Abstract
To gain insights into the cellular processes required for intracellular bacterial pathogenesis, we previously developed a generalisable screening approach to identify small molecule compounds that alter Listeria monocytogenes infection. In this report, a small molecule library enriched for compounds affecting neurological functions was screened and 68 compounds that disrupted L. monocytogenes infection of macrophages were identified. Many of these compounds were known antimicrobial agents, however 26 compounds were novel inhibitors of intracellular infection. Two of the compounds chosen for further study, the antipsychotic drug thioridazine and the calcium channel blocker bepridil, exhibited dose-dependent inhibition of vacuolar escape and intracellular replication of L. monocytogenes during infection of murine macrophages. These results suggest that clinically approved neurological drugs may provide a novel source of anti-infective agents that are suitable for development as therapeutics against intracellular bacterial infections.
Keywords: Listeria monocytogenes, Small molecule screen, Intracellular infection, Bepridil, Neurological compounds, Thioridazine
1. Introduction
Listeria monocytogenes is an intracellular bacterial pathogen that causes significant morbidity and mortality in immunocompromised individuals, infants and the elderly. Infections typically occur through ingestion of contaminated foods and most often result in gastroenteritis. However, infections can also cause more serious outcomes such as septicaemia, meningitis and death [1]. Although antibiotics can be used successfully to treat L. monocytogenes infection, the mortality rate is often 20–30% [2], the highest fatality rate of any food-borne pathogen.
For several decades, L. monocytogenes has been studied as a model organism for understanding host-pathogen interactions leading to virulence as well as acquired cell-mediated immunity to intracellular pathogens, thus the cell biology of L. monocytogenes infection is well characterised. Following entry into host cells, bacteria initially reside within a membrane-bound vacuole, however L. monocytogenes rapidly escapes this compartment to enter the host cell cytosol where replication occurs. Cytosolic bacteria use actin-based motility to move and spread from cell to cell without contacting the extracellular environment [3]. Whilst the majority of studies into the pathogenesis of L. monocytogenes have employed classical genetic-based approaches [1], chemical genetic strategies have recently been applied to elucidate the biological processes governing infection in many pathogenic systems [4]. As small molecules have the ability to affect pathways within both the bacterium and the host cell, numerous insights into the requirements for host-pathogen interactions during intracellular infection can be achieved. We have previously developed a microscopy-based screening approach to identify small molecules that disrupt intracellular infection by L. monocytogenes. This approach was used in a limited capacity to screen a small collection of known bioactive compounds in order to identify molecules that may potentially be developed as anti-infectives for intracellular bacterial pathogens [5].
We previously identified the neurological compound pimozide as an inhibitor of L. monocytogenes entry into host cells, vacuole escape and cell-to-cell spread of bacteria [5]. In this report, we have further examined the effects of neurological compounds on intracellular bacterial infection. A small molecule library consisting of 1040 compounds that was enriched for molecules affecting neurological processes was screened and 68 compounds that disrupted intracellular infection of murine bone marrow-derived macrophages (BMMs) by L. monocytogenes were identified. A list of all 1040 compounds in this collection can be found at http://iccb.med.harvard.edu/screening/compound_libraries/ninds.htm. Because the library contains many US Food and Drug Administration (FDA)-approved compounds (75% of the collection), the potential to identify novel therapeutic uses of clinically approved drugs is enhanced. This is of particular significance as the emergence of multiple antibiotic-resistant strains of pathogens necessitates the development of improved anti-infectives as therapeutic strategies. Of the 68 identified compounds, 26 displayed novel anti-infective capability. Further examination of two of the compounds, thioridazine and bepridil, indicated that these drugs decreased intracellular infection by L. monocytogenes in a dose-dependent manner by significantly inhibiting vacuole escape. This study demonstrates that clinically approved classes of compounds with novel anti-infective capabilities can be readily identified and potentially developed as next-generation therapeutics for intracellular bacterial infections.
2. Materials and methods
2.1. Bacterial strains
Wild-type L. monocytogenes strain 10403S was grown in brain-heart infusion (BHI) medium (Difco, Detroit, MI) at 30 °C without shaking prior to infection of host cells. Listeria monocytogenes 10403S expressing green fluorescent protein (strain DH-L1252) was grown in a similar manner and was used to infect host cells for small molecule screening and vacuole escape assays.
2.2. Cell culture
Murine BMMs were prepared as previously described [5]. Briefly, femurs and tibias were removed from 4–8-week-old BALB/c mice (Jackson Laboratories, Bar Harbor, ME) and bone marrow cells were flushed from the bones and cultured for 6 days in BMM medium [complete Dulbecco’s Modified Eagle Medium (DMEM) and 30% L929 cell supernatant]. On Day 6, BMM cells were harvested, washed with phosphate-buffered saline (PBS) and plated at an appropriate density for 18–24 h prior to infection experiments. All cell cultures were maintained at 37 °C in a 5% CO2-air atmosphere.
2.3. Small molecule screen
A small molecule screen of the National Institute of Neurological Disorders and Stroke (NINDS) (MicroSource Discovery Systems Inc., Gaylorsville, CT) compound library was conducted as previously described [5]. Briefly, BMMs were seeded in clear-bottom 384-well plates (Corning-Costar, Corning, NY) for 14–16 h and then incubated with the compounds for 2 h followed by infection with L. monocytogenes strain DH-L1252 [multiplicity of infection (MoI) of 1:2]. After 1 h of incubation, gentamicin was added to kill extracellular bacteria and infected BMMs were incubated for an additional 14 h and then fixed and stained. Fluorescence microscopy images were acquired at two positions per well using an ImageXpress™ high-content cell analysis system (Molecular Devices, Downingtown, PA) and analysed with MetaMorph image analysis software (Molecular Devices). The NINDS library was screened three times in duplicate at the National Screening Laboratory for the Regional Centers of Excellence in Biodefense and Emerging Infectious Diseases (NSRB) (http://nsrb.med.harvard.edu) at Harvard Medical School (Boston, MA).
2.4. Extracellular growth assay
Wild-type L. monocytogenes 10403S was grown for 14–16 h in BHI medium at 30 °C without shaking and then back-diluted 1:20 into fresh BHI broth containing 25 μM of an NINDS compound (MicroSource Discovery Systems Inc.) or 0.001% dimethyl sulphoxide (DMSO) (in equivalent volumes). Cultures were grown at 37 °C with shaking and the optical density at 600 nm was measured at various time points to assess bacterial growth.
2.5. Intracellular growth assay
Intracellular growth assays were conducted as previously described [5]. Briefly, 4 × 105 BMMs/well were plated in a 24-well plate for 24 h. Cells were pre-treated for 2 h with medium containing 25 μM of compound (thioridazine or bepridil) or 0.001% DMSO followed by infection with L. monocytogenes 10403S (MoI 1:2). At 1 h post infection, 5 μg/mL gentamicin was added. The number of intracellular bacteria was determined at various time points by lysis of BMMs followed by plating of dilutions on Luria-Bertani (LB) agar and incubating for 16–20 h at 37 °C to enumerate the total number of intracellular bacteria.
2.6. Assessment of vacuole escape
Vacuolar escape was assessed as described previously [5]. Briefly, BMMs seeded on glass coverslips for 24 h were incubated in medium containing 25 μM of compound or 0.001% DMSO for 2 h. BMMs were then infected with L. monocytogenes strain DH-L1252 (MoI 10:1) for 30 min followed by the addition of 50 μg/mL gentamicin. At 90 min post infection, the coverslips were washed and fixed. Host cell F-actin was stained with Texas Red®-X phalloidin (Invitrogen, Carlsbad, CA) followed by staining with Hoechst 33342 (Invitrogen). Coverslips were analysed by fluorescence microscopy and images were acquired using MetaMorph imaging software (Molecular Devices). To quantify vacuole escape, >100 bacteria/coverslip were examined and the percentage of bacteria associated with F-actin was determined.
2.7. Dose-dependent inhibition of Listeria monocytogenes infection
The dose-dependent inhibition of intracellular infection was assessed using a derivation of the intracellular growth assay described above. BMMs were seeded 24 h prior to infection at 4 × 105 BMMs/well in a 24-well plate. Cells were washed with PBS and pre-treated for 2 h with medium containing two-fold dilutions of compound (thioridazine or bepridil ranging from 390 nM to 50 μM) or 0.001% DMSO. BMMs were infected (MoI 1:2) with L. monocytogenes 10403S in the presence of the indicated concentration of compound or 0.001% DMSO. At 1 h post infection, 5 μg/mL gentamicin was added to each well. Then, 4 h following infection the number of intracellular bacteria was determined by removing the medium from the wells, washing with PBS and lysing host cells in 500 μL of 0.1% Triton X-100 in PBS. Dilutions of host cell lysates were plated on LB agar and grown for 16–20 h at 37 °C to enumerate the total number of intracellular bacteria.
2.8. Statistical analysis
To assess the statistical significance of the vacuole escape assay, repeated measure one-way analysis of variance (ANOVA) was calculated using Prism software (GraphPad, San Diego, CA). A P-value of <0.05 was considered significant.
3. Results and discussion
3.1. Small molecule screen to identify neurological compounds with anti-infective activity
Previous research from our laboratory identified the anti-infective activity of the neurological drug pimozide. This unusual finding led us to pursue further the identification of neurological compounds exhibiting antimicrobial ability. Using a similar approach as previously described [5], we screened the NINDS small molecule library at the National Screening Laboratory for the Regional Centers of Excellence in Biodefense and Emerging Infectious Diseases (NSRB) at Harvard Medical School. This library contains 1040 compounds known to affect neurological activity.
From the NINDS library screen, 68 compounds were identified that disrupted L. monocytogenes infection in BMMs. These compounds were grouped based on defined biological function. The largest subgroup of compounds identified included known antibiotics or antibacterial agents (Table 1). In addition to antibiotics and antiseptics, antifungal, antiprotozoal and antihelminthic drugs were identified that were inhibitory to L. monocytogenes infection. Moreover, 26 compounds that exhibited unanticipated anti-infective activity were also identified (Table 2). The majority of these compounds were antipsychotic drugs or modulators of calcium pathways. Interestingly, whilst our previous work identified the antipsychotic drug pimozide as having anti-infective properties, and pimozide has been shown to antagonise calcium binding [6], it should be noted that inhibition of calcium binding did not appear to be necessary for pimozide anti-infective activity [5].
Table 1.
Compounds with predicted antimicrobial activity. Forty-two compounds identified from the National Institute of Neurological Disorders and Stroke (NINDS) library screen were previously identified as antimicrobial agents and are classified by known activity. All compounds listed resulted in decreased Listeria monocytogenes infection with three exceptions
| Compound | Defined activity |
|---|---|
| Antibiotic | |
| Hetacillin potassium | β-Lactam antibiotic |
| Erythromycin propionate lauryl sulfate | Macrolide antibiotic produced by Streptomyces erythreus |
| Spiramycin | Macrolide antibiotic |
| Oleandomycin phosphate | Antibiotic produced by Streptomyces antibioticus |
| Lasalocid sodium | Antibiotic |
| Oxytetracycline | Antibiotic produced by Streptomyces rimosus |
| Azithromycin | Macrolide antibiotic |
| Phenethicillin potassium | Antibiotic |
| Chlortetracycline | Antibiotic produced by Streptomyces aureofaciens |
| Erythromycin | Macrolide antibiotic |
| Doxycycline hydrochloride | Broad-range antibiotic, bacteriostatic |
| Roxithromycin | Macrolide antibiotic |
| Metampicillin sodium | β-Lactam antibiotic |
| Clofoctol | Antibiotic, bacteriostatic |
| Patulin | Antibiotic; mycotoxin |
| Bacampicillin hydrochloride | Antibiotic |
| Minocycline hydrochloride | Antibiotic |
| Clindamycin hydrochloride | Antibiotic, bacteriostatic |
| Rifampicin | Antibiotic |
| Meclocycline sulfosalicylate | Antibiotic, bacteriostatic |
| Trimethoprim | Antibiotic, bacteriostatic |
| Methacycline hydrochloride | Antibiotic |
| Helenine | Antibiotic produced by Penicillium funiculosum |
| Erythromycin ethylsuccinate | Macrolide antibiotic |
| Penicillin V potassium | Antibiotic, bactericidal |
| Tetracycline hydrochloride | Antibiotic |
| Mitomycin C | Antineoplastic antibiotic produced by Streptomyces caespitosus |
| Phenethicillin potassium | Antibiotic |
| Antiseptic | |
| Benzalkonium chloride | Topical antiseptic |
| Thimerosal | Antiseptic; preservative |
| Methylbenzethonium chloride | Topical antiseptic |
| Cetylpyridinium chloride | Topical antiseptic |
| Cetrimonium bromide | Topical antiseptic |
| Merbromin a | Topical antiseptic |
| Acriflavinium hydrochloride a | Antiseptic |
| Antifungal | |
| Phenylmercuric acetate | Fungicide; bactericide |
| Econazole nitrate | Antifungal |
| Ketoconazole b | Antifungal |
| Haloprogin | Broad-spectrum topical antifungal |
| Thiram | Fungicide; pesticide |
| Antihelminthic/antiprotozoal | |
| Mefloquine | Antimalarial |
| Quinacrine hydrochloride | Antihelminthic; antiprotozoal |
Two compounds (a) resulted in overall increased infection of host cells, while addition of one compound (b) resulted in increased infection of host cells, but with fewer cells infected. The compound library was screened three times in duplicate.
Table 2.
Compounds with novel anti-infective activity. Twenty-six compounds identified from the National Institute of Neurological Disorders and Stroke (NINDS) library screen were grouped by previously defined activity. All compounds listed resulted in decreased Listeria monocytogenes infection. The compound library was screened three times in duplicate
| Compound | Defined activity |
|---|---|
| Antipsychotic/psychotic | |
| Thioridazine hydrochloride | Antipsychotic for treatment of schizophrenia |
| Prochlorperazine edisylate | Antipsychotic for treatment of vertigo; antiemetic |
| Clomipramine hydrochloride | Antidepressant |
| Triflupromazine hydrochloride | Antipsychotic; D2 dopamine antagonist |
| Trifluoperazine hydrochloride | Antipsychotic for treatment of schizophrenia |
| Perphenazine | Antipsychotic; antiemetic |
| Methiothepin maleate | Antipsychotic; 5-HT1 and 2 serotonin antagonist |
| Fluphenazine hydrochloride | Antipsychotic; dopamine receptor antagonist |
| Cannabinol | Cannabinoid receptor agonist; inactive as psychotomimetic |
| δ-8-Tetrahydrocannabinol | Psychotomimetic; antiemetic; antiglaucoma |
| Calcium | |
| Amiodarone | Vasodilator; calcium channel (type l) blocker |
| Bepridil | Calcium channel blocker; antiarrhythmic |
| W-7 hydrochloride | Calmodulin antagonist |
| Proadifen hydrochloride | Cytochrome P450 inhibitor; Ca2+ antagonist; antiarrhythmic |
| Perhexiline maleate | Coronary vasodilator; Ca2+ antagonist |
| Hormone | |
| Tamoxifen | Selective oestrogen receptor modulator |
| Clomiphene citrate | Selective oestrogen receptor modulator |
| Anaesthetic | |
| Dyclonine hydrochloride | Topical anaesthetic |
| Oxethazaine | Local anaesthetic |
| Antineoplastic | |
| Floxuridine | Antineoplastic; antimetabolite |
| Celastrol | Antineoplastic; anti-inflammatory |
| Anticholinergic | |
| Drofenine hydrochloride | Anticholinergic; antispasmodic |
| Dicyclomine hydrochloride | Anticholinergic; antispasmodic |
| Anti-inflammatory | |
| Oxyphenbutazone | Anti-inflammatory; NSAID |
| Celecoxib | Anti-inflammatory; NSAID; COX-2 inhibitor |
| Parthenolide | Anti-inflammatory |
NSAID, non-steroidal anti-inflammatory drug; COX-2, cyclooxygenase-2.
3.2. Inhibition of extracellular growth of Listeria monocytogenes
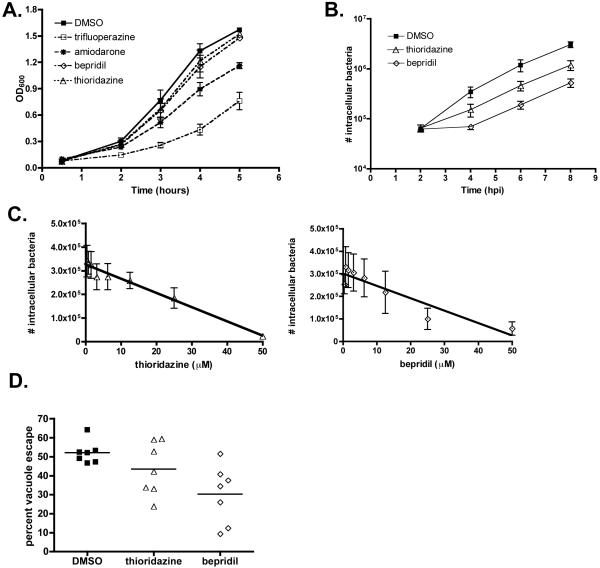
The anti-infective activities of the antipsychotic drugs thioridazine and trifluoperazine and the calcium inhibitors amiodarone and bepridil were further examined as these compounds are used clinically. Trifluoperazine and amiodarone partially inhibited the extracellular growth of L. monocytogenes in broth culture (Fig. 1A). Since we were most interested in compounds that inhibited intracellular infection, further efforts were focused on thioridazine and bepridil, two compounds that did not significantly inhibit extracellular growth of L. monocytogenes. Thioridazine is an antipsychotic drug that has been used to treat schizophrenia for over 40 years [7]. Moreover, antimicrobial activity of thioridazine both against intracellular and extracellular Staphylococcus aureus and Mycobacterium tuberculosis has been reported [8-10]. Bepridil is a calcium channel blocker used to treat angina pectoris [11] but has also been shown to reduce inflammation and pathology in a murine model of multiple sclerosis [12] and may also decrease axonal injury in other neuroinflammatory diseases [13].
Fig. 1.
Growth of Listeria monocytogenes in the presence of compounds identified from the National Institute of Neurological Disorders and Stroke (NINDS) library. (A) Extracellular growth of L. monocytogenes in brain-heart infusion broth supplemented with 25 μM of the indicated compound or 0.001% dimethyl sulphoxide (DMSO). The mean and standard error of the mean (S.E.M.) of three individual experiments is presented. OD600, optical density at 600 nm. (B) Intracellular growth of L. monocytogenes in murine bone marrow-derived macrophages (BMMs) is decreased in the presence of thioridazine or bepridil. The mean of six individual experiments performed in triplicate is shown. hpi, hours post infection. (C) BMMs were treated with two-fold dilutions of thioridazine or bepridil for 2 h prior to infection with L. monocytogenes. At 4 h post infection, the number of intracellular bacteria was determined. In each panel, the mean and S.E.M. of four individual experiments performed in triplicate is shown. A line of linear regression is also shown in each panel. (D) Vacuole escape of L. monocytogenes is significantly decreased in the presence of thioridazine or bepridil. The percentage of cytosolic bacteria (associated with F-actin) is reported. Each point represents the mean of one experiment in which at least 100 bacteria were counted.
3.3. Inhibition of intracellular growth of Listeria monocytogenes
To assess the effects of thioridazine and bepridil on the intracellular growth of L. monocytogenes, BMMs were treated with 25 μM of either compound or 0.001% DMSO prior to and during infection. BMMs treated with thioridazine or bepridil exhibited a reduction in intracellular bacteria, with a greater decrease in bacterial numbers observed in BMMs treated with bepridil (Fig. 1B). The intracellular infection analysis showed that the number of bacteria present at 2 h post infection was similar in drug-treated and untreated BMMs, suggesting that there was no defect in bacterial uptake into host cells. The decreased number of intracellular bacteria observed in drug-treated BMMs over the subsequent 6 h of the infection suggested a partial inhibition of the ability of bacteria to escape from the phagocytic vacuole [14].
3.4. Dose-dependent inhibition of Listeria monocytogenes infection
To determine the dose-dependent effects of thioridazine and bepridil, BMMs were treated with a range of concentrations of either compound prior to and during infection with L. monocytogenes. As shown in Fig. 1B, the inhibitory effects of the compounds could be observed by 4 h post infection, therefore this time point was chosen for the dose-dependency studies. The inhibitory effects on intracellular infection of both thioridazine and bepridil (Figs. 1C and 1D) were dependent on the dose administered, with a greater reduction in the number of intracellular bacteria observed as the concentration of each compound was increased to 50 μM.
3.5. Vacuole escape of Listeria monocytogenes is inhibited by neurological compounds
We next determined the frequency of vacuole escape during infection in the presence of the inhibitory compounds. Vacuolar and cytosolic localised L. monocytogenes were quantified and the percentage of bacteria that had escaped the phagosome was determined. A decrease in vacuole escape of L. monocytogenes was observed in cells treated with either thioridazine or bepridil (Fig. 1E). DMSO-treated control cells yielded an average 52.2% vacuole escape frequency, whereas thioridazine- and bepridil-treated cells yielded 43.5% and 30.3% vacuole escape, respectively. The decrease in the means of each group was statistically significant as determined by a repeated measure one-way ANOVA [F (2,12) = 3.962; P < 0.05]. These data suggest that both compounds inhibited L. monocytogenes intracellular infection by reducing the efficiency of vacuole escape.
4. Conclusions
In this study, we report the anti-infective activity of compounds associated with inhibition of neurological functions. Treatment of host cells with thioridazine or bepridil decreased the ability of L. monocytogenes to escape the phagocytic vacuole to initiate intracellular replication. Currently, the mechanism of inhibition of vacuole escape is unclear. Bepridil is a calcium inhibitor and thioridazine is an antipsychotic. Prior reports have shown that antipsychotic compounds can function as calcium channel antagonists [6], and calcium fluxes within host cells following infection by L. monocytogenes have been implicated in modulating bacterial entry and vacuole escape [15]. Therefore, additional studies into the effects on calcium fluxes during L. monocytogenes infection of drug-treated cells may yield further insights into the mechanism of inhibition. None the less, because of the prevalence of antibiotic resistance in many bacterial species, identification of clinically approved compounds with additional physiological effects provides an opportunity to develop these drugs further for alternate safe therapeutic uses. This study demonstrates that anti-infective activity may be found amongst many existing collections of small molecule compounds and suggests that neurological compounds may represent an untapped reservoir of antimicrobial drugs.
Acknowledgments
Small molecule screening facilities were provided by the National Screening Laboratory for the Regional Centers of Excellence in Biodefense and Emerging Infectious Diseases (NSRB). The authors would like to thank Sean Johnston and Melody Tsui of the NSRB for technical assistance.
Funding
This work was supported by the National Institutes of Health [grants AI53669 (to DEH), AI69772 (to LAL) and AI057159 (NSRB)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None declared.
Ethical approval
Not required.
References
- [1].Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9:1236–43. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [3].Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kawasumi M, Nghiem P. Chemical genetics: elucidating biological systems with small-molecule compounds. J Invest Dermatol. 2007;127:1577–84. doi: 10.1038/sj.jid.5700853. [DOI] [PubMed] [Google Scholar]
- [5].Lieberman LA, Higgins DE. A small-molecule screen identifies the antipsychotic drug pimozide as an inhibitor of Listeria monocytogenes infection. Antimicrob Agents Chemother. 2009;53:756–64. doi: 10.1128/AAC.00607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gould RJ, Murphy KM, Reynolds IJ, Snyder SH. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc Natl Acad Sci USA. 1983;80:5122–5. doi: 10.1073/pnas.80.16.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fenton M, Rathbone J, Reilly J, Sultana A. Thioridazine for schizophrenia. Cochrane Database Syst Rev. 2007;3:CD001944. doi: 10.1002/14651858.CD001944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ordway D, Viveiros M, Leandro C, Arroz MJ, Amaral L. Intracellular activity of clinical concentrations of phenothiazines including thioridiazine against phagocytosed Staphylococcus aureus. Int J Antimicrob Agents. 2002;20:34–43. doi: 10.1016/s0924-8579(02)00110-3. [DOI] [PubMed] [Google Scholar]
- [9].Ordway D, Viveiros M, Leandro C, Bettencourt R, Almeida J, Martins M, et al. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:917–22. doi: 10.1128/AAC.47.3.917-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Ingen J, van der Laan T, Amaral L, Dekhuijzen R, Boeree MJ, van Soolingen D. In vitro activity of thioridazine against Mycobacteria. Int J Antimicrob Agents. 2009;34:190–1. doi: 10.1016/j.ijantimicag.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [11].Shapiro W. Comparative efficacy of bepridil versus placebo in angina pectoris: treatment and withdrawal studies. Am J Cardiol. 1992;69:43D–9D. doi: 10.1016/0002-9149(92)90958-2. [DOI] [PubMed] [Google Scholar]
- [12].Brand-Schieber E, Werner P. Calcium channel blockers ameliorate disease in a mouse model of multiple sclerosis. Exp Neurol. 2004;189:5–9. doi: 10.1016/j.expneurol.2004.05.023. [DOI] [PubMed] [Google Scholar]
- [13].Kapoor R, Davies M, Blaker PA, Hall SM, Smith KJ. Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann Neurol. 2003;53:174–80. doi: 10.1002/ana.10443. [DOI] [PubMed] [Google Scholar]
- [14].Dancz CE, Haraga A, Portnoy DA, Higgins DE. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J Bacteriol. 2002;184:5935–45. doi: 10.1128/JB.184.21.5935-5945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wadsworth SJ, Goldfine H. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun. 1999;67:1770–8. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]