Abstract
Depression is a frequent comorbidity of temporal lobe epilepsy (TLE); however its mechanisms remain poorly understood and effective therapies are lacking. Augmentation of hippocampal interleukin-1β (IL-1β) signaling may be a mechanistic factor of both TLE and clinical depression. We examined whether pharmacological blockade of hippocampal interleukin-1 receptor exerts antidepressant effects in an animal model of comorbidity between TLE and depression, which developed in Wistar rats following pilocarpine status epilepticus (SE). In post-SE animals, depression-like state was characterized by behavioral equivalents of anhedonia and despair; dysregulation of the hypothalamo-pituitary-adrenocortical axis; compromised raphe-hippocampal serotonergic transmission. Two-week long bilateral intrahippocampal infusion of human recombinant Interleukin-1 Receptor antagonist (IL-1ra) improved all of the examined depressive impairments, without modifying spontaneous seizure frequency and without affecting normal parameters in naïve rats. These findings implicate hippocampal IL-1β in epilepsy-associated depression, and provide a rationale for the introduction of IL-1β blockers in the treatment of depression in TLE.
Keywords: Temporal lobe epilepsy, depression, comorbidity, brain inflammation, Interleukin-1β, hippocampus
Introduction
Depression represents one of the most common comorbidities of temporal lobe epilepsy (TLE), and has profound negative impact on the quality of life of TLE patients (Kanner, 2003; Kondziella et al., 2007). However, the causes and mechanisms of depression in TLE remain poorly understood, partly due to the lack of proper animal models. We previously reported that rats which develop chronic epilepsy following pilocarpine status epilepticus (SE) exhibited set of interictal disorders congruent with depression. Specifically, the following depressive impairments were documented in post-SE animals: behavioral equivalents of anhedonia (i.e. loss of the ability to experience pleasure) and despair; dysregulation of hypothalamo-pituitary-adrenocortical (HPA) axis; compromised raphe-hippocampal serotonergic transmission (Mazarati et al., 2008; 2009a). As the pilocarpine model reproduces both epileptic and depressive states, it may serve as a model of comorbidity between TLE and depression and as such, can be used both for studying mechanisms of this condition and as a screening platform for therapeutic interventions. Since depression is a multisymptomatic and a multifactorial disorder, further studies are necessary to advance the validation of this model; however the impairments established up-to-date allow initial experimental therapy studies.
One factor which may contribute to depression in TLE is hippocampal tissue inflammation, and particularly, enhanced interleukin-1β (IL-1β) signaling. Indeed, activation of hippocampal IL-1β and its receptor (IL-1R) have been established hallmarks of TLE both in clinical and experimental settings, and have been implicated in mechanisms of epileptogenesis (Bartfai et al., 2007; Ravizza et al., 2008; Vezzani et al., 2002; 2008; Vezzani and Granata, 2005). At the same time, IL-1β and other inflammatory cytokines may lead to depression conceivably via inducing perturbation in the HPA axis, as suggested by clinical observations, and confirmed by experimental studies (Capuron and Dantzer, 2003; Dunn et al., 2005; Leonard, 2006). Particularly, the dysregulation of the HPA axis (which represents a neuroendocrine hallmark of depression; Kondziella et al., 2007; Swaab et al., 2005) can be induced by the direct activation of hippocampal IL-1β signaling (Melik Parsadaniantz et al., 1999).
Such connections between epilepsy and IL-1β on the one hand, and IL-1β and depression on the other hand, prompted us to examine whether protracted pharmacological blockade of hippocampal IL-1R exerts antidepressant effect in the post-SE model of comorbidity between epilepsy and depression.
Methods
Subjects
The experiments were performed in male Wistar rats (Charles River, Wilmington, MA), fifty days old at the beginning of the study, in accordance with the policies of the National Institutes of Health.
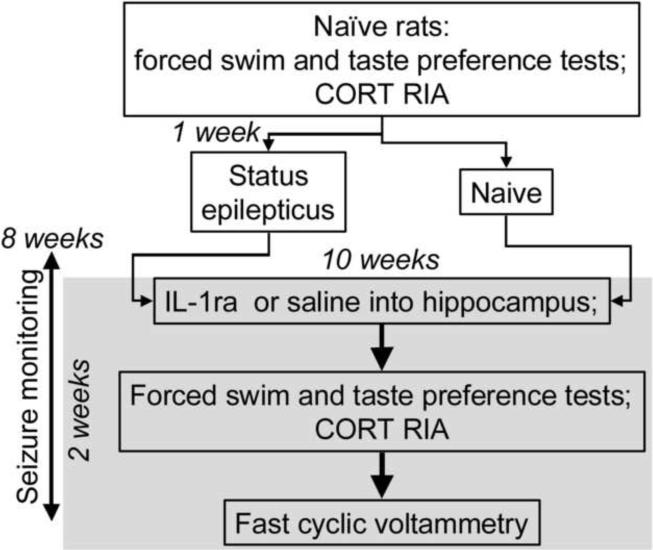
Study design is outlined in Fig. 1. Detailed description of procedures is provided in earlier publications (Mazarati et al., 2008; 2009a).
Figure 1. Experimental design.
Explanations are in the Methods section.
Forced swim test (FST)
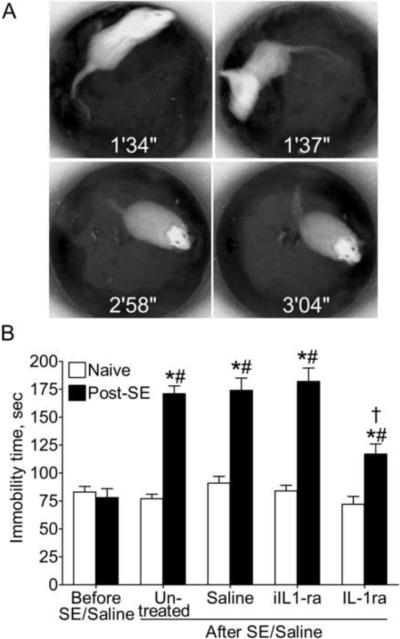
FST allows examining state of despair, and is based on the innate ability of rats to adopt active strategies in the inescapable stressful situation (Pucilowski and Overstreet, 1993). FST consisted of a single five-minute swimming session in the tank (height - 60 cm, diameter 30 cm) filled with water at 22°–25°C. Swimming behavior was videotaped and analyzed offline. Cumulative immobility time (Fig. 2A) was calculated. The increased immobility time indicates the state of despair (Mazarati et al., 2008; Pucilowski and Overstreet, 1993).
Figure 2. Effects of IL-1ra treatment on the forced swimming behavior.
A. Sample snapshots taken from pre-recorded video during FST. Time after the start of the test is indicated on each image. Examples of active swimming which reflects active escape strategies are presented at 1 min 34 s and 1 min 37 s. Note change in the rat's position in the tank which occurred during the 3 s period, and the fuzziness of images due to the animal's movement. Examples of immobility when animals move only enough to avoid drowning, are presented at 2 min 58 s and 3 min 04 s. Note that the animal's position in the tank did not change during 6 s of recording, and that the body is positioned vertically in the water. B. Immobility time in naive and post-SE animals - untreated, treated with saline, heat-inactivated IL-1ra (iIL-1ra) or active IL-1ra. Note the increase in the cumulative immobility time in untreated, saline-treated and iIL-1ra – treated post-SE animals, and its partial reversal following IL-1ra administration. Data are presented as Mean±SEM. *- p<0.05 After SE vs. Before SE (repeated measure ANOVA+Neuman Keuls post hoc test); #- p<0.05 Post-SE vs. Naive; †- p<0.05 Post-SE IL-1ra vs. Post-SE saline (One-Way ANOVA+Neuman Keuls post hoc test).
Taste preference test
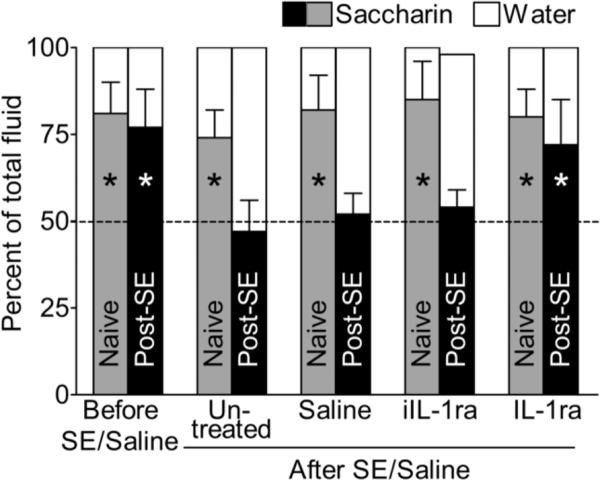
This test for anhedonia is based on the innate preference of rodents towards sweets (Pucilowski et al., 1993). Taste preference was examined using saccharin solution consumption test (Mazarati et al., 2008; Pucilowski et al., 1993). The rat's cage was supplied with two 250 ml graduated bottles, one filled with water and another – with 0.1% saccharin solution. Taste preference was expressed as percent of the volume of saccharin solution of a total volume of fluid (saccharin plus water) consumed over 24 hours. The loss of preference for saccharin (i.e. consumption of statistically equal volumes of saccharin and water) is indicative of anhedonia (Mazarati et al., 2008; Pucilowski et al., 1993).
Plasma corticosterone (CORT) assay
Measurement of baseline plasma CORT level and dexamethasone/corticotropin releasing hormone (DEX/CRH) test were performed upon the completion of behavioral tests (Johnson et al., 2006; Mazarati et al., 2009a; Pohorecky et al., 2004; Steimer et al., 2007). Between 8:00 AM and 10:00 AM, 50–100 μl of blood was collected from the tail vein into the EDTA – coated tubes. The animals were then injected into the tail vein with DEX (Sigma; 0.03 mg/kg). Six hours later blood was collected again, and animals were injected into the tail vein with CRH (Sigma; 50 ng/kg); two blood samples were taken 30 and 60 min after CRH injection. CORT was detected in 10 l plasma samples, using Immunochem™ Double Antibody Corticosterone 125I RIA kit (MP Biomedicals, Orangeburg, NY). Dysregulation of the HPA axis in depression consists of the elevated baseline CORT level; failure of DEX to suppress CORT; exacerbated and prolonged increase of CORT in response to CRH (the latter two impairments constitute positive DEX/CRH test) (Johnson et al., 2006; Mazarati et al., 2008; 2009a; Pohorecky et al., 2004; Zobel et al., 2004).
Status epilepticus (SE)
Two days after DEX/CRH test, animals received intraperitoneal injection of LiCl (130 mg/kg, Sigma, St. Louis, MO) and twenty four hours later - subcutaneous injection of pilocarpine (40 mg/kg, Sigma). Behavioral seizures were monitored during SE. SE was characterized by continuous limbic seizures starting 10–15 min after pilocarpine injection. Three and eight hours after seizure onset, rats were injected intraperitoneally with diazepam (5 mg/kg) and phenytoin (50 mg/kg) in order to alleviate further seizures and to decrease mortality. In control animals pilocarpine was substituted with saline.
Monitoring of spontaneous seizures
Due to the complexity of the drug delivery system, monitoring of spontaneous seizures was limited to video. Beginning from 8 weeks after SE, and until the end of experiments, animals' behavior was continuously recorded using digital camera. Seizures were analyzed off-line. Two seizure types were considered: focal seizures (motor arrest, facial twitches and mastication), and generalized clonic or clonic-tonic seizures (all body clonus, rearing or rearing and falling) (Mazarati et al., 2009a). Seizure monitoring served two purposes: first – for the selection of proper time points for the FST and CORT assays (as described below); second – for the evaluation of effects of treatment on seizure frequency. For the second purpose, the cumulative number of spontaneous seizures was calculated during two consecutive two-week periods: one immediately preceding the start of IL-1ra treatment, and another during the IL-1ra treatment. Data obtained for untreated post-SE rats as well as for rats treated with IL1-ra or saline were analyzed for each of the groups by comparing seizure counts before treatments with those during treatments.
Treatment with Interleukin-1 receptor antagonist (IL-1ra)
On order to achieve sustained blockade of hippocampal IL-1R, we used continuous intrahippocampal delivery of human recombinant IL-1ra (Amgen, Thousand Oaks, CA, Vezzani et al., 1999; Vezzani et al., 2000) by means of ALZET osmotic pumps. Earlier studies proved that IL-1ra preserves its stability and physiological activity when used in conjunction with the ALZET pump at 37°C for as long as four weeks (Knedla et al., 2009; Sandberg et al., 1993); after two weeks of incubation in the ALZET pump at 37°C, IL-1ra retained nearly 100% of its initial activity (Knedla et al., 2009).
Ten weeks after SE, animals underwent surgical implantation of system for continuous intrahippocampal infusion of IL-1ra. The system (Durect Corporation, Cupertino, CA) consisted of the ALZET osmotic pump model 2002 (delivery rate 0.5 μl/hr, duration 14 days, volume 200 μl) connected to the guide cannula via a polypropylene catheter (Brain Infusion Kit I). Osmotic pumps had been filled with the 140 μg of IL-1ra diluted in sterile saline, so that animals received 10 μg of the drug per day; the dose was based on earlier reports (Vezzani et al., 1999; 2000, 2002). The pumps then were activated and primed in sterile saline for 24 hours at 37°C according to the manufacturer's recommendation. Afterwards, each animal was implanted with two systems to provide bilateral infusion. Under isoflurane anesthesia, guide cannulas were stereotaxically implanted bilaterally into the hippocampi (4.3 mm posterior and 4 mm lateral from Bregma, 3.8 mm ventral from brain surface). Osmotic pumps were placed subcutaneously between the shoulders. Control groups included untreated animals; animals infused with the heat-inactivated IL-1ra (exposed to 70°C water bath for 30 min; 140 μg in 0.2 ml); and animals infused with saline (0.2 ml; both control treatments were administered using same delivery system as in experimental subjects). At the end of drug delivery (i.e. just before fast cyclic voltammetry experiments described below), pumps were removed, and the residual volume of content was aspirated and measured, in order to confirm the delivery of a test agent. The residual volume in the pumps was between 0 and 20 μl (i.e. did not exceed 10% of the original volume).
Behavioral test and CORT assay during IL-1ra treatment
During the days 10–13 after the beginning of IL-1ra infusion, the animals were subjected to FST, taste preference test and plasma CORT assay. To avoid possible immediate effects of seizures on FST, the test was only performed upon verification (by reviewing video recordings) that no seizures had developed for at least 6 hours prior to the test. For CORT assay, blood was only collected upon verification that animals had not developed seizures for at least 6 hours prior (Mazarati et al., 2009a).
Fast cyclic voltammetry (FCV)
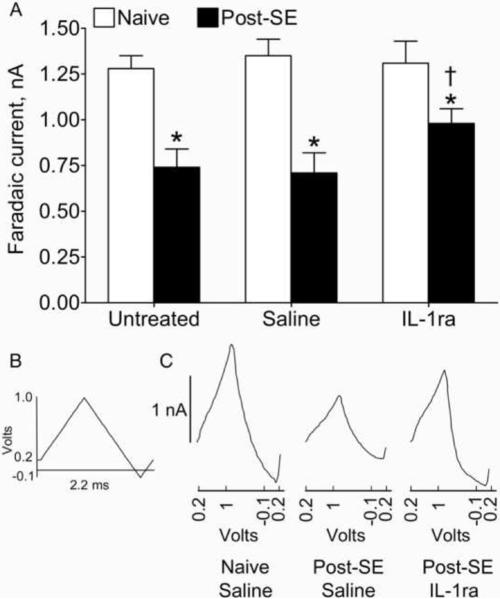
FCV allows measuring strength of the raphe-hippocampal serotonergic transmission (Bunin and Wightman, 1998; Jackson et al., 1995; Mazarati et al., 2008); it was preformed 14 days after the beginning of IL-1ra administration. Under urethane anesthesia (1.25 g/kg), animals were implanted with a bipolar electrode into dorsal raphe (7.8 mm posterior from Bregma, midline, 6.5 mm ventral from brain surface) and with a nafion-coated carbon fiber electrode (World Precision Instruments, Sarasota, FL) into the hippocampus (4.3 mm posterior to Bregma, 3 mm lateral from midline, 3.6 mm down from brain surface). The release of serotonin (5-HT) in the hippocampus was induced by electrical stimulation of raphe (bipolar square wave pulses, 100 Hz, 200 ms, 0.35 mA; Bunin and Wightman, 1998; Mazarati et al., 2008). The amount of 5-HT released in response to the stimulation was measured by applying oxidizing current to the carbon fiber electrode (scanned consecutively from 0.2 V to 1 V, −0.1 V and 0.2 V, at a rate of 1000 V/s, Fig. 5B). Oxidative peaks were acquired before and after raphe stimulation, using POT500 scanning potentiostat (World Precision Instruments) coupled with the AcqKnowledge software (BIOPAC, Goleta, CA). The difference between peaks before and after stimulation, known as faradaic current, reflects concentration of 5-HT oxidative product quinone and is directly proportionate to the amount of 5-HT (Wrona and Dryhurst, 1987). Compromised raphe-hippocampal serotonergic transmission (evident as the decrease of the amplitudes of faradaic currents) represents a biochemical hallmark of depression (Mann et al., 1989; Murphy et al., 1978). Since FCV is a non-survival procedure, it was done at one time point.
Figure 5. Effects of IL-1ra treatment on 5-HT release in the raphe-hippocampal pathway.
A. Statistical analysis. Mean±SEM amplitude of faradaic currents recorded from the hippocampus in response to dorsal raphe stimulation was lower in untreated and saline-treated post-SE animals, than in respective naïve subjects, thus indicating compromised raphe-hippocampal serotonergic transmission. This deficit was partially reversed by IL-1ra treatment. *- p<0.05 Post-SE vs. Naive; †- p<0.05 post-SE IL-1ra vs. post-SE saline and post-SE untreated (One-Way ANOVA + Neuman-Keuls post hoc test). B. The waveform of oxidizing current applied to hippocampal carbon fiber electrode. C. Sample oxidative peaks acquired from Saline-treated Control rat, post-SE Saline-treated and post-SE IL-1ra treated animals.
Data analysis
Data were analyzed using Prizm software (GraphPad, San Diego, CA). The study included the following groups. Twenty four control animals (i.e. not subjected to SE), which subsequently were divided into IL-1ra-treated, saline-treated and untreated groups (8 animals per group). Twenty seven post-SE animals, also subsequently divided into IL-1ra-treated, saline-treated and untreated groups (9 animals per group). Additionally, 6 post-SE rats were treated with the heat-inactivated IL-1ra. Statistical tests are described in respective figure legends.
Results
Effects of IL-1ra on FST (Fig. 2B)
Treatment with IL-1ra had no effect on behavior in control animals as compared both with themselves at the beginning of the study, and with saline-treated naïve rats. Untreated and saline-treated post-SE animals, as well as those infused with the heat-inactivated IL1-ra, showed statistically significant increase in the immobility time during FST, as compared both with themselves prior to SE, and with respective controls.
Administration of IL-1ra to post-SE subjects resulted in significant shortening of the immobility time, as compared with both untreated and saline-treated post-SE rats. However, immobility time remained significantly longer than in control subjects.
Effects of IL-1ra on taste preference (Fig. 3)
Figure 3. Effects of IL-1ra treatment on saccharin consumption.
Naive animals preferred saccharin over tap water, while untreated, saline-treated and heat-inactivated IL-1ra (iIL-1ra) – treated post-SE rats consumed equal amount of each fluid. IL-1ra restored preference towards saccharin. Data for saccharin are presented as Mean±SEM. *- p<0.05 saccharin solution vs. tap water (t-test).
Control and experimental animals prior to SE preferred saccharin solution over tap water with the ratio of approximately 4:1. In control rats, IL-1ra did not affect preference towards saccharin. Post-SE rats (untreated, treated with saline and with the heat-inactivated IL1-ra) consumed statistically equal amounts of saccharin and water. This redistribution between saccharin and water consumption was not accompanied by changes in the intake of total volume of fluid (i.e. saccharin plus water; not shown). In post-SE animals, IL-1ra completely restored preference towards saccharin.
Since intrahippocampal infusion of heat-inactivated IL1-ra had not ameliorated behavioral symptoms of depression, this control treatment was not included in further studies.
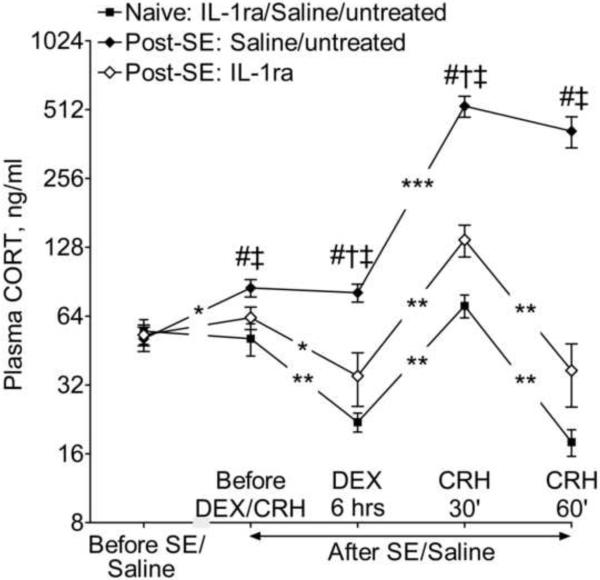
Effects of IL-1ra on the HPA axis (Fig. 4)
Figure 4. Effects of IL-1ra treatment on baseline plasma CORT concentration and on DEX/CRH test.
Since in all three groups of naïve animals all examined parameters were statistically similar for the first and the second assays, in order to simplify the presentation of the findings, the outcome of DEX/CRH test is presented only for the second assay (i.e. during IL-1ra treatment) and data in naive groups are combined for the three treatments (i.e. IL-1ra, saline, and untreated). In saline-treated and untreated rats, SE led to the increase of baseline plasma CORT level; failure of DEX to decrease CORT concentration; and the exacerbated and longer-lasting response to CRH (since the responses in two groups were statistically close, the data are combined for presentation purposes). IL-1ra decreased baseline plasma CORT level and partially normalized the outcome of DEX/CRH test. Data are presented as Mean±SEM. Even though data for some groups were combined, statistical comparisons were done between respective groups: #- p<0.05 Post-SE-Saline and Post-SE untreated vs. respective Naïve controls and at respective time points; †- p<0.05 post-SE IL-1ra vs. Naive IL-1ra; ‡- p<0.05 post-SE IL-1ra vs. post-SE Saline and post-SE untreated (One-Way ANOVA + Neuman-Keuls post hoc test). *- p<0.05; **- p<0.01; ***- p<0.001 comparison within the same groups, for two adjacent time points between which the symbols are located (Repeated measure ANOVA + Neuman-Keuls post hoc test).
In all control groups (i.e. IL-1ra, saline and untreated) baseline CORT level and the outcome of DEX/CRH test were statistically similar during the first (i.e. at the beginning of the study) and the second assays (i.e. 12 weeks later). DEX decreased CORT level by 3.5 fold. CRH induced the 4-fold rise in CORT at 30 min; the latter returned to the pre-CRH level at 60 min.
Both untreated and saline-treated post-SE animals showed interictal increase of baseline CORT (20% as compared with controls and themselves prior to SE). DEX failed to decrease concentration of CORT; after CRH injection, the rise of CORT was steeper (7-fold) and longer-lasting (present at 60 min) than in control rats. IL-1ra significantly decreased baseline CORT level in post-SE subjects as compared with saline-treated and untreated post-SE animals; CORT concentrations were statistically similar between IL-1ra – treated post-SE rats and control subjects. IL-1ra partially restored the response to DEX and mitigated the exacerbated response to CRH.
Effects of IL-1ra on 5-HT release from the hippocampus (Fig. 5)
In controls, intrahippocampal infusion of IL-1ra did not alter amplitudes of faradaic currents recorded from the hippocampus after dorsal raphe stimulation. In untreated and saline-treated post-SE rats, the amplitude of faradaic currents was significantly lower than in controls, which pointed towards compromised raphe-hippocampal serotonergic transmission. The latter was partially restored by IL-1ra, although it was still significantly lower than in control subjects.
Effects of IL-1ra on seizures (Table 1)
Table 1. Behavioral spontaneous seizure count in post-SE rats.
Numbers indicate cumulative number of behavioral seizures for individual animals over 2 two-week periods: before and during IL1-ra and saline treatments (or respective period in untreated rats). No differences were observed between the two periods for each of the groups (p>0.05, both Wilcoxon and Mann-Whitney tests for paired and unpaired comparisons), and across the groups (p>0.05, Kruskal-Wallis test). No differences were observed in the direction in which frequency changed (i.e. decreased, increased and remained unchanged) among any pairs of groups (p>0.05, Fisher's exact test).
| Treatment group | Before treatment | During treatment | Changes in seizure count during treatment vs. before treatment |
|---|---|---|---|
| IL1-ra | 3 | 0 | Decreased- 4 Increased- 3 Unchanged-2 |
| 2 | 1 | ||
| 11 | 5 | ||
| 4 | 0 | ||
| 6 | 8 | ||
| 33 | 42 | ||
| 9 | 71 | ||
| 0 | 0 | ||
| 4 | 4 | ||
| Saline | 7 | 3 | Decreased- 4 Increased- 5 |
| 1 | 0 | ||
| 4 | 3 | ||
| 56 | 2 | ||
| 13 | 14 | ||
| 5 | 12 | ||
| 0 | 2 | ||
| 3 | 39 | ||
| 0 | 7 | ||
| Untreated | 11 | 4 | Decreased- 5 Increased-4 |
| 9 | 0 | ||
| 10 | 4 | ||
| 4 | 0 | ||
| 42 | 4 | ||
| 4 | 61 | ||
| 0 | 15 | ||
| 0 | 33 | ||
| 4 | 7 |
For all groups of post-SE animals (IL-1ra, saline and untreated) the number of seizures documented before and during IL-1ra administration was statistically similar. All animals exhibited both focal and generalized seizures; the latter type constituted at least 75% of all documented seizures. The distribution between the two seizure types was statistically similar before and during IL-1ra administration, as well as in untreated and saline-treated rats during the first and the second observation periods (data not shown). Furthermore, retrospective correlation analysis did not reveal any statistical correlation between frequency of spontaneous seizures observed during the two weeks of treatment with IL-1ra and the immobility time in the FST at the end of IL-1ra treatment (Spearmen coefficient of correlation r=−0.085, p>0.05).
Effects of different treatment regimens
Since the administration of IL-1ra at 10 μg/day neither completely reversed all depressive symptoms, nor reduced seizure frequency, we attempted to examine effects of the compound at 30 μg/day (n=4). Two-three days after the onset of IL-1ra infusion, animals showed lack of grooming, motor exaltation (e.g. jumping in response to touch or moving the cage), and progressive weight loss. For this reason, these studies were interrupted.
Another group of post-SE animals (n=5) was injected with IL-1ra acutely bilaterally into the hippocampus, using chronically implanted guide cannulas and Hamilton microsyringe, at 1 μg (i.e. equivalent to the amount delivered over 2.5 hours in chronic experiments). The animals were subjected to FST 2 weeks before and 30 min after IL-1ra injection. This treatment did not improve behavioral deficit in the FST (not shown).
Discussion
Protracted blockade of hippocampal IL-1R attenuated behavioral, endocrine and biochemical hallmarks of depression following SE. This effect did not appear to be a consequence of seizure modification, since seizure frequency was neither altered by the treatment, nor statistically correlated with the outcome of behavioral test under IL-1ra administration. IL-1ra improved the examined parameters of depression in epileptic rats selectively, without affecting responses in naïve animals. Antidepressant action of IL-1ra was not due to an immediate blockade of IL-1R, but apparently involved chronic downstream mechanisms, since acute drug injection exerted no antidepressant effect.
The study confirmed our findings that post-SE animals exhibit interictal depression-like impairments (Mazarati et al., 2008; 2009a). Behavioral depressive deficits are not results of non-specific motor and sensory impairments induced by SE: despite the increased immobility time in the FST, and the loss of preference towards saccharin, SE impairs neither the animal's ability to swim, nor the normally present aversion to bitter quinine (Supplementary on-line material).
High incidence of depression among epilepsy patients, as well as reciprocal connection between the two conditions has led to the hypothesis that depression and epilepsy share certain mechanisms (Kanner, 2003; Kondziella et al., 2007). We suggest that hippocampal IL-1β may be a factor contributing to depression in TLE. Indeed, IL-1β signaling is enhanced in the hippocampus during the course of TLE, and may contribute to epileptogenesis (Bartfai et al., 2007; Ravizza et al., 2008; Vezzani and Granata, 2005). At the same time, brain inflammation, and particularly the augmentation of IL-1β signaling induced depression in experimental setting (Dunn et al., 2005). For example, inflammation induced both by lipopolysaccharide and by IL-1β was accompanied by behavioral symptoms of depression (Dunn and Swiergiel, 2005; O'Connor et al., 2009; Yirmiya, 1996); lipopolysaccharide-induced depression was reversed by IL-1ra (Konsman et al., 2008). Clinical observations also provide evidence for the association between inflammatory cytokines and depression (Capuron and Dantzer, 2003; Dunn et al., 2005; Leonard, 2006; van den Biggelaar et al., 2007).
Mechanisms through which the augmented hippocampal IL-1β signaling leads to depression need further investigation. The former has been proven to induce perturbations in the HPA axis (Melik Parsadaniantz et al., 1999), as seen under conditions of depression (Capuron and Dantzer, 2003; Dunn et al., 2005; Leonard, 2006), and in post-SE animals (Mazarati et al., 2009a). In our studies, the dysregulation of the HPA axis following SE was mitigated by the blockade of hippocampal IL-1R. The observed effect of IL-1ra was consistent with previously reported increase of plasma CORT level by the IL-1β injected in the rat hippocampus (Melik Parsadaniantz et al., 1999). As the DEX/CRH test reflects reduced inhibitory feedback control within the HPA axis (Pariante and Miller, 2001; Watson et al., 2006), partial improvement of the response to DEX after intrahippocampal IL-1ra treatment was an apparent result of the normalization of the afferent component of the HPA autoinhibitory loop (De Kloet and Reul, 1987; Melik Parsadaniantz et al., 1999). Normalization of the response to CRH may be explained based on the finding that the activation of hippocampal IL-1β stimulated transcription and processing of proopiomelanocortin (POMC, precursor of adrenocorticotropic hormone, ACTH) in the anterior pituitary (Melik Parsadaniantz et al., 1999). It can be surmised that the sustained blockade of hippocampal IL-1β in post-SE subjects would mitigate the enhanced POMC transcription and cleavage; thus the administration of CRH to the IL-1ra – pretreated post-SE rats would target corticotropes in which ACTH production would have been normalized, the latter being reflected in the mitigated rise in plasma CORT level.
Although the dysregulation of the HPA axis has been an accepted hallmark of clinical depression (Kondziella et al., 2007; Steimer et al., 2007; Watson et al., 2006; Zobel et al., 2004), exact mechanistic connection between the two are not known. One hypothesis links the dysregulation of the HPA axis to the deficiency of serotonergic transmission. Raphe-hippocampal serotonergic pathway plays important role in regulating mood and is compromised in depression (Kondziella et al., 2007), as well as in the pilocarpine model (Mazarati et al., 2008). Among possible mechanisms involved in the raphe-hippocampal serotonergic deficiency in depression is the augmentation of mechanisms which inhibit 5-HT release. Specifically, depression is characterized by the upregulation of raphe 5-HT1A autoreceptors (Boldrini et al., 2008; Stockmeier et al., 1998) – a component of short feedback autoinhibitory loop involved in the control of 5-HT release (Aghajanian et al., 1990; Riad et al., 2000); thus the upregulation of raphe 5-HT1A receptors would result in the decrease of 5-HT release. The discussed mechanism may contribute to depression in TLE patients as well: recent study involving positron emission tomography using a selective 5-HT1A ligand showed increased binding affinity of raphe 5-HT1A receptors in patients with TLE and concurrent depression, and its positive correlation with the severity of clinical symptoms of depression (Lothe et al., 2008).
Enhanced level of circulating glucocorticoids may upregulate raphe 5-HT1A autoreceptors via glucocorticoid receptor-mediated genomic mechanisms (Bellido et al., 2004; Man et al., 2002). The upregulation of raphe 5-HT1A autoreceptors and the involvement of glucocorticoids in this process are yet to be shown directly in the pilocarpine model. However, our in-progress studies found that post-SE animals exhibited enhanced sensitivity to a selective 5-HT1A blocker WAY100635 injected locally into raphe, as judged by effects of the compound in the FST; furthermore, protracted pharmacological blockade of raphe glucocorticoid receptors by mifepristone improved behavioral symptoms of depression in post-SE rats without affecting these parameters in naïve animals (Mazarati et al., 2009b; Supplementary on-line material) and reversed the enhanced sensitivity to the 5-HT1A ligand (personal unpublished data). These findings suggest that hypersensitization of raphe 5-HT1A receptors may be indeed occurring in the pilocarpine model, and that increased exposure of raphe glucocorticoid receptors to circulating CORT may contribute to the 5-HT1A hypersensitivity and depression.
Attenuation of depressive symptoms by IL-1ra was not an epiphenomenon of its anticonvulsant effects. While this finding emphasizes the importance of IL-1β signaling as specific mechanism of depression associated with epilepsy, it may also be surprising, considering the discussed role IL-1β in epileptogenesis. Several explanations can be offered on this matter. First, it should be acknowledged that our experimental protocol may not be suitable for reliable examination of antiepileptic therapies. In our laboratory the pilocarpine model is refined in such a way that frequency of recurrent seizures is relatively low due to aggressive anticonvulsant interventions during SE (Mazarati et al., 2008; 2009a) in order to afford examination of interictal phenomena, and as such it may prohibit adequate examination of antiepileptic treatments. Second, IL-1ra did not affect severity of limbic seizures under intrahippocampal administration, although it was anticonvulsant when administered into the lateral brain ventricle (Vezzani et al., 1999; 2002), or systemically (Marchi et al., 2009); in such case, a broader exposure of brain to IL-1ra may be required for the drug to exert anticonvulsant effect. Third, it is plausible that a higher dose of IL-1ra would be antiepileptic; however this possibility could not be explored due to the described side effects. On a related note, our finding that depression in post-SE animals could not be completely abolished by IL-1ra, may be either due to the fact that the dose was not saturated to maximal therapeutic level, or that the exposure of hippocampal tissue to IL-1ra was limited to the sites of injection, or that other mechanisms (inflammatory and non-inflammatory alike) are involved in the described phenomenon. The latter explanations seems likely: even considering inflammation only, other changes (e.g. proliferation of microglia, disruption of the blood-brain barrier; Ravizza et al., 2008; Vezzani and Granata, 2005) occur in TLE. Another inflammatory cytokine, tumor necrosis factor-α, has been directly implicated in the enhanced neuronal excitability ensuing after a systemic inflammatory challenge resulting in decreased seizure threshold (Riazi et al 2008; Galic et al 2008).
The observed dissociation between antidepressant effects of IL-1ra and lack of its effects on seizure frequency is not surprising. Indeed, both clinical (Gilliam et al., 2007) and experimental (Mazarati et al., 2008) evidence suggest that the severity of depression in TLE does not necessarily depend on frequency of seizures.
The importance of antidepressant action of IL-1ra is further emphasized by the fact that in the pilocarpine model, selective serotonin reuptake inhibitor (SSRI) fluoxetine was ineffective in mitigating depressive impairments (Mazarati et al., 2008). This finding on itself is not unexpected, as up to 1/3 of depression patients do not respond to SSRI (Barbui et al., 2002). In fact, our studies may offer an explanation as to the lack of antidepressant effects of fluoxetine in post-SE rats. A plausible mechanism through which SSRIs induce antidepressant effect is desensitization of raphe 5-HT1A autoreceptors and subsequent strengthening of the raphehippocampal serotonergic transmission (Chaput et al., 1986; Le Poul et al., 1995). It is conceivable that the ongoing hippocampal inflammation and continuing positive drive of the HPA axis on raphe overrides fluoxetine-mediated desensitization of 5-HT1A autoreceptors. In such case, adjunctive therapy of IL-1R antagonists together with conventional antidepressants may be worth exploring.
In conclusion, the present study implicates hippocampal IL-1β in mechanisms of epilepsy-associated depression, and provides a rationale for the introduction of IL-1R antagonists in the treatment of depression in TLE patients. Presently IL-1ra is used for the treatment of autoimmune diseases, such as rheumatoid arthritis (Kalliolias and Liossis, 2008), and its central bioavailability in the healthy brain upon systemic administration is questionable. However, epilepsy is accompanied by the breakdown of the blood-brain barrier (BBB; Marcon et al., 2009; Ravizza et al., 2008; Rigau et al., 2007), which, in turn would likely render brain tissue accessible to compounds, which normally do not permeate BBB. Indeed, experimental findings showed that peripherally administered IL-1ra exhibited neuroprotective effects in animal models of stroke and traumatic brain injury (Relton et al., 1996; Sanderson et al., 1999), that is in conditions accompanied by the BBB leakage.
Supplementary Material
Acknowledgment
The work was supported by research grants MH079933 from the National Institutes of Health (A. Mazarati) and 132081 from Epilepsy Foundation of America (A. Mazarati). IL-1ra was a gift from Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. discussion 103. [DOI] [PubMed] [Google Scholar]
- Barbui C, Hotopf M, Garattini S. Fluoxetine dose and outcome in antidepressant drug trials. Eur J Clin Pharmacol. 2002;58:379–386. doi: 10.1007/s00228-002-0497-7. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Sanchez-Alavez M, Andell-Jonsson S, Schultzberg M, Vezzani A, Danielsson E, Conti B. Interleukin-1 system in CNS stress: seizures, fever, and neurotrauma. Ann N Y Acad Sci. 2007;1113:173–177. doi: 10.1196/annals.1391.022. [DOI] [PubMed] [Google Scholar]
- Bellido I, Hansson AC, Gomez-Luque AJ, Andbjer B, Agnati LF, Fuxe K. Corticosterone strongly increases the affinity of dorsal raphe 5-HT1A receptors. Neuroreport. 2004;15:1457–1459. doi: 10.1097/01.wnr.0000130542.06764.7f. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28:6904–6913. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam FG, Maton BM, Martin RC, Sawrie SM, Faught RE, Hugg JW, Viikinsalo M, Kuzniecky RI. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology. 2007;68:364–368. doi: 10.1212/01.wnl.0000252813.86812.81. [DOI] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD, Liossis SN. The future of the IL-1 receptor antagonist anakinra: from rheumatoid arthritis to adult-onset Still's disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs. 2008;17:349–359. doi: 10.1517/13543784.17.3.349. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- Knedla A, Riepl B, Lefevre S, Kistella S, Grifka J, Straub RH, Gay S, Scholmerich J, Muller-Ladner U, Neumann E. The therapeutic use of osmotic minipumps in the severe combined immunodeficiency (SCID) mouse model for rheumatoid arthritis. Ann Rheum Dis. 2009;68:124–129. doi: 10.1136/ard.2007.086116. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Alvestad S, Vaaler A, Sonnewald U. Which clinical and experimental data link temporal lobe epilepsy with depression? J Neurochem. 2007;103:2136–2152. doi: 10.1111/j.1471-4159.2007.04926.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–276. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, Ryvlin P. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008;131:2765–2782. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MS, Young AH, McAllister-Williams RH. Corticosterone modulation of somatodendritic 5-HT1A receptor function in mice. J Psychopharmacol. 2002;16:245–252. doi: 10.1177/026988110201600310. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry Suppl. 1989:7–14. [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon J, Gagliardi B, Balosso S, Maroso M, Noe F, Morin M, Lerner-Natoli M, Vezzani A, Ravizza T. Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis. Neurobiol Dis. 2009;34:121–132. doi: 10.1016/j.nbd.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, Sankar R. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–2083. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Shin D, Kwon YS, Bragin A, Pineda E, Tio D, Taylor AN, Sankar R. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis. 2009a;34:457–461. doi: 10.1016/j.nbd.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Shin D, Bragin A, Tio D, Taylor A, Sankar R. Hyperactivity of hypothalamo-pituitary-adrenocortical axis in a rat model of temporal lobe epilepsy: a mechanism of comorbidity between epilepsy and depression. Epilepsia. 2009b;50(Suppl 10):68. [Google Scholar]
- Melik Parsadaniantz S, Dauge V, Roques BP, Kerdelhue B. Acute intrahippocampal injection of human interleukin-1beta stimulates the anterior pituitary POMC transcription and increases plasma levels of ACTH and corticosterone in the male rat. Neuroendocrinology. 1999;69:77–87. doi: 10.1159/000054405. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Campbell L, Costa JL. The brain serotonergic system in the affective disorders. In: Lipton MA, DiMascio A, Killam KF, editors. Psychopharmacology: A generation of progress. Raven Press; New York: 1978. pp. 1235–1247. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Baumann MH, Benjamin D. Effects of chronic social stress on neuroendocrine responsiveness to challenge with ethanol, dexamethasone and corticotropin-releasing hormone. Neuroendocrinology. 2004;80:332–342. doi: 10.1159/000083682. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH. Effect of chronic antidepressant treatment on responses to apomorphine in selectively bred rat strains. Brain Res Bull. 1993;32:471–475. doi: 10.1016/0361-9230(93)90293-k. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of Interleukin-1 Receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138:206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A. Treatment with an interleukin-1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes. 1993;42:1845–1851. doi: 10.2337/diab.42.12.1845. [DOI] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Steimer T, Python A, Schulz PE, Aubry JM. Plasma corticosterone, dexamethasone (DEX) suppression and DEX/CRH tests in a rat model of genetic vulnerability to depression. Psychoneuroendocrinology. 2007;32:575–579. doi: 10.1016/j.psyneuen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Smith MS, Ferrier IN, Young AH. The dex/CRH test--is it better than the DST? Psychoneuroendocrinology. 2006;31:889–894. doi: 10.1016/j.psyneuen.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Dryhurst G. Oxidation chemistry of 5-hydroxytryptamine. 1. Mechanism and products formed at micromolar concentrations. J Org Chem. 1987;52:2817–2825. [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zobel A, Wellmer J, Schulze-Rauschenbach S, Pfeiffer U, Schnell S, Elger C, Maier W. Impairment of inhibitory control of the hypothalamic pituitary adrenocortical system in epilepsy. Eur Arch Psychiatry Clin Neurosci. 2004;254:303–311. doi: 10.1007/s00406-004-0499-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.