Abstract
Here we make use of neural ablation to investigate the properties of the TrpV1-expressing neurons in the trigeminal and dorsal root ganglia of mice. Resiniferotoxin (RTX), a potent TrpV1-agonist, administered either by direct injection in the ganglion or intrathecally killed approx. 70% of TrpV1-cells and resulted in modest thermal analgesia. Interestingly, after carageenan injection in the hind-paw, the analgesic effects of RTX were dramatically increased with mice now paradoxically showing far less response to heat applied at sites of inflammation. This additional carageenan and RTX-induced analgesia was transient, lasting less than 2 days, and likely resulted from deafferation of remaining TrpV1-neurons. Remarkably although RTX affected sensitivity to heat, mechanical sensitivity (both of normal and inflamed tissue) was completely unaltered by toxin-mediated silencing of the TrpV1-sensory input. Thus our data demonstrate that TrpV1-neurons are selectively tuned nociceptors that mediate responses to thermal but not mechanical pain and insinuate a labeled line model for somatosensory coding.
Keywords: Resiniferotoxin, TrpV1, nociception, pain, thermal sensation
Introduction
In mammals a family of Trp-ion channels has been implicated in mediating thermosensation (Jordt et al., 2003). TrpV1, the capsaicin receptor, plays an important role in sensation of noxious heat (Caterina et al., 1997). TrpV1−/− mice have reduced thermal sensitivity but importantly loss of TrpV1 does not eliminate responses to noxious heat (Woodbury et al., 2004). TrpV1 is also believed to play a role in increased pain sensitivity following inflammation at least in part because inflammation induced phosphorylation of TrpV1 results in increased sensitivity of this ion-channel to temperature (Bhave et al., 2002; Cesare et al., 1999; Nilius et al., 2005; Numazaki et al., 2002). Indeed, carageenan induced inflammation strongly enhances the response of normal mice to noxious heat but does not sensitize TrpV1−/− mutants (Caterina et al., 2000). Thus attention has focused on TrpV1 and TrpV1 cells for their role in pain sensation and as targets for pain therapies. Notably, TrpV1 antagonists have been shown to be efficacious in animal models of chronic pain (Szallasi et al., 2007; Wong and Gavva, 2009) but, as yet, have not been exploited in human pain management.
An alternate strategy for pain management involves ablation of TrpV1-expressing cells, or TrpV1-fibers. This approach has a long history: more than 25 years ago, capsaicin injection was reported to dramatically decrease the level of inflammatory mediators (e.g. substance P) and pain sensation for the life of animals (Jancso et al., 1977; Nagy et al., 1981; Yaksh et al., 1979). More recently, the potent TrpV1 agonist, resiniferotoxin (RTX) has been demonstrated to kill TrpV1-expressing neurons by affecting long term Ca2+-entry (Olah et al., 2001). RTX has also been used therapeutically in animals and has been reported to alleviate chronic pain with no effects on discriminative touch or proprioception (Brown et al., 2005; Karai et al., 2004; Neubert et al., 2003). In a rat models, RTX was shown to eliminate many TrpV1 neurons, dramatically decrease responses to capsaicin and thermal pain (Karai et al., 2004) and also to affect mechanosensation (Tender et al., 2008).
Here, we set out to determine the function of TrpV1-cells and explored the use of RTX as a means to selectively eliminate these neurons in mice. We carried out a detailed investigation into the efficacy of RTX in ablating sensory neurons and demonstrate that although a large subset of TrpV1 neurons are killed by a single injection, other TrpV1-cells are not affected even by repeated RTX-treatments. RTX reduces but does not eliminate capsaicin responses and thermal sensation of normal mice. In contrast, after inflammation RTX was far more effective and resulted in a profound loss of thermal sensation without any corresponding effect on mechanosensation (including to painful stimuli). Thus our data strongly support a model where TrpV1-neurons selectively mediate thermal nociception and a distinct labeled-line transmits mechanical pain (see also (Cavanaugh et al., 2009)).
Results
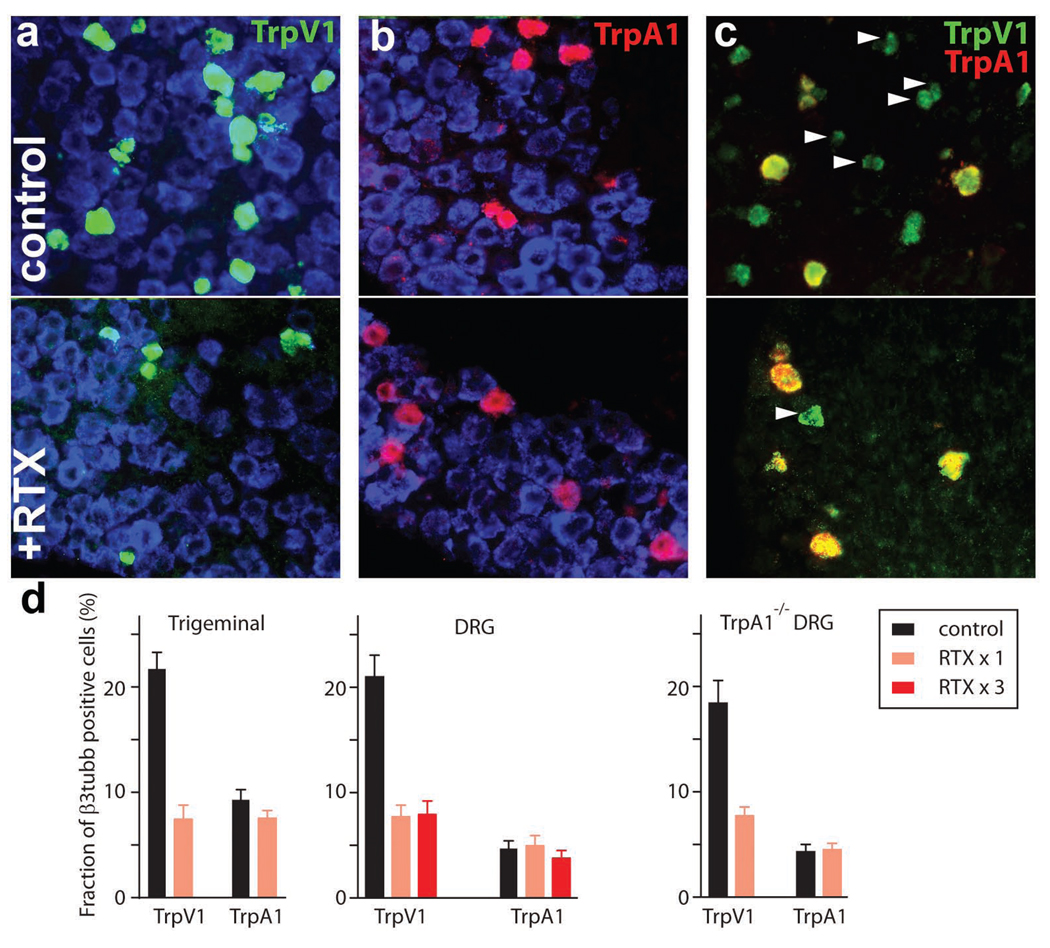
RTX is a potent TrpV1 agonist that has been reported to effectively kill TrpV1-expressing cells in vitro and selectively ablate capsaicin sensitive sensory neurons in animal models (Karai et al., 2004). Thus RTX is an attractive agent to help reveal the role of TrpV1-neurons in vivo and to generate ganglia depleted in this subset of neurons for transcriptome analysis. In initial experiments, we administered large amounts of RTX to mice (10-fold higher doses than previously reported effective in long-term alleviation of pain) by injecting the toxin directly into the trigeminal ganglion or intrathecally to target dorsal root ganglia (Karai et al., 2004). Figure 1 demonstrates that such RTX treatment was effective at reducing the number of TrpV1-expressing sensory neurons both in the trigeminal and dorsal root ganglia with approximately 70% of TrpV1-neurons killed by a single injection. However, RTX never completely ablated this population of cells. Indeed, repeated intrathecal injection of RTX was no more effective at killing TrpV1-neurons than a single dose (Fig. 1).
Figure 1. RTX kills a large subset of TrpV1-positive neurons.
Double-label in situ hybridization was used to examine RTX induced cell ablation. Trigeminal neurons were identified using an antisense β3-tubulin probe (blue, a, b); upper panels (a–c) show control sections from untreated ganglia. Trigeminal RTX treatment (lower panels, a–c) dramatically reduced the fraction of neurons containing TrpV1 (green, a) but not TrpA1 (red, b). After RTX-treatment (c, lower panel) TrpV1, TrpA1 double positive neurons but almost no TrpV1 positive, TrpA1 negative cells (arrowheads) remain. (d) Quantitation of the effects of RTX on the proportion of neurons expressing TrpV1 or TrpA1 in trigeminal and DRG ganglia of normal and TrpA1−/−-mice; mean ± SEM (n=9).
TrpV1-expressing neurons are heterogeneous. For example, many TrpV1-cells have small cell bodies, yet others are amongst the largest diameter neurons in the dorsal root and trigeminal ganglia (Ma, 2002). Similarly, in situ hybridization and immunohistochemistry reveal heterogeneity with respect to molecular markers including neuropeptides, GPCRs and ion-channels (Guo et al., 1999; Moriyama et al., 2003; Negri et al., 2006; Story et al., 2003). Notably, our data demonstrate that RTX treatment had little effect on the number of neurons expressing TrpA1 (Fig. 1), a molecule that we (and others, e.g. (Story et al., 2003) have shown is selectively expressed in a population of large diameter TrpV1-cells. To rule out a change in expression pattern of TrpA1 after RTX-treatment, we used double label in situ hybridization to show that all remaining TrpA1-cells are also TrpV1 positive (Fig. 1c, lower panel). Therefore it appears that a subset of large diameter, TrpA1-positive TrpV1-sensory neurons are resistant to RTX treatment. Interestingly, using TrpA1-knockout mice we have shown that TrpA1 itself is not required for the RTX-resistance of this subset of TrpV1 cells (Fig. 1d and Fig. S1 demonstrating the specificity of the probes used for detection).
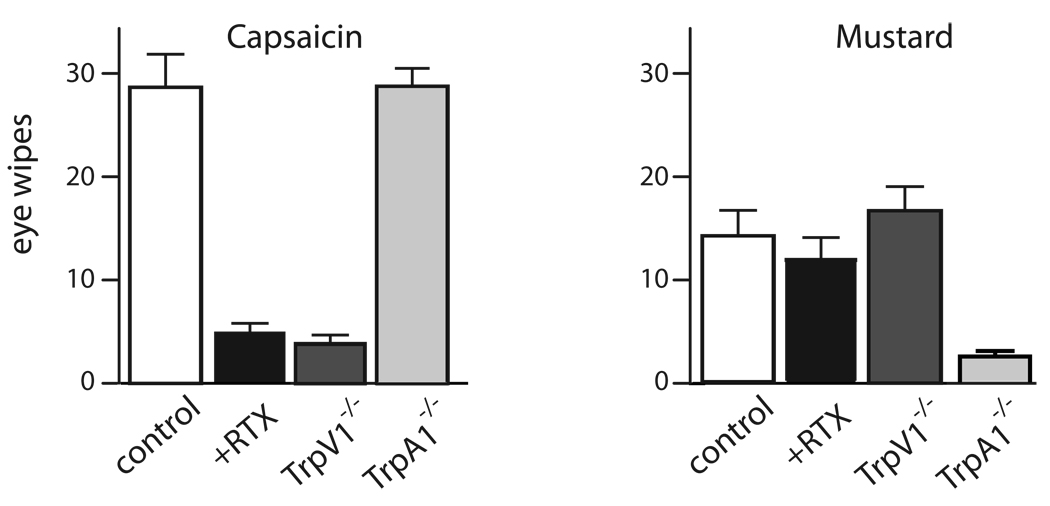
Our data (Fig. 1) predict that RTX treated animals should selectively lose responses to TrpV1 but not to TrpA1-agonists. Therefore we tested responses to both capsaicin and mustard oil in the well characterized eye-wipe assay. As expected, Fig. 2 shows that RTX treatment dramatically decreases the sensitivity of mice to the TrpV1-agonist, capsaicin but not to its TrpA1-counterpart, mustard oil. Similar results (data not shown) were obtained using an assay of oral aversion (Mueller et al., 2005) and with direct capsaicin injection into the hind paw (see experimental procedures). Therefore, both at a structural and function level, the TrpA1-subset of TrpV1-cells are largely unaffected by exposure of sensory ganglia to RTX.
Figure 2. RTX treatment differentially affects TrpV1 and TrpA1 mediated responses.
Eye wipe responses to 100 µM capsaicin and 10 mM mustard oil demonstrate that trigeminal RTX treatment dramatically reduces irritation caused by TrpV1 but not TrpA1 agonists. As a control we also demonstrated that TrpV1−/− and TrpA1−/−-mice selectively lost responses to capsaicin and mustard oil respectively; mean ± SEM (n>8 animals tested two times).
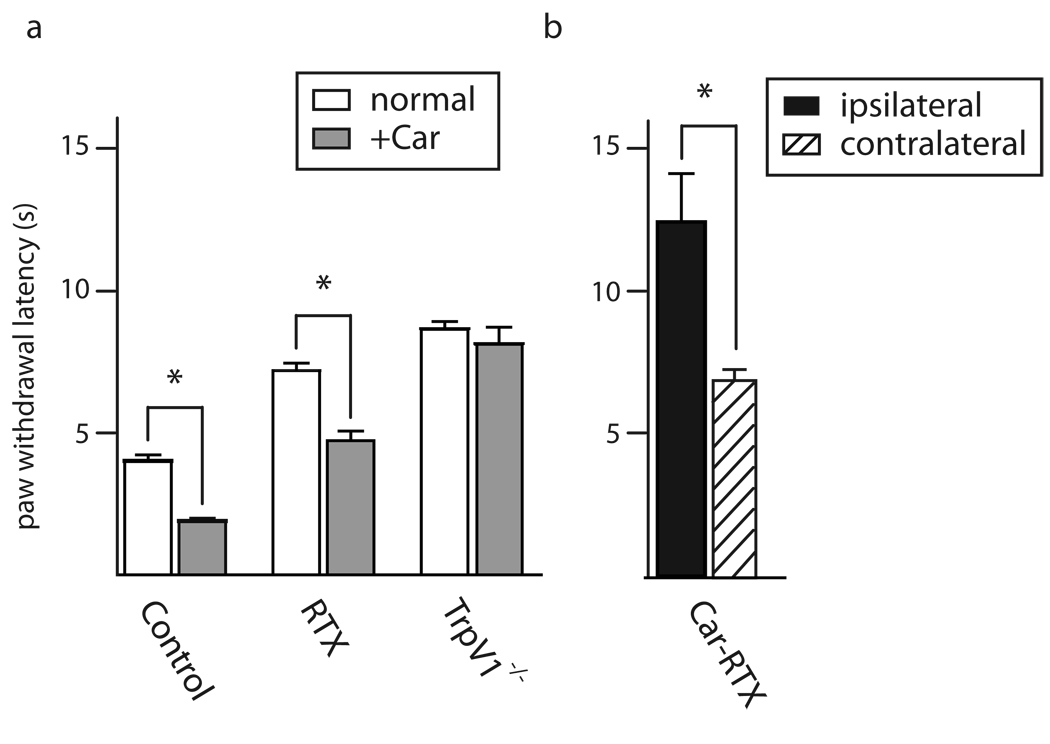
What are the roles of these two subsets of TrpV1-cells in pain sensation? First we measured thermal responses of mice injected intrathecally with RTX using the Hargreave’s test. As shown in Fig. 3, RTX significantly reduces the sensitivity of mice to thermal pain, increasing their reaction time by a factor of about two. Although this is a relatively modest decrease in thermal sensitivity given the extent to which RTX-treatment depletes TrpV1-expressing neurons, the effect is approximately the same as knockout of TrpV1, a major thermosensor that defines these neurons (Fig. 3). Given that RTX-treatment does not eliminate TrpV1/TrpA1-positive cells it is likely that these neurons mediate residual thermal responses and the increased time required for paw withdrawal may simply reflect a decrease in density of peripheral processes responding to heat.
Figure 3. RTX treatment and inflammation alter thermal sensation.
Paw withdrawal latency was used to monitor thermal sensitivity of uninflamed (open bars) and inflamed (carageenan treated, +Car, filled bars) tissue (a). For uninflamed tissue, intrathecal RTX treatment or knockout of TrpV1 reduced thermal sensitivity approx. 2-fold. In inflamed tissue, the effectiveness of RTX was determined by when it was administered. RTX prior to inflammation, (gray bar) was approx. 2-fold less effective than knockout of TrpV1. In contrast, (b) injection of RTX after inflammation (black bar) much more dramatically reduced thermal sensitivity and time for withdrawal of the inflamed paw was now significantly greater that for the contralateral uninflamed paw (stippled bar); mean ± SEM (n=5 animals each tested ≥3 times) * P<0.001, unpaired Student’s t-test.
The level of protection against thermal pain afforded by RTX injection in mice (Fig. 3) appeared relatively small in comparison with reports of its analgesic effects in animal models (Brown et al., 2005). Therefore we investigated whether RTX, like TrpV1-knockout had more prominent affects on thermosensation after inflammation. Fig. 3 shows that carageenan induced inflammatory pain increased sensitivity in the Hargreave’s test by a factor of 2 in control animals. Importantly RTX-treated animals also showed a similar sensitization after inflammation (in contrast in rat no carageenan induced allodynia was reported by (Karai et al., 2004)) whereas thermal responses in TrpV1−/−-mice were almost completely unchanged after carageenan injection (Caterina et al., 2000). Taken together our data demonstrate that RTX-treatment is not as effective as a prophylactic protection against inflammatory pain as might have been predicted from its use in pain therapy (Brown et al., 2005). However, when used therapeutically, RTX is administered to animals already suffering pain. To mimic this, we investigated whether carageenan induced inflammation altered the efficacy of RTX. Thermal responses of mice in which RTX was administered after carageenan were now dramatically reduced, and these mice were significantly less sensitive to thermal pain than TrpV1−/−-mice. Remarkably, thermal sensitivity at the site of inflammation was actually lower than for the opposite, uninflammed paw.
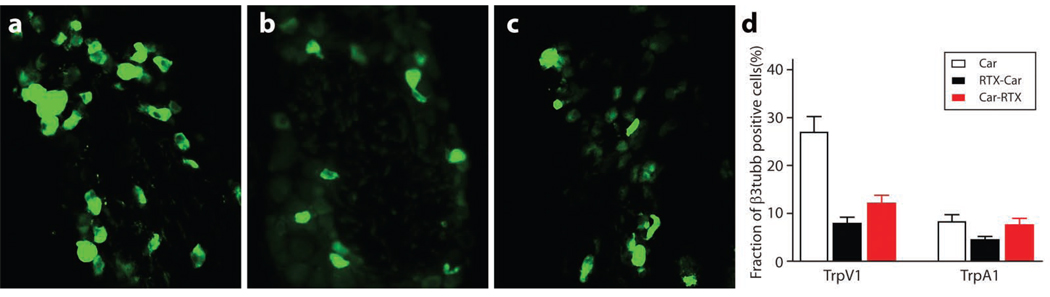
A possible explanation for the increased effectiveness of RTX in thermal protection after inflammation is that inflammatory mediators increase the proportion of TrpV1-neurons killed by the toxin. Therefore, immunohistochemistry and in situ hybridization of the DRGs from animals treated with carageenan (in the hind paw) and intrathecal RTX were used to monitor TrpV1 and TrpA1-positive neurons (Fig. 4). As previously reported (Amaya et al., 2003), peripheral inflammation increases the proportion of TrpV1 positive cells in the relevant lumbar DRGs of control mice (Fig. 1, Fig. 4 and S2). However, neither the absolute number nor the proportion of TrpV1 (or TrpA1) positive neurons remaining in the L4/5 DRG after RTX treatment was significantly affected by the order of injection of toxin and carageenan. A second possibility is that consequences of RTX treatment are more than the killing of a subset of TrpV1 neurons and that these additional effects are affected by inflammation. What could these effects be? It is well known that capsaicin, another TrpV1-agonist, causes transient or permanent deafferation of sensory neurons at doses where it does not lead to cell death (see (Lynn, 1990). Therefore we hypothesized that RTX-treatment might also destroy axonal processes of the TrpV1/TrpA1-cells that survive toxin treatment and that this deafferation is greatly enhanced by inflammation.
Figure 4. RTX mediated cell ablation is not affected by peripheral inflammation.
Immuno-localization and in situ hybridization for TrpV1 and TrpA1 in DRG demonstrate that carageenan induced inflammation does not change the effectiveness of RTX mediated neural ablation. Shown are immunohistochemistry for TrpV1 in the relevant lumber DRG of mice: (a) control carageenan alone; (b) RTX then carageenan; (c) carageenan then RTX; (d) quantitation of double label in situ hybridization of TrpV1 or TrpA1 versus β3-tubulin; mean ± SEM (n=9).
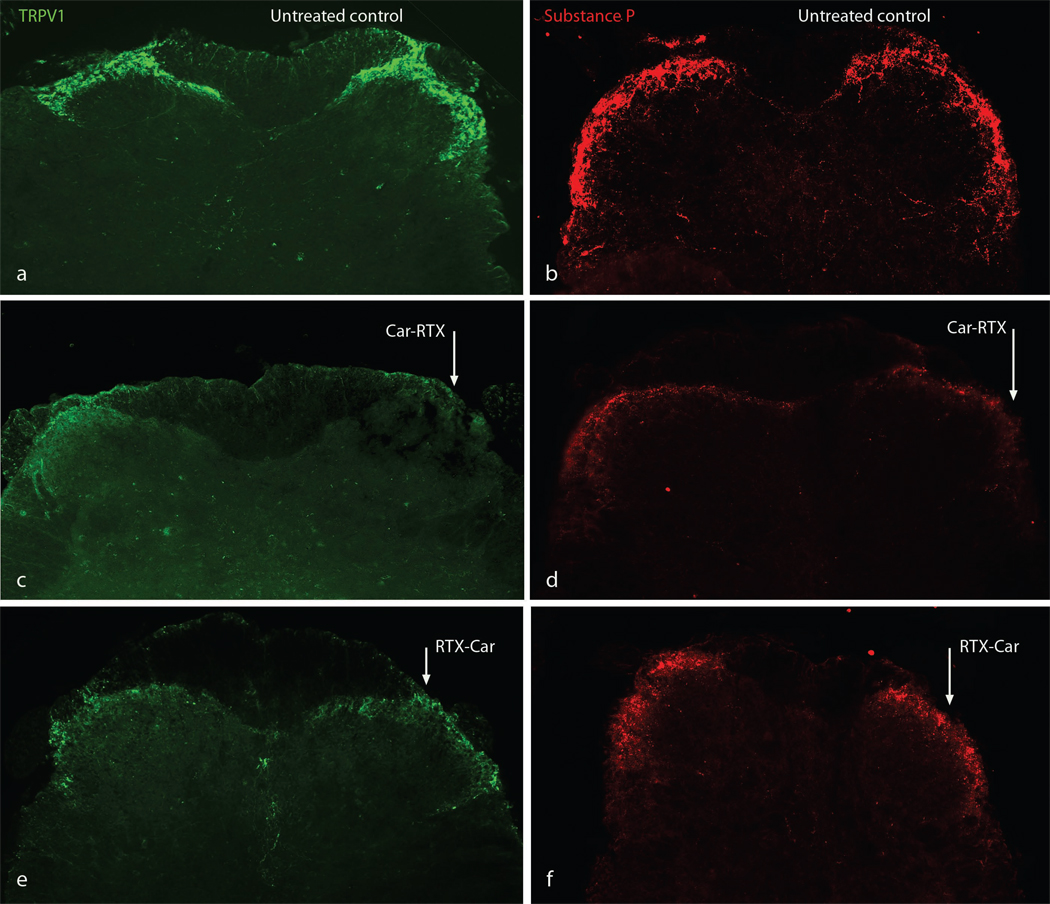
To directly monitor the axons of TrpV1-positive neurons, we carried out immunohistochemistry of the dorsal horn and stained for TrpV1 and the TrpV1-cell specific marker substance P. As reported previously, control mice showed prominent TrpV1 staining in lamina I/II of the dorsal horn (Guo et al., 1999) while staining was absent in tissue of TrpV1−/−-mice (data not shown). As expected, RTX decreased the intensity of TrpV1- and substance P-staining in the dorsal horn, but importantly staining was still observable (compare Fig. 5a and b to e and f). However in mice treated with carageenan prior to RTX injection essentially no residual TrpV1-staining was observed in the dorsal horn 1 day after RTX injection (Fig. 5c and d, ipsilateral to inflammation, arrowed). This additional loss of TrpV1- and substance P-staining strongly supports the postulate that RTX treatment after inflammation results in a profound loss of thermosensation because the TrpV1-cells that are not killed by RTX have had axonal connections destroyed by toxin treatment.
Figure 5. TrpV1 afferents in the dorsal horn are eliminated by RTX treatment after peripheral inflammation.
TrpV1 (a, c, e) and substance P (b, d, f) immunostaining in the dorsal horn of mice revealed sensory efferents in laminae I and II. The level of immunostaining was dramatically reduced after RTX treatment (compare untreated controls a and b with RTX treated c, d, e and f). Peripheral tissue inflammation by carageenan injection after RTX treatment (e and f, right side arrowed, RTX-Car) reduced TrpV1- and substance P-immunostaining. However, inflammation before RTX treatment (c and d, right side arrowed, Car-RTX and d) essentially eliminated all stained fibers in the dorsal horn.
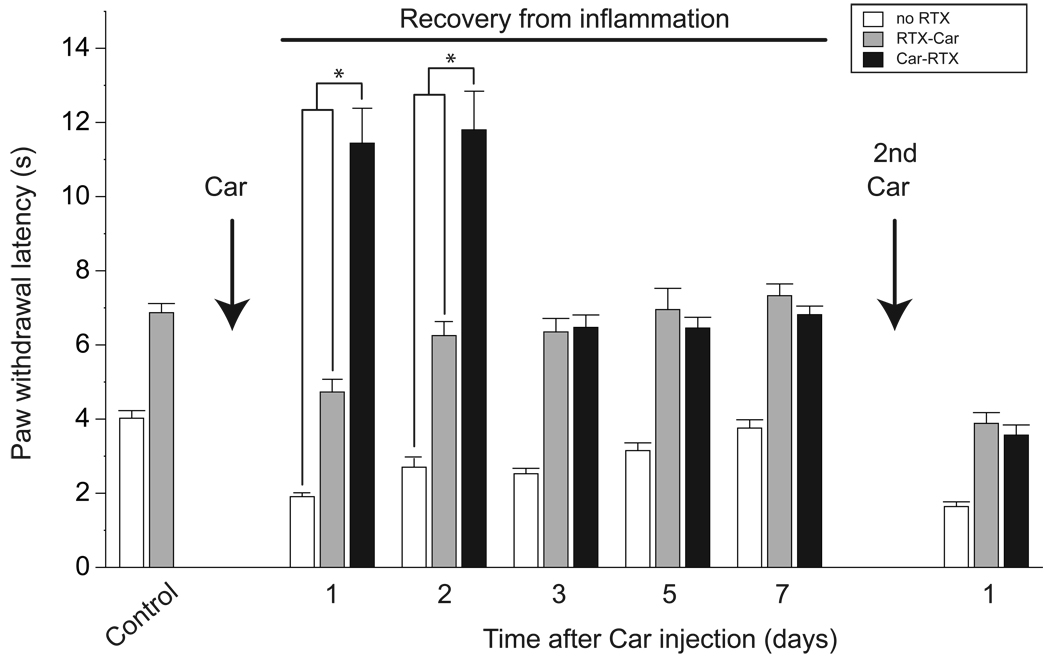
In contrast to the neurotoxicity of RTX, we envisioned that deafferation might be a transient process as sensory nerves often re-grow after peripheral injury. Indeed, within 7 days of carageenan then RTX treatment (Car-RTX) substantial recovery of TrpV1 staining in the dorsal horn was observed (data not shown). Notably, we also found that the response of Car-RTX mice to heat remained dramatically reduced for 28h after RTX treatment but then recovered rapidly to match the responses the RTX-Car group (Fig.6). Moreover, once thermal sensitivity had returned to the level of the control RTX-only mice, additional inflammatory challenges produced an equivalent sensitization to thermal pain in both groups of RTX-treated mice (Fig. 6). Therefore our data strongly substantiate the hypothesis that after inflammatory insult, RTX causes widespread but transient deafferation of TrpV1-fibers that are not killed by the toxin and thus prevents almost all TrpV1-neuron mediated responses.
Figure 6. RTX treatment after inflammation induces both a long-lasting and a transient decrease in thermal sensitivity and inflammatory hyperalgesia.
Paw withdrawal latency was used to monitor thermal sensitivity both before and at intervals after injection of carageenan (Car) into the paw. In control animals (open bars, no RTX), inflammatory hyperalgesia declined over a period of seven days but could be re-evoked by a second carageenan injection. RTX-treatment prior to inflammation (gray bars) decreased thermal sensitivity with responses paralleling those of controls at all time points. Injection of RTX after inflammation (black bars) initially resulted in a dramatic and significant reduction of thermal sensitivity; * P<0.001 compared to untreated and RTX-Car treated mice (unpaired Student’s t-test), however, by 3 days after inflammation, responses were indistinguishable from those of animals treated with RTX before carageenan. Subsequent inflammatory challenges increased (rather than decreased) thermal sensitivity; means ± SEM (n=5 animals each tested ≥3 times).
Previous reports have not provided a clear view as to whether RTX-treatment results in mechanosensory deficits. On the one hand, when used to treat pain, RTX has been suggested to spare discriminative touch sensation and proprioception (Karai et al., 2004). On the other hand there are also reports that both thermal and mechanical sensitivity is reduced after RTX treatment (Tender et al., 2008). The CAR-RTX mice now provide a powerful model to answer the question, are TrpV1-neurons required to sense mechanical pain? Therefore we tested mechanical nociception using two well characterized assays in the RTX-Car and Car-RTX-treated mice (before, during and after inflammation). Figure 7 demonstrates that RTX mediated ablation and deafferation of TrpV1-sensory neurons have no effect on the sensitivity of mice to mechanical stimuli. Thus we conclude that TrpV1-neurons are very selectively required for thermal but not mechanical pain.
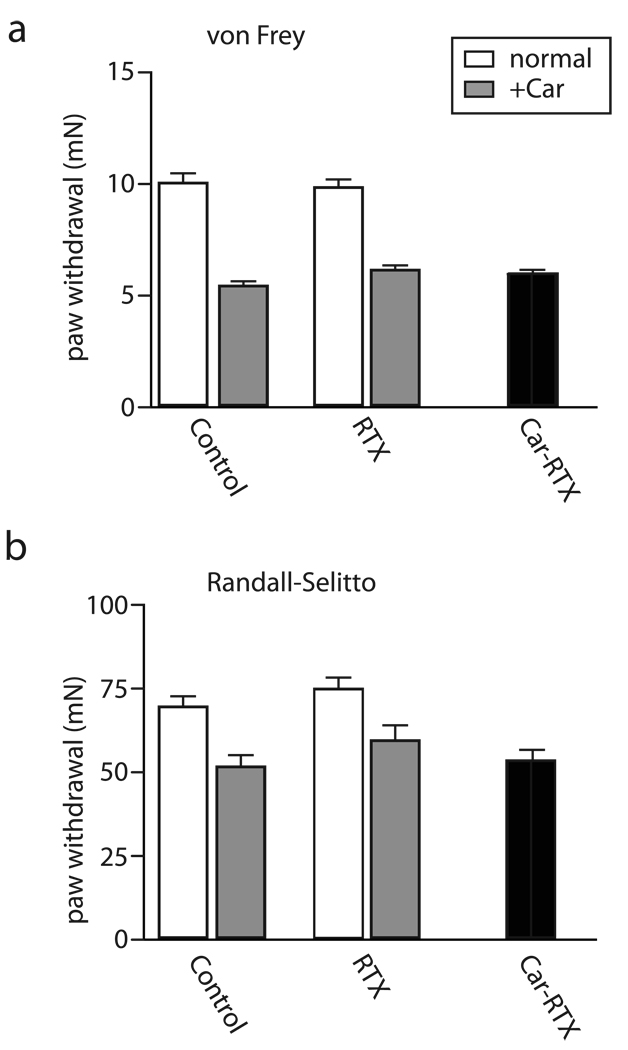
Figure 7. Mechanical stimulation is not affected by RTX mediated neural ablation and deafferation.
Paw withdrawal to von Frey filaments (A) and tail flinch responses to pinch (B) were used to monitor mechanical pain in normal (open bars) and inflamed (carageenan treated, +CAR, filled bars) tissue. Inflammation reduced the threshold stimulation required for both types of responses. However, intrathecal RTX treatment did not alter mechanical responses either in normal or inflamed tissue. Notably even RTX administration after inflammation (Car-RTX), which dramatically attenuates thermal pain (Fig. 3), had no effect on responses to either type of mechanical stimulation (black bars); mean ± SEM (n=5 animals each tested 3≥ times).
Discussion
Recently considerable attention has focused on RTX as a novel therapeutic agent for treating chronic pain by ablation of TrpV1-expressing cells in select sensory ganglia. Here we demonstrate that although RTX treatment is effective at killing many TrpV1-expressing sensory neurons, a population of large diameter TrpA1/TrpV1-expressing cells (approx 30% of the total TrpV1-cells in the trigeminal ganglion) is resistant to the toxin (Fig. 1 and Fig. 2). We have shown that TrpA1 is simply a marker of these neurons and is not required to protect them from RTX-mediated cell death and speculate that it may simply be the larger volume of this subset of sensory neurons that makes them less sensitive to toxin. For example, the surface/volume ratio decreases as diameter increases, thereby reducing the relative effect of gating a cell surface channel; moreover sequestration of Ca2+ into more abundant intracellular stores may also help protect these cells from permanent damage.
It has been well established that inflammation affects the thermal and chemical sensitivity of TrpV1 (Julius and Basbaum, 2001), and that this is largely responsible for the decreased heat tolerance after carageenan injection. Protein kinase-A and C mediated phosphorylation of TrpV1 (Bhave et al., 2002; Cesare et al., 1999; Numazaki et al., 2002) shifts its voltage dependence (Nilius et al., 2005) and consequently increases its responses to all stimuli at the periphery. Here we demonstrate that inflammation dependent changes in neural sensitivity also extend more centrally: RTX differentially affects the neurons at the level of the ganglion before and after inflammation (Fig 3, Fig 5 and Fig 6). Our data are consistent with increased sensitivity of TrpV1 extending to the cell bodies of the neurons innervating inflamed tissue, and, as a result, RTX-treatment of these ganglia induces profound thermal desensitization (Fig. 3). Direct detection of the TrpV1-neurons (Fig 4) proves that RTX does not kill a significant number of additional TrpV1-neurons innervating the paws of mice that had been injected with carageenan. Taken together, with the transient nature of this thermal analgesia (Fig. 5 & Fig. 6), it appears that like capsaicin (see reviews; (Fitzgerald, 1983; Lynn, 1990)) RTX can either induce cell death or deafferation depending on the timing/conditions of exposure (indeed other treatments like CFA induced inflammation may produce similar effects). The very profound relief of chronic pain symptoms reported following RTX injection into ganglia of dogs (Brown et al., 2005) likely reflects such an increased sensitivity of the ganglion neurons. Future studies will thus be needed to establish whether RTX is effective at treating all types of chronic pain and it will be important to establish if this is related to ablation and/or deafferation of sensory neurons.
For the past 50-years, a central dogma in pain biology has been that broadly tuned nociceptive C-fibers are responsible for sensing all types of pain. Molecular biology has recently identified a number of molecules thought to play roles in diverse forms of nociception e.g. thermosensation (Jordt et al., 2003), detection of noxious chemicals (Bautista et al., 2006; Bautista et al., 2008; Kwan et al., 2006), itch (Han et al., 2006), etc. Of these, TrpV1 is believed to be the major sensor of noxious heat, yet TrpV1−/− mice only exhibit relatively mild loss of thermal responses (Caterina et al., 2000; Eckert et al., 2006) and Fig. 3). Notably, we show that ablation and deafferation of these neurons has a much more pronounced phenotype demonstrating that there are additional mechanisms for thermodetection selectively localized to these cells. In contrast, RTX-treatment has no effect on mechanosensation (Fig. 7). Thus we conclude that different subsets of sensory neurons must mediate thermal and mechanical pain. Interestingly, results of capsaicin injection reported 30 years ago (Yaksh et al., 1979) are completely compatible with our current findings –loss of heat but not mechanical sensitivity– although at that time, the molecular and cellular basis for this finding could not be unequivocally established. It is also relevant to compare our findings with other recent results e.g. ablation of Nav1.8 expressing cells (Abrahamsen et al., 2008) implicated these cells in mechanosensation and cold responses but not noxious heat. Very recently, another subset of cells have been shown to mediate pleasant touch (Loken et al., 2009) while ablation of Mrgprd-cells has been shown to affect mechanosensation but not temperature responses (Cavanaugh et al., 2009). In addition, it has been shown that δ-opioids selectively inhibit mechanical pain while μ-opioids affect thermosensation (Scherrer et al., 2009). Indeed Cavanaugh et al., 2009 (published while this manuscript was in preparation) used an alternate strategy to eliminate TrpV1 cells responses and also concluded that these neurons are required for responses to noxious heat but not mechanical pain. In this case capsaicin did not lead to cell death of TrpV1-expressing neurons like RTX treatment but lead to a reported loss of function of all TrpV1 afferent; this is likely because of the differences in affinity and the doses of drug used. Interestingly, all these results parallel those reported for mammalian taste where each modality, sweet, sour, salty, bitter and umami (savory) is mediated by a distinct labeled line (Chandrashekar et al., 2006). In combination our data and the results from a variety of other approaches substantiate a new view of somatosensation (and pain): selectively tuned subsets of sensory neurons respond specifically to different types of stimuli.
Materials and Methods
Animal models
Mice were 25–30g (2–4 month old) male C57/BL6, TrpV1−/− (Caterina et al., 2000) or TrpA1−/− (Kwan et al., 2006). Procedures followed the NIH Guidelines for the care and use of laboratory animals, and were approved by the National Institute of Dental and Craniofacial Research Animal Care and Use Committee. Trigeminal microinjection: Injections (200ng RTX, LC Laboratories in 2µl PBS) were performed essentially as described previously (Karai et al., 2004). Experiments were initiated approximately three weeks after surgery. Intrathecal injections: 200 ng of RTX in 10 µl PBS containing 0.05 % ascorbic acid, and 7 % Tween 80 was administered to the L4/L5 intraspinal space (Mestre et al., 1994). Carrageenan injection: The plantar surface of a hind paw or the tail was injected with 20 µl of a solution of 2% carageenan in PBS (Sigma). We determined that carageenan injection induced similar inflammation in all animals, and monitored recovery by measuring paw edema using a plethismometer device (IITC Life Science, USA). To investigate the effects of inflammation on RTX treatment, RTX was administered 24 h after carageenan and behavioral testing was initiated 4h later. In control experiments on mice without inflammation we established no change in thermal sensitivity was observed in time points from 4h to 5 weeks after RTX injection.
Behavioral Assays
Chemical sensitivity: eye wipe assays were performed to investigate the afferent functions of the ophthalmic branch of the trigeminal nerve. Capsaicin and mustard oil induced eye-wipes were video recorded and counted for 1 min after delivery of 50 µl of solution (100 µM capsaicin or 10 mM mustard in PBS). In addition, we carried out an oral avoidance test for capsaicin and mustard oil essentially as described previously (Mueller et al., 2005).Peripheral responses to capsaicin injection in the hind paw were recorded as described in (Caterina et al., 2000). Thermal responses: We used a semi-automated Hargreaves test (Ugo Basile Varese, Italy) to measure thermal responses. Animals were habituated for 30 min in individual chambers prior to experiments. A radiant heat source was focused on the plantar surface of the hind paw, and the time from the initiation of the radiant heat until paw withdrawal was measured. A maximum cut-off of 20 s was used to prevent tissue damage. Mechanical responses: Threshold-force induced paw withdrawal was measured using an automated von Frey apparatus (Ugo Basile Varese, Italy); in addition a modified Randall-Sellito device (IITC Life Sciences, USA) was used to automatically measure responses when pressure was applied to the tail.
In situ hybridization and immunohistochemistry
In situ hybridization (ISH) was performed at high stringency (washed 30 min, 0.2× SSC, 70°C) as described previously (Hoon et al., 1999). To test for specificity and sensitivity of our experiments with TrpA1 KO animals we performed ISH with both full-length TrpA1 probe and exon 22–24 TrpA1 cRNA (exons deleted in TrpA1 KO animals). Figure S1 shows that while there are no positive cells that hybrididize with the exon 22–24 probe the full-length probe detects the same number of cells as wild-type animals. A similar result was seen with TrpV1 KO animals and their respective deleted exon and full-length cRNA probes.
For double labeling we used fluorescein and digoxigenin labeled probes that were detected with antibodies coupled to horseradish peroxidase and alkaline phosphatase together with tyramide-FITC and fast-red (Adler et al., 2000). For immunohistochemistry, mice were perfused and excised tissue fixed for a further 1h in 4% PFA. Tissue was prepared for sectioning by soaking in 30% sucrose/PBS (overnight at 4°C) before freezing. Immunostaining was carried out using a 1:500 dilution of anti-TrpV1 (Chemicon) and an Alexa-488 donkey anti-rabbit secondary antibody (Invitrogen). Images were collected using a Microphot FX microscope (Nikon) and processed with Adobe Photoshop.
Supplementary Material
In situ hybridization specificity was examined using probes to TrpA1 and TrpV1 genes. Dorsal root ganglia neurons that express TrpA1 and TrpV1 full length (FL) coding sequence probes and those that express the part of the gene that corresponds to the deletion site in their respective knockout animals (exons 22–24 for TrpA1 KO and exon 13 for TrpV1 KO) were identified. The proportion of neurons expressing TrpV1 or TrpA1 was the same in wild-type (WT), TrpA1 KO and TrpV1 KO. Whereas, probes to FL TrpA1 or TrpV1 show similar number of positive cells in KO animals as in wild-type, the probe to TrpA1 exon 22–24 showed no hybridization to TrpA1 KO DRG tissue and TrpV1 exon 13 similarly showed no positives in TrpV1 knockouts demonstrating the specificity of this approach to define specific populations of neurons.
Comparison of the number of neurons in dorsal root ganglia that express TrpV1 under normal conditions and after carrageenan induced inflammation. Upper panels show representative merged images from double label in situ hybridization experiments of TrpV1-expressing neurons (red) compared to β3-tubulin expressing neurons (green). Results from normal (left) and carrageenan stimulated tissue (CA; right) show an increased number of TrpV1-expressing cells following inflammation. Lower panels show quantitation of double label in situ hybridization of TrpV1 versus β3-tubulin; mean ± SEM (n=9) shows.
Acknowledgements
We thank N. Ryba, T. Usdin and S. Gutkind for helpful discussions and critical review of the manuscript. This work was supported by the intramural research program of the NIH, NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science (New York, N.Y. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain research. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nature neuroscience. 2008;11:772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science (New York, N.Y. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009 doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Eckert WA, 3rd, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Capsaicin and sensory neurones--a review. Pain. 1983;15:109–130. doi: 10.1016/0304-3959(83)90012-x. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. The European journal of neuroscience. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Current opinion in neurobiology. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. The Journal of clinical investigation. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature neuroscience. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–69. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- Ma QP. Expression of capsaicin receptor (VR1) by myelinated primary afferent neurons in rats. Neuroscience letters. 2002;319:87–90. doi: 10.1016/s0304-3940(01)02537-x. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. Journal of pharmacological and toxicological methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Emson PC, Iversen LL. A re-evaluation of the neurochemical and antinociceptive effects of intrathecal capsaicin in the rat. Brain research. 1981;211:497–502. doi: 10.1016/0006-8993(81)90980-x. [DOI] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104:219–228. doi: 10.1016/s0304-3959(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? The Journal of physiology. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. The Journal of biological chemistry. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. The Journal of biological chemistry. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nature reviews. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tender GC, Li YY, Cui JG. Vanilloid receptor 1-positive neurons mediate thermal hyperalgesia and tactile allodynia. Spine J. 2008;8:351–358. doi: 10.1016/j.spinee.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain research reviews. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science (New York, N.Y. 1979;206:481–483. doi: 10.1126/science.228392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In situ hybridization specificity was examined using probes to TrpA1 and TrpV1 genes. Dorsal root ganglia neurons that express TrpA1 and TrpV1 full length (FL) coding sequence probes and those that express the part of the gene that corresponds to the deletion site in their respective knockout animals (exons 22–24 for TrpA1 KO and exon 13 for TrpV1 KO) were identified. The proportion of neurons expressing TrpV1 or TrpA1 was the same in wild-type (WT), TrpA1 KO and TrpV1 KO. Whereas, probes to FL TrpA1 or TrpV1 show similar number of positive cells in KO animals as in wild-type, the probe to TrpA1 exon 22–24 showed no hybridization to TrpA1 KO DRG tissue and TrpV1 exon 13 similarly showed no positives in TrpV1 knockouts demonstrating the specificity of this approach to define specific populations of neurons.
Comparison of the number of neurons in dorsal root ganglia that express TrpV1 under normal conditions and after carrageenan induced inflammation. Upper panels show representative merged images from double label in situ hybridization experiments of TrpV1-expressing neurons (red) compared to β3-tubulin expressing neurons (green). Results from normal (left) and carrageenan stimulated tissue (CA; right) show an increased number of TrpV1-expressing cells following inflammation. Lower panels show quantitation of double label in situ hybridization of TrpV1 versus β3-tubulin; mean ± SEM (n=9) shows.