- 26.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 27.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 29.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: The Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longstreth W, Jr., Larsen EK, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: The Cardiovascular Health Study. Am J Epidemiol. 2007;165:78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 32.Sharrett AR. A review of population-based retinal studies of the microvascular contribution to cerebrovascular diseases. Ophthalmic Epidemiol. 2007;14:238–242. doi: 10.1080/09286580701396712. [DOI] [PubMed] [Google Scholar]
- 33.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 34.Larson EB. Evidence, Guidelines, Performance Incentives, Complexity, and Old People: A Clinician's Dilemma. J Am Geriatr Soc. 2009;57:353–354. doi: 10.1111/j.1532-5415.2008.02125.x. [DOI] [PubMed] [Google Scholar]
- 35.Molander L, Lovheim H, Norman T, et al. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. J Am Geriatr Soc. 2008;56:1853–1859. doi: 10.1111/j.1532-5415.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 36.Ohira T, Shahar E, Chambless LE, et al. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 37.Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: The Framingham Study. Arch Intern Med. 2001;161:2343–2350. doi: 10.1001/archinte.161.19.2343. [DOI] [PubMed] [Google Scholar]
- 38.Straus SE, Majumdar SR, McAlister FA. New evidence for stroke prevention: Scientific review. JAMA. 2002;288:1388–1395. doi: 10.1001/jama.288.11.1388. [DOI] [PubMed] [Google Scholar]
- 39.Shinkawa A, Ueda K, Kiyohara Y, et al. The Hisayama Study. Silent cerebral infarction in a community-based autopsy series in Japan. Stroke. 1995;26:380–385. doi: 10.1161/01.str.26.3.380. [DOI] [PubMed] [Google Scholar]
- 40.Tanizaki Y, Kiyohara Y, Kato I, et al. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke. 2000;31:2616–2622. doi: 10.1161/01.str.31.11.2616. [DOI] [PubMed] [Google Scholar]
- 41.Stemmermann GN, Hayashi T, Resch JA, et al. Risk factors related to ischemic and hemorrhagic cerebrovascular disease at autopsy: The Honolulu Heart Study. Stroke. 1984;15:23–28. doi: 10.1161/01.str.15.1.23. [DOI] [PubMed] [Google Scholar]
- 42.Humpel C, Marksteiner J. Cerebrovascular damage as a cause for Alzheimer's disease. Curr Neurovasc Res. 2005;2:341–347. doi: 10.2174/156720205774322610. [DOI] [PubMed] [Google Scholar]
- 43.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 44.Kalaria RN. Linking cerebrovascular defense mechanisms in brain ageing and Alzheimer's disease. Neurobiol Aging. 2008;30:1512–1514. doi: 10.1016/j.neurobiolaging.2007.10.020. Epub 2008 Jan 10. [DOI] [PubMed] [Google Scholar]
- 45.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 46.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]