Abstract
Objectives
To approximate methods for human transcranial magnetic stimulation (TMS) in rats, we tested whether lateralized cortical stimulation resulting in selective activation of one forelimb contralateral to the site of stimulation could be achieved by TMS in the rat.
Methods
Motor evoked potentials (MEP) were recorded from the brachioradialis muscle bilaterally in adult male anesthetized rats (n=13). A figure-of-eight TMS coil was positioned lateral to midline. TMS intensity was increased stepwise from subthreshold intensities to maximal machine output in order to generate input-output curves and to determine the motor threshold (MT) for brachioradialis activation.
Results
In 100% of the animals, selective activation of the contralateral brachiradialis, in the absence of ipsilateral brachiradialis activation was achieved, and the ipsilateral brachioradialis was activated only at TMS intensities exceeding contralateral forelimb MT. With increasing TMS intensity, the amplitudes of both the ipsilateral and contrlateral signals increased in proportion to TMS strength. However the input-output curves for the contralateral and ipsilateral brachioradialis were significantly different (p<0.001) such that amplitude of the ipsilateral MEP was reliably lower than the contralateral signal.
Conclusions
We demonstrate that lateralized TMS leading to asymmetric brachioradialis activation is feasible with conventional TMS equipment in anesthetized rats.
Significance
These data show that TMS can be used to assess the unilateral excitability of the forelimb descending motor pathway in the rat, and suggest that rat TMS protocols analogous to human TMS may be applied in future translational research.
Keywords: transcranial magnetic stimulation, TMS, rat, motor evoked potential, MEP
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a nearly quarter-century-old method for noninvasive focal cortical stimulation that is in wide use in humans for studying cortical plasticity and cortical excitability (Barker et al. 1985; Barker et al. 1987; Hallett 2007). TMS in humans is commonly applied over the motor cortex such that a reliable unilateral motor evoked potential (MEP) can be elicited in the contralateral arm and hand muscles (Kobayashi and Pascual-Leone 2003; Fitzgerald et al. 2006). The MEP features, such as its peak amplitude or its integrated voltage, can then be measured as markers of regional cortical excitability, and the MEP latency can be used to assess conduction along central motor pathways. Presently, motor cortex TMS methods are applied in a wide range of studies in healthy subjects and in patients with disorders of cortical excitability or corticospinal integrity. Epilepsy, stroke, motor neuron disease, and multiple sclerosis are among the diseases where motor cortex TMS methods appear useful to measure the extent of disease, or the effect of therapeutic interventions (Ziemann 2004; Rossini and Rossi 2007; Frye et al. 2008).
In recent years, TMS research has been extended to the rat (Luft et al. 2001; Aydin-Abidin et al. 2008; Rotenberg 2008), a common translational research animal model. Yet, although human in human TMS experiments TMS strength is most often calibrated to unilaterally activate the upper limb motor cortex, the majority of published TMS data in rats are derived from experiments where MEPs were recorded from the hindlimbs (Luft et al. 2001; Luft et al. 2002). Forelimb TMS-induced MEPs have been demonstrated in rats, but the capacity to induce unilateral forelimb MEPs and to measure their threshold for activation by TMS has not been described (Kamida et al. 1998; Nielsen et al. 2007). That is, relatively focal TMS of the rat motor cortex similar to human focal TMS that results in upper limb MEPs selectively contralateral to the site of stimulation has not been shown. Such regional stimulation in the rat would be useful in many experimental protocols where unilateral hemispheric stimulation is desired.
Among the present limitations for focal TMS in rats is the relatively broad volume of electrical current which can be induced by a conventional TMS coil which in a rat brain may translate to simultaneous stimulation of the motor cortex in both hemispheres and /or of the distal components of descending motor pathways, and thus limit the translational relevance of methods intended to model relatively focal human TMS. Improving focality of rat TMS by construction of miniature TMS coils has been difficult as this requires restricting the amount of current discharged into them, in order to prevent the risk of coil rupture (Cohen and Cuffin 1991). However, the lack of focality offered by conventional TMS coils may be overcome with eccentric coil positioning such that only a fraction of the electromagnetic field is applied to one hemisphere (Luft et al. 2001). Accordingly, to better approximate human focal TMS methods in rats and to facilitate future translational rat TMS experiments, we conducted the present study to test whether lateralized TMS coil placement can lead to asymmetry of the brachioradialis MEP in the rat.
MATERIALS AND METHODS
Animal preparation
Thirteen adult male Long-Evans rats (243 ± 37g; mean ± s.d.) were used for the present experiment. All animals were housed in a temperature-controlled animal care facility with a 12/hour light-dark cycle. All procedures were approved by and in accordance with the guidelines of the Animal Care and Use Committee at Children’s Hospital (Boston, MA) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were anesthetized with intraperitoneal sodium pentobarbital and placed into a stereotactic frame within 30 minutes after injection. Sodium pentobarbital was administered in divided doses starting with 50 mg/kg. After placement into the earbars, rats were assessed clinically at 15 minute intervals and additional sodium pentobarbital was administered in increments equal to 20–30% of the initial dose until a clinical state of deep sedation with preserved withdrawal from foot pinch was achieved. All efforts were made to minimize animal suffering and the number of animals used. Rat body temperature was maintained with a circulating water heater.
Electromyography
MEPs were recorded with monopolar uninsulated 27G stainless steel needle electrodes (Axon Systems Inc., Hauppauge, NY) inserted into the belly of each brachioradialis muscle. Location of the brachioradialis was determined by palpation through with the forelimb in extended position. A reference electrode was positioned distally in the paw between the 3rd and 4th digit. Each animal was electrically grounded via a single needle electrode placed in the tail. EMG signal was band pass filtered 100 to 1000 Hz and amplified X1000 (AM Systems Model 1700; Sequim, WA). EMG signal was digitized with 40 kHz sampling and stored for post hoc analysis (AD Instruments Colorado Springs, CO). To eliminate averaging of positive and negative voltages of polyphasic signals, all MEP voltages were converted to absolute values and integrated for measures of power (area under the curve).
Transcranial Magnetic Stimulation
A MagStim Rapid magnetic stimulator equipped with one booster and a figure-of-eight coil (outside lobe diameter = 20 mm, inside lobe diameter = 10 mm; combined long axis length = 110 mm; Magstim Company, Whitland, UK) was used for the present study. To minimize the probability that electrical current would be induced by the magnetic stimulator in the metal stereotactic frame and conducted to the rat, the ear bars and snout clamp were covered with paraffin film before each experiment. The TMS coil was held in a micromanipulator and secured to the stereotactic frame (Figure 1), which permitted movement of the coil in three axes. TMS intensity was recorded as percent machine output (%MO), with 100% corresponding to the maximal amplitude electrical current conducted through the magnetic coil, and to the maximal strength of the generated magnetic field.
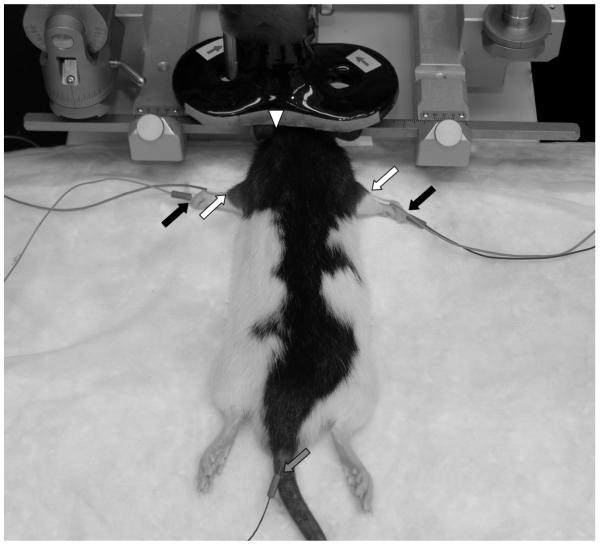
Figure 1. Rat and TMS Coil Setup.
Anesthetized rats were placed into an electrically isolated metal rodent stereotactic frame. EMG was recorded from the brachioradialis muscle bilaterally as the figure-8 TMS coil was positioned with its center anterior and lateral to bregma. Active (white arrow), reference (black arrow) and ground (gray arrow) electrodes are indicated. The TMS coil midline is marked by a white triangle. Note, direction of electrical current (arrows on coil) is constant in with the coil positioned over either the left or the right hemisphere.
TMS sessions conducted with the coil in a relatively constant position with the center 12.50 ± 0.84 mm (mean ± s.d.) lateral (mirrored either left or right) to midline, 3.58 ± 0.26 mm posterior to bregma and in light contact with the scalp, which in our experience approximates a 2.5 mm distance between an adult rat brain and coil surface. Since surgical exposure of the bregma would require a deeper anesthetic state than could be tolerated in our experiment, the anterior-posterior bregma coordinates were calculated relative to the interaural line and used a the zero referent according to methods described by Whishaw and colleagues (1977). The slight variation in coil center position in the present experiment reflects a small variance in skull width and curvature of the snout. Once the TMS coil was positioned, an input-output curve was generated by systematically adjusting the stimulator intensity in steps of 5% MO from 40 – 100 % MO with a 7 second inter-pulse interval.
Statistics
All data were reviewed post hoc by visual inspection. Individual EMG traces with obvious electrical artifact (< 0.1% of total) were eliminated from analysis. Peak-to-peak amplitude, motor threshold (MT) and integrated amplitude were computed automatically in the 5–30 msec time window, as our pilot data indicated a 4 msec maximal duration of TMS artifact, and approximately 25 msec maximal latency to onset of the MEP in rats. Values from ten consecutive sweeps were averaged for the comparison of MEP voltages at any one TMS intensity. Means of values derived from the MEP ipsilateral and contralateral to site for stimulation at multiple TMS intensities were compared by paired 2-tailed t-tests, and proportions were compared by chi squared test. Contribution of laterality and stimulus intensity to MEP amplitude were compared by two-way ANOVA.
RESULTS
Motor Threshold and the Contralateral MEP
Rapid motor threshold (MT) measures were obtained from the input-output curve in each animal post hoc by automated analysis. We designated forelimb MEPs as “contralateral” if the signal was from the brachioradialis in the limb opposite to the stimulated hemisphere, and “ipsilateral” if the signal was from the brachioradialis in the limb on the same side to the stimulated hemisphere.
Based on pilot brachioradialis TMS-derived MEP data in our laboratory and on published reports establishing motor hindlimb MEPs in rats, we defined MT as the stimulation intensity (% MO) at which a MEP >15 μV in amplitude was recorded on ≥ 5 of 10 trials (Luft et al. 2001). With these criteria, we identified average MT of 86.2 ± 8.9 % MO (mean ± s.d.). We also found a left-right discrepancy such that average left hemisphere (right brachioradialis) MT was 81.4 ± 8.0 % MO (n=7), and the right hemisphere (left brachioradialis) average MT was 91.7 ± 7.5 % MO (n=6), significantly greater than left (p = 0.03; t = 2.42). However, as TMS coil positions were essentially constant over the left and right hemisphere, the possibility that the left-right discrepancy reflects a difference in size or location of the relevant portions of the motor systems should be considered and further study will be required to validate this finding.
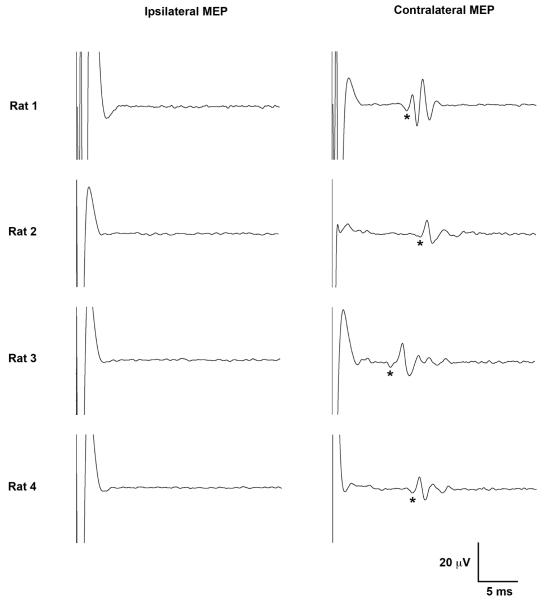
At 100% MT, contralateral brachioradialis MEP onset latencies, defined as time to the start of the first positive deflection, ranged from 7.6 to 12.4 ms (mean 10.4 ± 1.5 ms). The MEPs generally had a complex and polyphasic morphology with one or two prominent peaks followed by a variable number of lower amplitude peaks (Figure 2), resembling in appearance and reliable lateralization the cortically-derived MEPs seen with direct electrical stimulation (Schlag et al. 2001).
Figure 2. Representative Motor Evoked Potential (MEPs).
Lateralized ipsilateral (left) and contralateral (right) MEPs recorded from the brachioradialis in four rats are shown. Each tracing is an average of five MEPs at 100% MT. Latency to the start of the first positive deflection (*) was used to calculate timing of MEP onset. Note, the polyphasic appearance of the MEP signal required rectification to absolute values for statistical integration of voltages. Upward trace deflections correspond to negative voltage.
Forelimb MEP Lateralization
The contralateral and ipsilateral forelimbs differed significantly with respect to the MT and the MEP size. Although MT (>15 μV MEP on ≥5 of 10 trials) was reached in the contralateral limb in 100% of the animals, MT criteria in the ipsilateral limb were met only in 15% (2 of 13) of the subjects. In the remaining rats, ipsilateral MT was not reached, even at 100% MO (Figure 3).
Figure 3. Lateralized Forelimb MEP Activation.
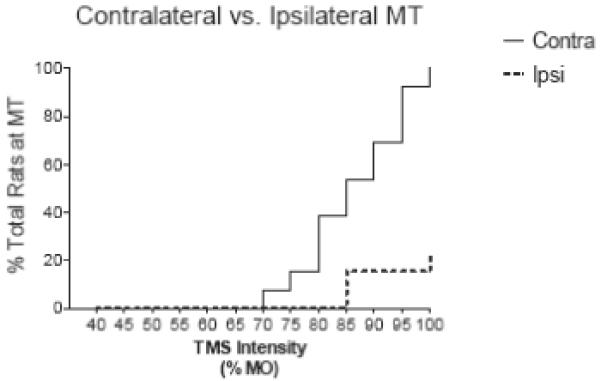
Asymmetry of the MEP response to eccentric TMS coil position is demonstrated in a Kaplan-Meier curve which shows a greater proportion of rats with contralateral (solid line) than ipsilateral (dashed line) activation at suprapthreshold stimulator intensities
The lateralization of the brachioradialis signal is also supported by the input-output curves which demonstrate that ipsilateral MEP amplitudes were smaller than contralateral ones. For the entire group of animals, at stimulator intensities ≥70% MO, peak as well as integrated MEP voltage was significantly greater for contralateral than for ipsilateral MEP (Figure 4A, 4B). Two-way ANOVA of these data reveals significant (P < 0.001) independent contributions of signal laterality and stimulus strength toward either the peak or integrated voltage MEP measure.
Figure 4. Ipsilateral-contralateral MEP Size Discrepancy.
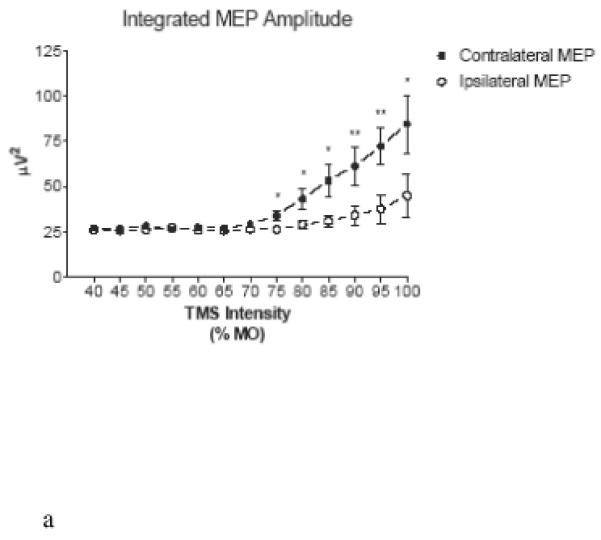
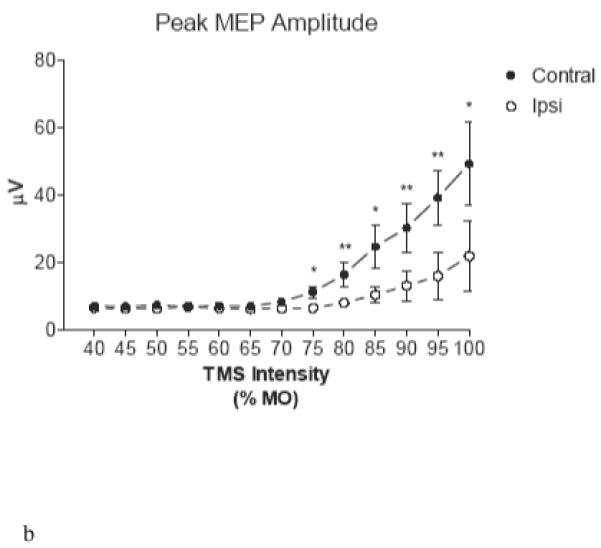
Input-output curves show consistently greater mean integrated (A) and maximal (B) voltage in the brachioradialis contralateral to the stimulated hemisphere at intensities > 70% MO. Error bars represent standard error of mean. (*) p<0.05; (**) p<0.01
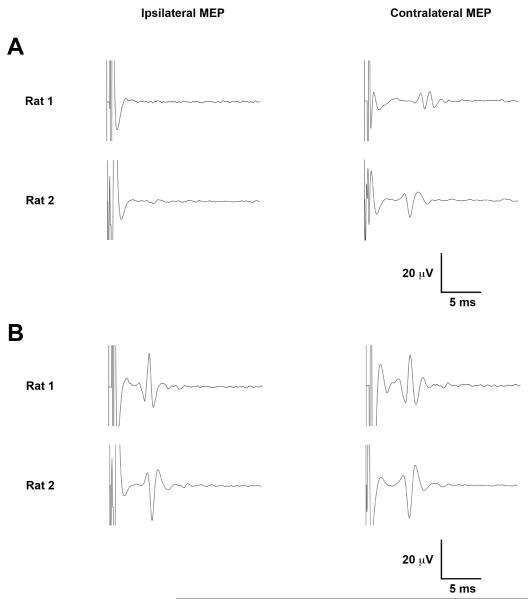
In two rats, a bilateral MEP (Figure 5) consistent with bilateral activation of descending motor pathways was identified, at stimulation intensities exceeding 100% MT. Although we did not observe isolated limb twitches with TMS in any animal, at TMS intensities approaching 100% MO, a visible twitch of the torso variably accompanied the bilateral MEP suggesting relatively broad motor activation with the higher TMS intensities.
Figure 5. Contralateral and Ipsilateral MEPs.
(A) Representative averaged sweeps from two rats show a predominantly contralateral MEP at 100% MT. (B) Bilateral MEPs are evident at stimulator of 112.5% MT (top) and 133.3 % MT (bottom). Five sweeps were averaged per tracing. Upward trace deflections correspond to negative voltage.
DISCUSSION
The present experiment provides the first demonstration that predominantly contralateral forelimb MEPs can be obtained with conventional TMS equipment that is adapted for rats. We also show that basic TMS-derived measures such as MT, an input-output curve, and an ipsilateral-contralateral MEP size ratio can be obtained from the rat brachioradialis. As 100% of subjects showed an ipsilateral-contralateral brachioradialis MEP discrepancy consistent with preferred activation of a contralateral motor pathway, these data support the hypothesis a reproducible asymmetric forelimb motor response can be achieved with a fixed eccentric TMS coil position. Although the current data do not exclude bilateral activation of extra-motor cortex, or motor connections beyond the brachioradialis, the reliable MEP asymmetry suggests that regional TMS is feasible in anesthetized rats. These data provide a step toward extending human TMS protocols to translational research where unilateral forelimb descending motor pathways can be interrogated in rats and in rat disease models.
The relatively low signal amplitude and high MT for the contralateral MEP, 100% MO in one rat may be an anesthesia effect. For instance, the paralytic capacity of sodium pentobarbital (Krampfl et al., 2000) may have contributed to the overall lower MEP amplitude in our study as compared to other published reports. This elevated threshold for activation may also account for the large fraction (85%) of rats who did not meet ipsilateral limb MT criteria. However, an overall lower MEP size does not affect the main observation of MEP asymmetry with eccentric position. We anticipate that in follow-up work to this study, simultaneous recording from multiple muscle groups in the forelimbs and in the hindlimbs may facilitate more detailed characterization of lateralized TMS effects in rats.
The broad MT range (Figure 3) in our data also suggests that follow-up experiments aimed to test systematically a range of anesthetics and doses to identify an optimal rat TMS protocol would also be useful. The application of EEG methods to monitor the depth of anesthesia as described by Luft and colleagues (2001) may also be beneficial in future experiments. Additionally, the capacity for relatively focal cortical activation with a large TMS coil suggests that development of a novel compact TMS coil to facilitate focal electromagnetic coupling between the stimulator and the rat brain (Liebetanz et al. 2003) would perhaps enable greater localization of the induced current in future work.
The origin of MEPs in rats remains a matter of interest that will require further investigation that is beyond the scope of the current experiment which is aimed to demonstrate asymmetry of the brachioradialis MEP with eccentric TMS coil positioning in rats. The strong lateralization of the MEPs in our study suggests that the signals originate within one hemisphere. Further, their polyphasic morphology suggests a cortical origin as these appeared similar in latency and morphology to published cortically-derived MEPs in rats that are seen with direct electrical stimulation (Schlag et al. 2001). However, as some signals had relatively early (<8 msec) latencies to onset, a subcortical origin of activation is plausible. We anticipate that further studies employing methods for focal brain and spinal cord lesion as well as precise electrical stimulation to test whether distinct cortical and subcortical forelimb MEP generators can be identified. Ultimately, the translational relevance of rat MEP data will have to be confirmed in follow-up studies since whether and to what extent rat corticomotor connectivity resembles human is an area of active investigation (Liang et al., 1991;Yang Liang et al., 1993; Alstermak et al., 2004).
Acknowledgements
This work was supported by Citizens United for Research in Epilepsy (AR), CIMIT (APL), and NIH grants K08NS055895 (AR), K24RR018875 (APL) and NS31718 (FEJ). The authors also appreciate a personal communication from Dr. Andreas Luft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alstermark B, Ogawa J, Isa T. Lack of Monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–9. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- Avdin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High-and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–61. doi: 10.1007/s00221-008-1356-2. [DOI] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jalinous R, Jarratt JA. Magnetic stimulation of the human brain and peripheral nervous system: an introduction and the results of an initial clinical evaluation. Neurosurgery. 1987;20:100–9. doi: 10.1097/00006123-198701000-00024. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN. Developing a more focal magnetic stimulator. Part I: Some basic principles. J Clin Neurophysiol. 1991;8:102–11. doi: 10.1097/00004691-199101000-00013. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Cristiani R, Bertini M, Curcio G, Ferrara M, Fratello F, et al. Handedness is mainly associated with an asymmetry of corticospinal excitability and not of transcallosal inhibition. Clin Neurophysiol. 2004;115:1305–12. doi: 10.1016/j.clinph.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Elalmis DD, Ozgunen KT, Binokay S, Tan M, Ozgunen T, Tan U. Differential contributions of right and left brains to paw skill in right-and left-pawed female rats. The International journal of neuroscience. 2003;113:1023–42. doi: 10.1080/00207450390204068. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Frye RE, Rotenberg A, Ousley M, Pascual-Leone A. Transcranial magnetic stimulation in child neurology: current and future directions. J Child Neurol. 2008;23:79–96. doi: 10.1177/0883073807307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G, Verdu E, Fores J, Lopez-Vales R, Navarro X. Functional and electrophysiological characterization of photochemical graded spinal cord injury in the rat. Journal of neurotrauma. 2003;20:501–10. doi: 10.1089/089771503765355568. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Kamida T, Fujiki M, Hori S, Isono M. Conduction pathways of motor evoked potentials following transcranial magnetic stimulation: a rodent study using a “figure-8” coil. Muscle Nerve. 1998;21:722–31. doi: 10.1002/(sici)1097-4598(199806)21:6<722::aid-mus3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Schelsinger F, dangler R, Johannes B. Phenobarbital has curare-like effects on adult-type nicotinic acetylcholine receptor channel currents. Anesth Analg. 90:970–4. doi: 10.1213/00000539-200004000-00036. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Liang F, Moret V, Wiesendanger M, Rouiller EM. CM connections in the rat: evidence from double-labeling of motoneuron and corticospinal axon axon arborizations. J Comp Neurol. 1991;311:356–66. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- Liang F, Rouiller EM, Wiesendanger M. Modulation of sustained electromyographic activity by single intracortical microstimuli: comparison of two forelimb motor cortical areas of the rat. Somatosensory & motor research. 1993;10:51–61. doi: 10.3109/08990229309028823. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Fauser S, Michaelis T, Czeh B, Watanabe T, Paulus W, et al. Safety aspects of chronic low-frequency transcranial magnetic stimulation based on localized proton magnetic resonance spectroscopy and histology of the rat brain. J Psychiatr Res. 2003;37:277–86. doi: 10.1016/s0022-3956(03)00017-7. [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, et al. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142:562–9. doi: 10.1007/s00221-001-0952-1. [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Cohen LG, Thakor NV, Hanley DF. Transcranial magnetic stimulation in the rat. Exp Brain Res. 2001;140:112–21. doi: 10.1007/s002210100805. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci. 2007;25:805–14. doi: 10.1111/j.1460-9568.2007.05326.x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–8. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Depositario-Cabacar D, Bae EH, Harini C, Pascual-Leone A, Takeoka M. Transient suppression of seizures by repetitive transcranial magnetic stimulation in a case of Rasmussen’s encephalitis. Epilepsy Behav. 2008 doi: 10.1016/j.yebeh.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Muller P, Birnbaum D, Harrington M, Riviello JJ, Pascual-Leone A, Jensen FE. Seizure suppression by EEG-guided repetitive transcranial magnetic stimulation in the rat. Clin Neurophysiol. 2008 doi: 10.1016/j.clinph.2008.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag MG, Hopf R, Redl H. Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in the rat. Somatosensory & motor research. 2001;18:106–16. doi: 10.1080/135578501012006219. [DOI] [PubMed] [Google Scholar]
- Wishaw IQ, Cioe JD, Previsich N, Kolb B. The variability of the interaural line vs the stability of bregma in rat stereotactic surgery. Physiology and Behavior. 1977;19:719–22. doi: 10.1016/0031-9384(77)90304-3. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–29. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]