Abstract
This review discusses the contributions of a newly considered form of plasticity, the ongoing production of new neurons from neural stem cells, or adult neurogenesis, within the context of neuropathologies that occur with excessive alcohol intake in the adolescent. Neural stem cells and adult neurogenesis are now thought to contribute to the structural integrity of the hippocampus, a limbic system region involved in learning, memory, behavioral control, and mood. In adolescents with alcohol use disorders, the hippocampus appears to be particularly vulnerable to the neurodegenerative effects of alcohol, but the role of neural stem cells and adult neurogenesis in alcoholic neuropathology has only recently been considered. This review encompasses a brief overview of neural stem cells and the processes involved in adult neurogenesis, how neural stem cells are affected by alcohol, and possible differences in the neurogenic niche between adults and adolescents. Specifically, what is known about developmental differences in adult neurogenesis between the adult and adolescent is gleaned from the literature, as well as how alcohol affects this process differently between the age groups. And finally, this review suggests differences that may exist in the neurogenic niche between adults and adolescents and how these differences may contribute to the susceptibility of the adolescent hippocampus to damage. However, many more studies are needed to discern whether these developmental differences contribute to the vulnerability of the adolescent to developing an alcohol use disorder.
Keywords: ethanol, alcoholism, progenitor, hippocampus, neurodegeneration
Introduction
Adolescence is a time of juxtaposition: adolescents are exploring and experimenting with many aspects of adult life, yet their brains are in a unique developmental state that renders them highly vulnerable to neurotoxicity (Smith, 2003; Spear, 2007). The age range of adolescence is decided culturally. Although in the U.S., adolescence is defined as the years between 13 and 19, the World Health Organization extends that to include those within 10-19 years of age (WHO, 2008). As adolescents explore and experiment with adult aspects of life, alcohol initiation and experimentation also begins. Annual surveys of drug and alcohol use have detailed disturbingly high rates of alcohol use and abuse among adolescents. Nearly 40% of 8th graders have tried alcohol, a value that rises throughout adolescence to 72% of 12th graders (Johnston et al., 2007). Remarkably, nearly half of high school seniors are considered current drinkers (drinking within the last 2 weeks) and over half of those students classify themselves as heavy drinkers (5+ drinks in one sitting in the last 2 weeks; Johnston et al., 2007). On average, adolescents drink less frequently than adults, however when adolescents drink, they consume alcohol in quantities similar to adults (Deas et al., 2000; Doremus et al., 2005; White et al., 2006). Between 6% of eighth graders to nearly a third of 12th graders report having been drunk within the last 30 days (Johnston et al., 2007). Considering the reduced sensitivity of adolescents to feeling intoxicated (e.g. Little et al., 1996), perhaps the reports of drinking five or more alcoholic beverages in one sitting are more telling, as those values are slightly higher among most adolescent age groups (Johnston et al., 2007). However, this statistic should also be tempered with data from college age students that the adolescent's definition of a drink may contribute to their underestimation of levels of intake (White et al., 2003). In other words, a significant population of adolescents drink alcohol and within that population, they are drinking to intoxicating and problematic levels.
Alcohol use and abuse clearly escalate throughout adolescence. One group estimates that of those adolescents who report alcohol use, 60-70% are binge drinkers (Zeigler et al., 2005). Unfortunately, the changes in brain structure and/or function that occur with repeated binge drinking are hypothesized to be a critical step between experimenting with alcohol and developing an alcohol use disorder (AUD; Crews et al., 2007; Crews, 1999; Koob and Le Moal, 1997). The risk of developing an AUD greatly increases with both a family history of alcohol use and an early onset of use (Grant and Dawson, 1997). For example, a teenager that begins to drink around the age of thirteen or earlier and who also has a family history of alcoholism, has an almost 60% likelihood of developing an AUD as an adult (Grant and Dawson, 1997). Unfortunately, the risk of developing an AUD is still higher for teenagers who begin to drink at an earlier age; the likelihood of developing an AUD is almost double, regardless of family history, when adolescents begin drinking around the age of 13 (Grant and Dawson, 1997). Indeed, approximately 40% of those diagnosed with an AUD developed their first symptoms between the ages of 15 and 19 (Brown et al, 2008; Deas et al., 2000).
This heightened risk for developing an AUD during adolescence suggests that there is something unique about the brain of adolescents or in their response to alcohol. In fact, both factors likely contribute: the adolescent brain is in a unique state and responds to alcohol distinctively compared to the adult (Smith, 2003; Spear, 2000; 2002). The adolescent brain is not just a little adult (Spear, 2000). The extent of neural modifications - from the molecular to cellular - support that this is a very unique and obviously dynamic time in development (Lenroot and Giedd, 2006). And though much of the structural foundation has been laid by adolescence, the fine-tuning of these connections via synaptic pruning is actively occurring during this period (e.g. Giedd et al., 1999; Sowell et al., 1999; reviewed in Lenroot and Giedd, 2006; Spear, 2007). Further, the adolescent brain responds to alcohol in ways that are very distinct from the adult (Spear, 2006). Understanding the adolescent's specific brain responses to alcohol within the context of ongoing plasticity is critical to comprehending how these events or this interaction results in the adolescent's vulnerability to developing an AUD (Crews et al., 2007; Smith, 2003; Spear, 2000; Spear, 2002). This review defines and considers the contributions of a newly considered form of plasticity, the ongoing production of new neurons or adult neurogenesis, to the neuropathologies that occur with excessive alcohol intake. Within this discussion, important questions are raised such as where the adolescent fits in with regard to developmental neurogenesis versus adult neurogenesis and how alcohol affects this process among the age groups. Finally, we glean several developmental differences from the literature that may impact the neurogenic niche and may underlie the adolescent's susceptibility to alcoholic neuropathology.
The Role of Alcohol-induced Neuropathology in AUDs
Excessive alcohol intake characteristic of AUDs causes progressive neurodegeneration, which is hypothesized to facilitate the critical steps or “downward spiral” between experimenting with alcohol and developing an alcohol use disorder (Crews et al., 2007; Crews 1999; Koob and Le Moal, 1997). Although alcohol causes global changes in the CNS, which is particularly evident when examining white matter tracts in human alcoholics, the alcoholic brain also suffers from atrophy of gray matter (Harper et al., 1987; De la Monte, 1988; Pfefferbaum et al., 1992; Pfefferbaum et al., 1997; Pfefferbaum et al., 2000). Some brain structures have heightened sensitivity to alcohol effects and/or toxicity. These regions, which include the frontal cortex and hippocampus, are among the most vulnerable to ethanol neurotoxicity (Pfefferbaum et al, 1997; Sullivan et al., 1995). Alcoholics exhibit a pattern of neurological symptoms suggestive of hippocampal neuropathology (Brandt et al., 1983; Parsons, 1993; Sullivan et al., 2000), which is supported by various assessments of brain structure to show that hippocampal integrity is altered in human alcoholics (Agartz et al., 1999; Bengochea and Gonzalo, 1990; Laakso et al., 2000; Sullivan et al., 1995). These regions, incidentally, are associated with cognitive functions such as a behavioral inhibition system that underlie many aspects of executive function and psychological regulation (Clark et al., 2008). Impairments in executive functions and/or behavioral inhibition from alcohol-induced neurotoxicity have been hypothesized as the critical link between excessive intake and the development of an AUD (Bechara et al., 2005; Clark et al., 2008; Crews et al., 1999; Koob and Le Moal, 1997).
Due to alcohol's many promiscuous effects on the CNS, multiple mechanisms underlie alcohol-induced neurodegeneration in these corticolimbic regions. For example, human postmortem and in vivo imaging studies consistently show reduced cortical white matter in the alcoholic brain (Harper et al., 1987; De la Monte, 1988; Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). Recent observations extend this effect to white matter microstructure, which suggests that white matter loss may be more severe than initially observed (Pfefferbaum et al., 2000) and suggests a mechanism of cortical disconnectivity that is associated with alcoholic cognitive deficits (Sullivan et al., 2005; Sullivan and Pfefferbaum, 2005). Although white matter loss is particularly evident in human alcoholics, the alcoholic brain also suffers from atrophy of cortical gray matter (Harper et al., 1987; De la Monte, 1988; Pfefferbaum et al., 1992; Pfefferbaum et al., 1997; Pfefferbaum et al., 2000). Alcohol-induced loss of grey matter has been attributed to reductions in neuronal number and size in addition to simplification of neuronal processes (Bengochea et al., 1990; Kril and Harper, 1989; Jensen and Pakkenberg, 1993).
Studies in animal models of AUDs parallel many of these observations in humans and allow for the direct link between alcohol, neurotoxicity and behavioral impairments (Lukoyanov et al., 1999; Riley and Walker, 1978; Walker et al., 1980). For example, alcohol produces cell loss and cell death in corticolimbic regions (Cadete-Leite et al., 1988; Crews et al., 2000; Lukoyanov et al., 2000; Paula-Barbosa et al., 1993; Zou et al., 1996). And, multiple studies have shown that the complexity of dendritic branching is attenuated in alcohol-exposed animals (Durand et al., 1989; He et al., 2005). Taken together, human and animal model studies strongly support that alcohol impairs multiple aspects of corticolimbic circuitry. Indeed, these modifications in response to alcohol and alcohol withdrawal range from degeneration of distinct neuronal populations to alcohol-induced changes in dendritic spine structure (Carpenter-Hyland et al., 2004; Carpenter-Hyland et al., 2006). Although multiple hypotheses exist about which aspects of alcohol-induced structural changes result in addiction, it is clear that structural changes and the resulting behavioral impairments are critical for the development of an AUD (Mulholland and Chandler, 2007).
Adolescent Susceptibility to Alcohol-induced Neuropathology
Mounting evidence from both human and animal research suggests that adolescents are more susceptible than adults to the neurotoxic effects of alcohol. This susceptibility has been observed across measures of cognitive performance and structural integrity (reviewed in Crews et al., 2007; Smith, 2003; Spear, 2007). For example, despite the shorter duration of alcohol problems or excessive intake, cognitive deficits are still detectable in adolescents diagnosed with an AUD (e.g. Brown et al., 2000). Alcohol-induced impairments have been reported for both adult and adolescent models of AUDs (e.g. Pascual et al., 2007), but very few compare ages for developmental differences in response or toxicity. In two animal studies that did compare adult and adolescent rats, the adolescent rats demonstrated greater impairments than adults on a hippocampal-dependent learning and memory task, the Morris Water Maze, as a result of excessive alcohol exposure (Schulteis et al., 2008; Sircar and Sircar, 2005). Although several groups have elegantly shown an enhanced amnestic effect of alcohol on memory formation or memory-like processes (e.g. Markwiese et al., 1998; Pyapali et al., 1999), the Schulteis and Sircar studies suggested that prior alcohol exposure either changed function or structure to result in these later deficits. Further, these studies also showed that memory impairments are either greater or more persistent in adolescent rats as compared to adult rats that only have modest or transient impairments (Schulteis et al., 2008; Sircar and Sircar, 2005). Interestingly, this developmental difference in susceptibility to memory impairment has only been demonstrated in a hippocampal-dependent task.
Human imaging studies, in addition to data from animal models of adolescent alcohol use and abuse, suggest that the hippocampus is especially vulnerable to alcohol effects, a vulnerability that appears to extend to neurotoxic consequences of excessive alcohol intake (White and Swartzwelder, 2004). Although much debate exists in the alcoholic neuropathology literature as to whether the hippocampus is damaged in adults with AUDs (Harper et al., 1987), evidence of hippocampal degeneration in adolescents diagnosed with an AUD is quite consistent. First, studies of neurocognitive functioning in alcohol abusing adolescents suggest impairment in behaviors associated with hippocampal processes. Human adolescents with a history of an AUD have deficits in verbal and spatial working memory, verbal and nonverbal retrieval, and attention (Brown et al., 2000; Tapert et al., 2002; Tarter et al., 1995). In addition, adolescent AUDs are associated with decreased IQ and academic achievements compared to matched controls (Tarter et al., 1995). Observations of cognitive deficits in hippocampal-related behaviors are supported by assessments of brain structural integrity. Several neuroimaging studies have shown that human adolescents with an AUD have altered brain morphology, including decreased hippocampal volume when compared to healthy cohorts (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005). Furthermore, hippocampal volume correlates positively with the age of onset and negatively to duration of use, which suggests the developing brain is more susceptible to alcohol-induced impairments (De Bellis et al., 2000).
Animal models of adolescent AUDs also allow for the direct link between alcohol, neurotoxicity and behavioral impairments (Smith, 2003; Spear, 2007). Animal models of binge drinking show that adolescents are more sensitive to alcohol-induced brain damage (Crews et al., 2000; Pascual et al., 2007). Specifically, adolescent rats show greater neurodegeneration than adults in frontal-anterior cortex regions as evidenced by amino cupric silver staining of dying cells (Crews et al., 2000). Recent studies have focused on elucidating specific biochemical and structural alterations that may make the adolescent brain more susceptible to the neurotoxic effects of alcohol, especially in the hippocampus. For example, the adolescent hippocampus is vulnerable to extensive changes in long-term protein expression, an effect that is absent in adults (Hargreaves et al., 2009). Intriguingly, proteins involved in plasticity were affected: chronic alcohol exposure caused long-term impairments in the expression of proteins related to cytoskeletal and synaptic functioning, cell cycle, and neurodegeneration (Hargreaves et al. 2009). These data support an earlier study that showed that low chronic alcohol exposure not only causes persistent structural changes in a variety of brain regions, such as the hippocampus, but immunoreactivity for two cytoskeletal proteins, Nf-200 and MAP-2, were also diminished (Evrard et al., 2006). Many of these cytoskeletal and synaptic proteins are involved in formation and maintenance of new neurons and synaptic connections, events that are critically involved in neurogenesis as well as maturation of hippocampal function (Carpenter-Hyland and Chandler, 2007; Crews et al., 2007; Hargreaves et al., 2009). These results further the idea that the unique state of plasticity of the adolescent brain may render it more sensitive to the neurotoxic effects of alcohol. Although the specific mechanisms responsible for causing altered morphology and impairment in these brain structures are unclear, a newly considered mechanism of structural plasticity, adult neurogenesis, may give insight to adolescent vulnerability to alcohol-induced brain damage.
Adult Neurogenesis
The phenomenon of neurogenesis, or the ability of the brain to generate new neurons, was thought originally to occur only during brain development in the fetus and young child. The observation that neurogenesis continued through juvenile stages and beyond was highly controversial during the latter half of the 20th century, even though Joseph Altman's original discovery of ongoing neurogenesis in the mid 1960s (Altman and Das, 1965) was supported by electron microscopy work in the late 1970's (Kaplan and Hinds, 1977). Wide acceptance of this phenomenon was prevented by dogma and technological limitations for nearly 20 more years until the advent of Bromo-deoxy-Uridine “pulse and chase” methods to label dividing cells (pulse) and observe their eventual phenotype (chase) with immunohistochemistry (Cameron and Gould, 1994; Cameron et al., 1993; Gross, 2000). The study of postnatal neurogenesis gained significant momentum in the mid 1990s, though the dogma was not overruled fully until the publication of reports showing that endogenous neural stem cells underlie constitutive neurogenesis in the adult brain (Doetsch et al., 1999; Palmer et al., 1997). The discovery that adult neural stem cells could be observed in the human brain finally swayed opinion to consider this phenomenon as a fundamental process in the brain (Eriksson et al., 1998). Neural stem cells and neurogenesis have been observed across several species, including all mammals examined to date, which suggests that it is a highly conserved process. Thus, it is now well accepted that neurogenesis continues in the postnatal and adult brain in at least two regions, along the lateral ventricles (subventricular zone) and in the dentate gyrus of the hippocampus (subgranular zone; Altman and Das, 1965; Doetsch et al., 1999). In the subventricular zone, neuroblasts migrate to the olfactory bulbs via the rostral migratory stream (Doetsch et al., 1999). The neurons generated along this path are primarily indicated in the function of olfaction (e.g. Ninkovic et al., 2007) and an equivalent site has only recently been identified in humans (Curtis et al., 2007). Hippocampal stem cells, meanwhile, reside in a thin band called the subgranular zone that runs along the inside of the dentate gyrus granule cell layer (Palmer et al., 1997). Neural stem cells of the hippocampus have been linked to nearly every function of the hippocampus including learning, memory, and mood and the associated disorders thereof (see Balu and Lucki, 2009 for review). This review focuses on neural stem cells and neurogenesis in the hippocampus because the hippocampus is a target of alcohol toxicity in the adolescent brain and because of this structure's relevance to learning, memory, and mood, all of which are impaired or altered in the AUDs (Canales, 2007; Eisch, 2002; Powrozek et al., 2004).
Components of Adult Neurogenesis - State of Knowledge for Adolescent Brain
Although it is now well accepted that the process of neurogenesis occurs throughout the lifetime of the organism, several aspects of neuron production change throughout development. Postnatal neurogenesis events are now referred to as adult neurogenesis in order to distinguish this phenomenon from its embryonic counterpart. The necessity of this distinction arises from the few but critical differences reported thus far between embryonic and adult forms of neurogenesis (for review see Temple, 2001). The rate of adult neurogenesis declines with age (Altman and Das, 1965, 1966; He and Crews, 2007; Manganas et al., 2007). Around mid adolescence new neuron production begins to asymptote according to rodent studies (Altman and Das, 1965, 1966) and decreases to barely detectable levels in aging (Kuhn et al., 1996; see also Drapeau and Abrous, 2008 for review). Intriguingly, the few studies that have estimated a rate of production were conducted in adolescent rats (e.g. Altman and Das, 1965; Cameron and McKay, 2001). As many as 250,000 new dentate granule cells, or 6% of the granule cell population, are produced each month (Cameron and McKay, 2001). However, this estimate was from 35-day old rats, which is equivalent to mid-adolescence (Spear and Brake, 1983). An 80% loss in granule cell production is observed from adolescence to young adulthood (e.g. 60-day old rats) which suggests that just over 1% to less than 0.4% of the granule cell population are generated monthly in young and middle-aged adults respectively (He and Crews, 2007; Lemaire et al., 2000). The net number of neurons produced is the culmination of several processes. Essentially, four processes are required for the successful production of a new neuron: neural stem cell proliferation, cell differentiation, cell migration, and cell survival and/or integration, each of these processes is regulated independently. For example, rates of neural stem cell proliferation are not predictive of the net number of new neurons because cell survival may compensate and affect outcome. Each component is discussed individually below and includes the current state of knowledge for these events in adolescent organisms:
Neural Stem Cell Proliferation
The replication of neural stem cells to form daughter cells is the process of proliferation. According to current definitions, there are two populations of neural stem cells that continue to proliferate throughout life to form new neurons in a normal adult brain: the subventricular zone of the anterior lateral ventricles (Doetsch et al., 1999), and the subgranular zone of the dentate gyrus of the hippocampus (Palmer et al., 1997). Two sites of constitutive neurogenesis, however, should not be interpreted to mean that only two sites of neural stem cells exist. Neural stem cells have been isolated from many other areas of the brain such as the cerebellum, cortex, hypothalamus, striatum, and substantia nigra (e.g. Chipperfield et al., 2005; Laywell et al., 2000; Lie et al., 2002; Reynolds and Weiss, 1992). However, neural stem cells only differentiate into neurons within a permissive environment, or a neurogenic niche (see Lathia et al., 2007 for review). Neural stem cells are considered multipotent progenitor cells, in that they can give rise to several other cell types, but those types are limited in number and, in this case, limited to cells found in neural tissue such as neurons and glia (Gardner, 2002). The division of stem cells is asymmetrical in that the daughter cells produced are not identical: both a stem cell and a more fate-restricted progenitor cell are produced. The stem cells go on to again replicate while progenitors may replicate or adopt a specific cell fate (for review see Crews et al., 2003; Gardner, 2002). Initially, the full stem-ness of hippocampal progenitor cells was actively debated (Seaberg and van der Kooy, 2002). However, overwhelming evidence from new rigorous investigations supports that the progenitors in the hippocampus have stem cell properties, namely they are multipotent and self-renew (e.g. Babu et al., 2007; Suh et al., 2007). Neural stem cells differ developmentally, that is between embryonic and adult, in their stem-ness and in their responsivity to a variety of neurogenic signals (see Lathia et al., 2007; Temple, 2001 for review). Although, the dentate gyrus subgranular zone consists of different types of stem and progenitor cells that react distinctly to different physiological stimuli (Kempermann et al., 2004a; Kronenberg et al., 2003), it is not known if any of these developmental differences extend to adolescents versus adults. The use of multiple markers and confocal microscopy is just beginning to examine whether there are developmental differences in stem cells or subtypes of progenitor cells in rodent studies in vivo (Hattiangady and Shetty, 2008).
Although identifying neural stem cells in vivo remains challenging, proliferating cells are easily identified by a variety of available markers. Cell proliferation, or dividing cells, can be visualized experimentally through BrdU labeling as briefly mentioned above. BrdU, a thymidine analog with a half life of 30 minutes (Packard et al., 1973), can be administered orally or through systemic injection, with injection being the most common (Taupin, 2007). Because BrdU is a thymidine analog, it is incorporated into cells in place of the thymine base pair as cells undergo DNA replication during the S phase of the mitotic cell cycle (see Figure 1). Thus any cell that is in S-phase in the presence of BrdU will incorporate it into the DNA. Dividing cells are essentially birthdated by this process. BrdU can then be visualized via immunohistochemistry on brain tissue sections. BrdU, however, does not indicate the phenotype of the dividing cell. The phenotype must be identified through a combination of cell type-specific (neuron-specific or glia-specific) plus BrdU in multiple fluorescence immunohistochemistry. Unfortunately, the dearth of specific neural stem cell markers has forced the field to rely upon location of cell cycle proteins or BrdU to examine neural stem cell proliferation, which has made it difficult to definitively state that agents that affect cell proliferation in the subgranular zone are truly affecting neural stem cell proliferation. Novel approaches are needed (e.g. recently-developed transgenic tools) to help elucidate both developmental differences in the stem cell population as well as how drugs or other agents affect stem cells and stem cell proliferation (Johnson et al., 2009).
Figure 1.
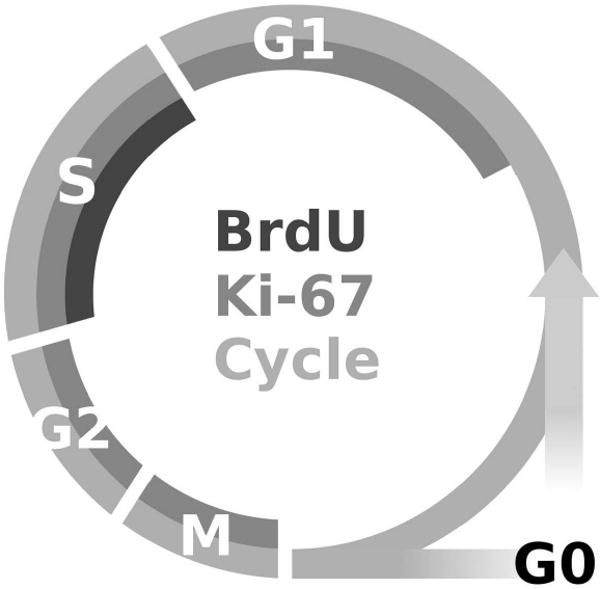
This figure is a cartoon representation of when two of the most commonly used cell cycle markers, Bromo-deoxy-Uridine (BrdU) and Ki-67, are observed with regard to the different stages of the cell cycle. Ki-67 is an endogenous marker of proliferation that is expressed throughout the active phases of cell division, specifically G1, S, G2 and M phases (Scholzen and Gerdes, 2000). BrdU, however, is only incorporated during synthesis phase (S-phase) of the cycle and therefore indicates that a cell is either in or has passed through the S-phase. Morris et al., 2009b proposes that in an adolescent model of an alcohol use disorder, that alcohol is arresting cells in the G1 phase of the cycle. A cell arrested at G1, would therefore be positive for Ki-67 but negative for BrdU, which would explain why a decrease in BrdU-positive cells, but no change in Ki-67 positive cells, were observed during alcohol intoxication. Notably, this difference in expression for BrdU versus Ki-67 is not observed in adult models. This difference may suggest a different mechanism of alcohol inhibition of proliferation in adults versus adolescents.
Cell proliferation, as measured by [3H]thymidine incorporation or its more commonly used analog, BrdU, decreases throughout postnatal development (see Figure 2; Altman and Das, 1965, 1966; He and Crews, 2007). Decreases in cell proliferation could be due to a lengthened or arrested cell cycle, more cells in quiescence, or fewer neural stem cells. Several independent observations support the latter. First, cell proliferation, measured by Ki-67 labeling, an endogenous cell cycle protein expressed in actively dividing cells (Scholzen and Gerdes, 2000), is decreased by 35% and 83% in young adult and aged rat dentate gyrus compared to adolescent rat dentate gyrus, respectively (Cowen et al., 2008; see also Figure 2). And, a recent human study of a proposed biomarker of neural stem cells suggested that neural stem cells decreased from at least preadolescence (aged 8-10) to mid adulthood (aged 30-35) in the hippocampus (Manganas et al., 2007). Although recent evidence suggests that cells may retain the ability to proliferate and differentiate into functional neurons, there appears to be a decrease in the number of neural stem cells in the aging brain (Ahlenius et al., 2009; Olariu et al., 2007). Furthermore, despite developmental changes in cell cycle kinetics between embryonic neurogenesis (cell cycle is ∼16 hours) and adult neurogenesis (cell cycle is ∼24 hours), the length of cell cycle does not change significantly from young adulthood to late adulthood (Lewis, 1978; Nowakowski et al., 1989; Olariu et al., 2007). In 9-week-old rats specifically, the length of the cell cycle is around 25 hours, which is similar to that reported for adults (Cameron and McKay, 2001). In sum, developmental differences in neural stem cell proliferation appear to be due to declining numbers of neural stem cells across the lifespan, but experiments with more specific stem cell markers or new approaches altogether are needed to best address this point.
Figure 2.
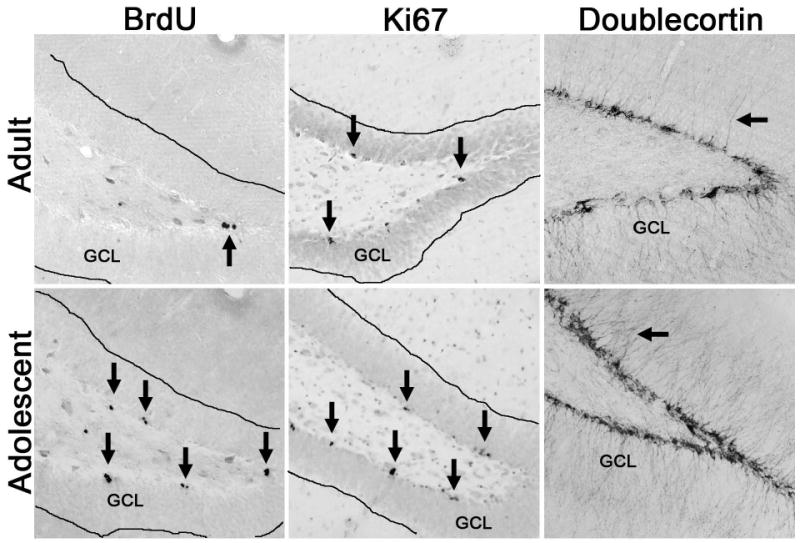
Representative photomicrographs of various neurogenesis-related markers (via immunohistochemistry) are shown and contrasted for adolescent versus adult rats. All images above are taken at the intersection of the superior and inferior blades of the dentate gyrus of the hippocampus. The images highlight that proliferation markers, BrdU and Ki-67, are increased in the adolescent brain compared the adult, which supports the higher rates of neural stem cell proliferation observed in the adolescent (He and Crews, 2007). The arrows in the BrdU and Ki-67 panels point towards clusters of dividing cells. In the doublecortin images, a thinner band of doublecortin-positive cell bodies is easily visualized in the adult panel. Further, the density immature granule cell neurons is also evident by more dendrites (arrows) projecting through the granule cell layer in the adolescent versus a single dendrite in the adult.
Differentiation and Migration
Soon after the neural stem cell divides to form the daughter progenitor, a fate choice is made as to whether the cell becomes a neuron or glia. The cell quickly begins expressing cell-type specific markers as it changes both morphologically and functionally into a neuron or glia. Immature neuron proteins such as β-Tubulin III or TUJ-1 or immature glia markers such as S100β are expressed within hours in a newly dividing cell. Neurons will progressively express neuron-specific markers such as polysialylated neural cell adhesion molecule (PSA-NCAM) and doublecortin to neuronal nuclei and finally calbindin as cells become mature neurons. Although newborn hippocampal progenitors may differentiate into neurons or glia, they primarily become neurons. Studies consistently report that around 60-80% of cells labeled with BrdU at birth, go on to express neuronal proteins and have neuronal characteristics when examined four weeks later (Kempermann et al., 2003). Intriguingly, this is one area where potential developmental differences between adults and adolescents can be gleaned from the literature, although no published work directly compares these ages to date. In work from the same author and using similar methodologies, it is clear that 75-80% of newborn cells adopt a neuronal fate in adult rodents whereas over 90% of newborn cells adopt a neuronal fate in adolescents (see Crews et al., 2006c; Morris et al., 2009b; Nixon and Crews, 2004). Further, in both adolescent reports, very few new astroglia were ever observed. Although, no direct comparison is made, based on the tight error bars and similar methods used, the most plausible interpretation is that fewer cells differentiate into neurons in adults as compared to adolescents. The functional implications of this developmental difference, though very intriguing, are not immediately clear.
Once the cell has adopted a fate, or differentiated, it begins to migrate. At this time, the progenitor cell moves into the granule cell layer of the dentate gyrus, where it may be incorporated into the granule cell layer and network. Some groups suggest that the age of the cell correlates with its location in the granule cell layer, with the oldest cells having migrated into the outer third of the granule cell layer. Expression of Doublecortin or PSA-NCAM, molecules used to identify neuroblasts or immature neurons, is associated with the ability of a newborn neuron to migrate (Cayre et al., 2009). This association has led some to speculate that reduced expression could in theory impact migration (e.g. He et al., 2005), however, these markers have not been investigated for any role in migration in adolescent brain per se. Similarly, although different events or conditions may disrupt migration, and alcohol exposure during fetal development alters migration (Charness et al., 1994), no reports exist to date that compare rates of migration of new born cells from adult versus adolescent rats. Thus, this area of inquiry may be ripe for discovery in adolescent versus adult differences in neurogenesis.
Survival and integration
The final processes that contribute to neurogenesis are whether the cell becomes integrated into the dentate gyrus and survives. Half of all newborn cells born do not survive, though those cells that remain four weeks after birth are considered stably incorporated into the granule cell layer (Dayer et al., 2003; Kempermann et al., 2003). Integration begins soon after the progenitor cell differentiates: it undergoes dendritic arborization, enhancing its synaptic connections with neighboring cells and within 4-10 days of birth, axonal connections are made to the CA3 field (Markakis and Gage, 1999). At 2 weeks post division, electrophysiological properties mirror that of immature granule cells, namely, excitatory synaptic input to these newborns neurons is GABAergic (Esposito et al., 2005; Tozuka et al., 2005). As the cell matures, dendritic spines begin to form and synaptic inputs become adult-like where glutamate is excitatory and GABA becomes inhibitory (Ge et al., 2006). By seven weeks of age, the electrophysiological properties of the newly generated neurons are almost indistinguishable from those of the mature neurons, which indicates that they are fully integrated into the hippocampal circuitry (van Praag et al., 2002).
Although cell survival machinery may be affected along development, age-related reductions in cell proliferation appear to drive the overall decrease in the production of new neurons (McDonald and Wojtowicz, 2005). For example, cell survival determined at 25 days following BrdU injection was decreased by 43% in adult rats and 86 - 92% in aged rats versus adolescents (Cowen et al., 2008; McDonald and Wojtowicz, 2005). However, if fewer cells were incorporating BrdU during proliferation, one would expect fewer cells to be visualized 25 days later. The mechanism of the age-related decrease in neurogenesis is an area of active investigation in the aging literature, though little exists on the opposite end of the spectrum comparing adolescents to adults.
Functional relevance of adult neurogenesis
Although it is now well accepted that adult born neurons are continuously integrated as granule cells of the dentate gyrus (Markakis and Gage, 1999; van Praag et al., 2002), the functional relevance of this phenomenon is less clear. Early studies underestimated the rate of adult neurogenesis, which contributed to a lack of appreciation for its potential significance to brain function (Gould, 2007). Cameron and McKay's (2001) more thorough estimation of rate coupled with the discovery of this process in humans has fueled the idea that such a metabolically costly process must be functionally significant in the adult brain (Eriksson et al., 1998; reviewed in Gould, 2007; Kempermann, 2008). The role of adult neurogenesis is strongly suggested by the numerous studies where aspects of neurogenesis are impaired by experimental manipulations, drugs or events/environments that also result in altered hippocampal integrity and deficits in hippocampal behaviors (Eisch, 2002; Santarelli et al., 2003; Saxe et al., 2006; Shors et al., 2001; Shors et al., 2002; Snyder et al., 2005). Further, strong evidence for a positive correlation between the number of new neurons and learning/memory performance have been observed across differences in rodent strains, environmental enrichment, hormonal manipulations, and age (Kempermann et al., 1997; Kuhn et al., 1996; Tanapat et al., 1999). Studies which attempt to selectively ablate adult neurogenesis, such as anti-mitotic drug administration, have provided the strongest evidence that neurogenesis is necessary for normal learning to occur (Shors et al., 2001). Unfortunately, much of this past work has been criticized for being correlative and many of these ablation strategies have other detrimental effects. Although a large body of lesion studies have shown that affecting the structural integrity of the dentate gyrus can profoundly alter hippocampal functions such as learning and memory (reviewed in Jarrard, 1993), not all hippocampal-dependent learning is impaired by reduced neurogenesis (e.g. Saxe et al., 2006; reviewed in Leuner et al., 2006; Zhao et al., 2008). Furthermore, many of these neurogenesis-promoting events impact other aspects of hippocampal structure such as synapse number and dendritic complexity. New discoveries have attempted to overcome these criticisms and have more directly shown that adult neurogenesis plays a significant role in hippocampal structure and function (e.g. Imayoshi et al., 2008). Recent theoretical papers that incorporate computational approaches corroborate in vivo studies that neurogenesis contributes to the hippocampus-dependent function of learning and memory (Aimone et al., 2009; see also Zhao et al., 2008 for review). Constant cell turnover in the dentate gyrus via neurogenesis may be necessary for recall fidelity of new memories (Deissertoth et al., 2004 and Chambers et al., 2004). Or, immature neurons may have their own role, specifically in encoding temporal associations (Aimone et al., 2006; 2009). Therefore, evidence continues to support at least a relationship between this phenomenon and learning, although these recent studies do not support a simple correlation between new neuron number and hippocampal learning (Leuner et al., 2006; Zhao et al. 2008). New tools and strategies to manipulate neurogenesis are forthcoming and should better elucidate this relationship and hone our understanding of adult neurogenesis in hippocampal function.
Another link between adult neurogenesis and hippocampal function is in the emerging role of new neurons in the pathophysiology of anxiety (Revest et al., 2009) and depression (Santarelli et al., 2003). Transgenic animals that specifically have impaired adult hippocampal neurogenesis demonstrate a variety of anxiety behaviors (Revest et al., 2009). Whereas, antidepressant drugs have been shown consistently to stimulate adult hippocampal neurogenesis (Malberg et al., 2000), neurogenesis appears to be required for the behavioral effects of antidepressants (Santarelli, et al., 2003; for review see Sahay and Hen, 2007). Intriguingly, depression and anxiety are associated with AUDs. Over half of adolescents with an AUD had a comorbid affective disorder (Bukstein et al., 1992). As well, early onset of an AUD is associated with more severe alcohol problems and young alcoholics are more likely to develop comorbid psychiatric disorder (Brown et al., 2008). This link is further supported by recent work in rodents showing that antidepressant treatment can prevent both alcohol-induced depression behavior and decreases in hippocampal neurogenesis (Stevenson et al., 2009). This paper furthered the notion that hippocampal neurogenesis may play a role in AUDs and especially that neural stem cells may contribute to addiction (for review see Canales, 2007; Eisch, 2002).
There are several perspectives from which neural stem cells and neurogenesis may contribute to addiction. First is the theory of addiction as a maladaptive form of learning and that adolescents may be particularly susceptible to experience-dependent learning processes (Carpenter-Hyland and Chandler, 2007). Indeed, integration of newborn neurons is experience-dependent and this process occurs in regions that are targets of alcohol neurotoxicity and are critical to learning and memory processes (Canales, 2007; Nixon, 2006). The second perspective relates to the self-medication hypothesis of abuse and addiction and that dysregulation of neural stem cells is correlated with psychiatric disorders (Eisch, 2002). Many groups suggest that people seek alcohol and other drugs of abuse as a way of alleviating anxiety or depression (Markou et al., 1998). A pre-existing condition of reduced neurogenesis that predisposes one to drug seeking or addiction coupled with excessive intake that further reduces this process potentially worsens hippocampal integrity (Canales, 2007). Impairment of hippocampal integrity, whether primary or secondary to excessive alcohol intake, is consistent with the downward spiral hypothesis of alcohol-induced brain damage contributing to addiction (Crews, 1999).
Alcohol and adult neurogenesis
Sites of neural stem cells and constitutive neurogenesis are also areas of the brain with the most consistent evidence of alcohol-induced dysfunction or damage, namely the frontal cortical regions and the hippocampus. As the hippocampus is a target of alcohol's neurodegenerative effects, and many now consider alterations in neural stem cells and neurogenesis as a mechanism that affects hippocampal integrity, it is not surprising that the majority of animal model studies have shown that alcohol intoxication inhibits adult neurogenesis (reviewed in Nixon, 2006). The discovery that alcohol reduces adult neurogenesis was expected thanks to a long history of work on alcohol and fetal neurogenesis within the Fetal Alcohol Spectrum Disorders literature (Crews et al., 2003; Luo and Miller, 1998). Many similarities are apparent across development for the effect of alcohol on neurogenesis, most especially that alcohol's effects on adult neurogenesis also varies due to the dose, duration and pattern of exposure, as well as the region examined. For example, acute doses of alcohol dose-dependently reduce cell proliferation in the subgranular zone of the dentate gyrus (Crews et al., 2006c; Jang et al., 2002) whereas high, chronic exposures reduce both cell proliferation and impair new cell survival (He et al., 2005; Nixon and Crews, 2002; Richardson et al., 2009). Some argue that chronic exposures do not consistently affect cell proliferation (e.g. Aberg et al., 2005; Herrera et al., 2003; Rice et al., 2004), however differences in blood alcohol concentrations, timing of investigations and interpretation of proliferation markers used may explain these differences (see comment on markers below). Further, after alcohol dependence and in abstinence, reactive increases in cell proliferation have been observed in adult rats (Nixon and Crews, 2004), an effect that is consistent with that reported for other models of brain injury such as stroke (ischemia) and traumatic brain injury (Dash et al., 2001; Liu et al., 1998). Some have even suggested that low, anxiolytic levels of alcohol may appear to promote proliferation versus controls by reducing stress-induced decreases in neurogenesis (Aberg et al., 2005). Thus, the time and the blood alcohol concentration at which cell proliferation is examined is exceptionally critical to understanding the effect of alcohol on the components of neurogenesis.
Whether the effects of alcohol on adult neurogenesis in the adult brain extend to the adolescent has recently been examined, but only in a few studies. According to multiple markers of neurogenesis, a similar decrease in neurogenesis, around 50%, is observed in adolescents, despite differences in duration or dose (Crews et al., 2006c; Morris et al., 2009b). When the various components of neurogenesis are examined independently, studies have consistently shown that alcohol dose-dependently decreases cell proliferation in the subgranular zone of the dentate gyrus of adolescent rats, though dose response studies have not been conducted in adult rodents (Crews et al., 2006c; Jang et al., 2002). Across several groups, alcohol inhibits cell proliferation in intoxicated adult rats at a surprisingly consistent rate of 40-50% in those models that can directly be compared (see Table 1 in Nixon, 2006), whereas in adolescents the rate is much more variable. Alcohol-induced decreases in BrdU labeling of cell proliferation vary between 20-50% versus control (Crews et al., 2006c; Jang et al., 2002; Morris et al., 2009b). Further, alcohol administration appears to differentially affect cell cycle markers in adolescent compared to adult animals. Whereas all studies to date show significant decreases in BrdU-labeling of proliferating cells in adolescent rats following a four-day alcohol binge, alcohol did not alter the number of Ki-67-positive cells between alcohol and control adolescent rats (Morris et al., 2009b). Ki-67 is expressed in actively dividing cells from G1-phase through M-phase (Scholzen and Gerdes, 2000; see Figure 1). This lack of alcohol effect on Ki-67-positive cells is in direct contrast to consistently reported alcohol-induced decreases in Ki-67 in adult rats, even with the same exposure model (Crews et al., 2006a; Leasure and Nixon, 2009). By examining the details of when the various cell cycle markers are expressed (see Figure 1), the lack of effect in Ki-67-positive cells coupled with a significant decrease in BrdU-labeling of cells in S-phase suggests that alcohol inhibits cell proliferation by altering or arresting the cell cycle in adolescents. Specifically, alcohol may arrest the cells in the G1 phase of the cell cycle, preventing cells from entering S-phase (Morris et al., 2009b). Alcohol arresting cells in G1 is consistent with alcohol's effect on the cell cycle in fetal neurogenesis (Li et al., 2001; Luo and Miller, 1998; Luo et al., 1999). Perhaps for neurogenesis, the adolescent brain is more similar to the developing brain than the adult; though much more work is needed to adequately address this question.
Similar to adult studies, acute doses of alcohol (5g/kg) inhibit cell proliferation without statistically impacting new cell survival (Nixon and Crews, 2002; Crews et al., 2006c). However, longer exposures, such as a four-day binge model, result in two effects: both reduced cell proliferation and impaired survival (Morris et al., 2009b; Nixon and Crews, 2002). Recent work has shown that reduced survival may be due to cell death because four-day binge exposure increased several cell death markers along the subgranular zone of the dentate gyrus (Morris et al., 2009b). In regard to cell differentiation, the majority of cells became neurons regardless of alcohol exposure, in both adult and adolescent models (He et al., 2005; Morris et al., 2009b). However, as mentioned previously, newborn cells differentiate into neurons in a slightly higher rate in adolescents (∼90%) than in adults (∼75%). This difference also reflects a lower rate of cells becoming glia in adolescents. At this time, it is not clear how this variation in differentiation alters hippocampal structure, function or even alcohol sensitivity in the adolescent rat. This difference is very exciting considering the recent attention on the role of glia in the AUDs.
Alcohol and neurogenesis: Relevance to neuropathology
A growing body of evidence indicates that impairing any of the processes in adult neurogenesis results in a reduction in the number of cells in the dentate gyrus. Though some struggle with the semantics of defining neurodegeneration to include the lack of cell generation, a recent study has clearly shown that inhibiting neurogenesis results in a dentate gyrus that is smaller, or has degenerated, compared to unaltered controls (Imayoshi et al., 2008). Others have long declared that the lack of neuron generation or regeneration is a form of neurodegeneration (Armstrong and Barker, 2001). Thus, impaired neurogenesis by events or agents such as excessive alcohol intake must be considered as a mechanism of neurodegeneration.
The consistent finding that alcohol reduces neurogenesis at a rate of inhibition that mirrors alcohol-induced cell loss in the dentate gyrus leads to the suggestion that hippocampal integrity is altered by alcohol's effect on multiple aspects of neurogenesis (Nixon and Crews, 2002; Walker et al., 1980). In both adolescent and adult rodent models, alcohol has been shown to significantly increase cell death in the dentate gyrus, but not to a large extent (Crews et al., 2000; Morris et al., 2009a; Obernier et al., 2002; Richardson et al., 2009). Cell death rates are estimated in the hundreds, whereas the rate of inhibition of neurogenesis estimates that thousands are cells are not produced. Indeed, these data strongly suggest that alcohol-inhibition of neurogenesis may be the primary mechanism by which the dentate gyrus degenerates, which resolves the historical conundrum of how the hippocampus degenerates without remarkable, observable cell death. Impaired neurogenesis as a mechanism of neurodegeneration is not mutually exclusive of the contribution of other neurodegenerative mechanisms (Armstrong an Barker, 2001). For instance, those cells that are born during intoxication have blunted dendritic trees (He et al., 2005), which could contribute to the loss of volume observed in AUDs (De Bellis et al., 2000; Sullivan et al., 1995). Retraction of processes or shrinking nuclei might also contribute to the loss of brain mass, but these events have not been investigated in adolescent models of AUDs (Ibanez et al., 1995).
Considering that the adolescent hippocampus is particularly sensitive to the damaging effects of alcohol when compared to the adult, one might question whether neural stem cells or neurogenesis could contribute to this enhanced sensitivity to damage. Though some groups have suggested as much for the frontal subventricular zone stem cells (Crews et al., 2006c), so few studies have been performed in adolescents that it is honestly difficult to answer this question. For example, only one timepoint has been investigated in a model of an AUD, but at that timepoint (during intoxication) percent-inhibition of cell proliferation is similar between adults and adolescents as is the percent of inhibition of neurogenesis (He et al., 2005; Morris et al., 2009b; Nixon and Crews, 2002). One could speculate that even though the rate of inhibition is similar, because adolescents produce more new neurons compared to adults (He and Crews, 2007), a larger number of cells would be “lost” or not born in the adolescent (e.g. 20% inhibition of 6,000 new cells a day in an adolescent is 1200 lost cells versus only 600 in a young adult that produces 3,000 cells a day). Perhaps total neuron loss contributes to the vulnerability of the hippocampus in the adolescent. It must be noted that there are no published reports to date regarding neurogenesis or the neurogenic niche after alcohol dependence in the adolescent. Although reactive neurogenesis has been observed in the adult binge model and this effect has been suggested to underlie hippocampal recovery in abstinence (Nixon and Crews, 2004), some models have shown permanent depletion of stem cells after prolonged dependence (Hansson et al., submitted; Richardson et al., 2009). Whether similar events occur in adolescents has not yet been described. Further, whether a sudden increase in the number of new neurons is a beneficial process in recovery or impedes hippocampal function is not known for either age (see Kempermann, 2004a for discussion). Studies with the immature neuron marker doublecortin suggest that neurogenesis returns to control levels in abstinence in adolescents (Morris et al., 2009b), but whether these cells become permanently incorporated into the granule cell layer or even function appropriately is not known. Considering that other measures of hippocampal integrity show long-term impairments in the adolescent (Evrard et al., 2006; Hargreaves et al., 2009), future studies need to investigate the long-term effects of alcohol on newborn neurons and the neurogenic niche.
Alcohol and neurogenesis: developmental differences in the neurogenic niche
Although neurogenesis differs across development, especially with regard to the potentiality and quantity of stem cells (Temple, 2001), the cell intrinsic signaling machinery required to produce a new neuron seems quite similar across ages. For example, the duration and timing of the cell cycle is similar across postnatal development to aging for adult neurogenesis (Olariu et al., 2007). However, a host of developmental differences in the environment that surrounds these neurogenic regions, or neurogenic niche, is just beginning to emerge (Lathia et al., 2007). Thus, it is really from the perspective of environmental regulation of neurogenesis process, meaning factors that make up and/or influence the neurogenic niche, where developmental differences and/or different vulnerabilities are predicted to exist. The remainder of this review will highlight known differences between adult and adolescent brain in their respective responses to alcohol and glean potential differences from developmental studies in areas relevant to alcohol effects on neurogenesis. Specifically, neurotransmitters, stress, inflammation and neurotrophic factors, all of which regulate neurogenesis, may differentially affect the components of adult neurogenesis in adolescents versus adults.
Neurotransmitter Influences
The influence of neurotransmitters on adult neurogenesis and the possible roles of neurotransmitters in the mechanism of alcohol-induced effects on adult neurogenesis have been reviewed previously (Cameron et al., 1998; Carpenter-Hyland and Chandler, 2007; Crews and Nixon, 2003). However, developmental differences in neurotransmission or alcohol-induced effects on neurotransmission have not been examined within the context of regulating adult neurogenesis. It is well established that adolescents have distinct pharmacological responses to the effects of alcohol as compared to the adult, effects that are suggested to underlie a unique sensitivity to alcohol within neurotransmitter systems. Specifically, a major factor that could lead to a differential effect of alcohol on adult neurogenesis in the adolescent compared to the adult is the influence of the neurotransmitters glutamate and gamma-amminobutryic acid (GABA). A large body of work has shown that both glutamatergic and GABAergic signaling are modulated by alcohol and that effects vary with age (Fadda and Rossetti, 1998; White and Swartzwelder, 2004).
Glutamate, the major excitatory neurotransmitter in the CNS, acts on both ion channel-coupled and G-protein coupled receptors. One of these ion channel-coupled receptors, the N-methyl-D-aspartate (NMDA) receptor is known to be responsible for multiple processes in the CNS including the proposed molecular mechanism of learning and memory, long-term potentiation (LTP). The NMDA receptor is a heterotetramer composed of three classes of subunits (obligatory NR1, plus NR2 and NR3). The composition of these receptors varies throughout the brain and also varies throughout brain development (Monyer et al., 1994; Pujic, 1993). Though it was originally thought that adult levels of expression and conformation were reached by adolescence, recent reports detail changes in NR2 subunit expression in the early adolescent period (Yoshimura et al., 2003) and that NR1 expression peaks in human adolescents (Henson et al., 2008). We note that this peak in NR1 expression correlates to a finding that adolescents have a greater sensitivity to alcohol inhibition of NMDA-related functions such as LTP (Pyapali et al., 1999). Although this greater sensitivity in adolescence was presumed related to developmental changes in NR2 subunit expression, this recent work suggests that unique subunit configurations may occur in adolescence to confer these developmental sensitivities (Henson et al., 2008). And, although the greater sensitivity of the adolescent to alcohol inhibition of NMDA-receptor mediated neurotransmission is thought to underlie the adolescent's greater memory impairment during alcohol intoxication (Pyapali et al., 1999; White and Swartzwelder, 2004), greater sensitivity to alcohol effects on the NMDA receptor could impact the receptor's other roles. For example, NMDA receptors have not been found on neural progenitors, but do emerge as the newborn neurons mature and integration of newborn neurons is NMDA receptor activity-dependent (Esposito et al., 2005; Tozuka et al., 2005; for review see Overstreet-Wadiche and Westbrook, 2006). Further, granule cell development in adult neurogenesis recapitulates that of ontogeny (Esposito et al., 2005). Therefore, one might speculate that any effects of alcohol exposure on the NMDA receptor during development may apply (e.g. Lee et al., 1994; Nixon et al., 2002; Nixon et al., 2004). Pharmacological antagonism of the NMDA receptor has been shown to promote neural stem cell proliferation and neurogenesis in the dentate gyrus (Cameron et al., 1995). This fairly consistent effect of NMDA antagonism increasing neurogenesis has been puzzling for the alcohol-neurogenesis field. Alcohol inhibits the NMDA receptor, which predicts that it might result in similar increases in cell proliferation and neurogenesis as an NMDA antagonist. However, alcohol intoxication very clearly decreases cell proliferation and neurogenesis (see Nixon, 2006 for review). Although, one might speculate that the reactive bursts in cell proliferation and neurogenesis that occur in abstinence after alcohol dependence could be due to chronic alcohol inhibition of the NMDA receptor, the timeline of effect is inconsistent with a mechanism of NMDA receptor antagonism (Nixon and Crews, 2004). In summary, there are many ways in which alcohol action on the NMDA receptor may alter adult neurogenesis, however many additional studies are needed to tease apart what appears as contradictory or inconsistent effects between alcohol, neurogenesis and the NMDA receptor.
GABA, the primary inhibitory neurotransmitter, also acts on both ionotropic (GABAA) and metabotropic (GABAB) receptors. The GABAA receptor, a Cl-permeable ion channel-coupled receptor that has many possible stoichiometries due to the 19 different gene products and its pentameric structure, is also developmentally regulated. In addition to the developmental changes that this receptor undergoes, alcohol differentially modulates GABA neurotransmission in adolescents compared to adults. GABAergic function is less sensitive to alcohol-potentiation in adolescence versus adults (Li et al., 2003; Li et al., 2006). Although this effect is thought to underlie the reduced sedative and motor impairing effects of alcohol in adolescents, this decreased sensitivity may impact other systems such as neurogenesis. For example, GABAA receptors have been identified on neural progenitor cells and immature neurons, and early synaptic integration of newborn neurons depends on GABA signaling (Ge et al., 2006; Tozuka et al., 2005). In a model proposed by Ge et al. (2006), it was suggested that newborn neurons integrate into the neuronal circuitry through ambient GABA levels. It could therefore be hypothesized that alcohol-induced potentiation of the GABAA receptor might significantly impact how newborn neurons are integrated into the dentate gyrus.
Alcohol withdrawal also affects both glutamate- and GABA-mediated neurotransmission and could impact the process of neurogenesis. For example, alcohol withdrawal may include seizures (Hall and Zador, 1997) and aberrant neurogenesis may result from a seizure (Parent et al., 1997). Further, many groups have reported differences in alcohol withdrawal seizures and seizure thresholds between adults and adolescents (Acheson et al., 1999). However, this age difference can be attributed to developmental differences in alcohol pharmacokinetics (Walker and Ehlers, 2009), such that when blood alcohol concentrations are similar between adult and adolescent rats, no differences in seizure severity or behavior are observed (Morris et al., 2009a). Severity of alcohol withdrawal seizures correlates with reactive neurogenesis following binge alcohol exposure, however when seizures are eliminated with repeated diazepam administration, reactive neurogenesis is still observed (Nixon and Crews, 2004). Unfortunately, neither reactive neurogenesis nor the role of withdrawal severity in neurogenesis have been examined in adolescent models as of yet.
Stress Response
Of the many systemic functions alcohol has been shown to affect, the hypothalamopituitary-adrenal (HPA) axis is a key modulator of hormonal events, especially during adolescence. During this developmental time period, the HPA axis undergoes changes in basal activity that are affected by the consumption of alcohol (Prendergast and Little, 2007; Silveri and Spear, 2004). The production of glucocorticoid hormones is regulated by the function of the HPA axis where corticotrophin-releasing hormone, secreted by the hypothalamus, stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH) that acts on the adrenal gland. These hormones act on glucocorticoid and mineralocorticoid receptors that produce profound effects on neuronal function during periods of high corticosterone release, such as that which occurs during alcohol administration and withdrawal (Prendergast and Little, 2007). Studies have shown that glucocorticoids may play a role in alcohol dependence and that stressful events early in life may contribute to the development of dependence (Piazza and Le Moal, 1998; Prendergast and Little, 2007; Spear, 2000). Studies suggests that the adolescent HPA axis responds to stressors quite differently than that of adults, which suggests that the decreased corticosterone release in adolescence may contribute to the reinforcing effects of alcohol (Piazza et al., 1993). Although alcohol-induced increases in plasma corticosterone are lower in younger animals compared to adults, the lower levels of glucocorticoid receptors in the adolescent brain suggest that alcohol still produces a spike in corticosterone (Piazza et al., 1993; Prendergast and Little, 2007; Silveri and Spear, 2004). Spikes or extended release of corticosterone contribute to neurodegeneration; specifically, corticosterone detrimentally impacts adult neurogenesis (Cameron and Gould, 1994). These data taken together imply that a age-specific regulation of glucocorticoid signaling might result in a unique neurodegenerative response in the adolescent brain. Compound this idea with data that corticosterone inhibition of neurogenesis is mediated by NMDA-mediated neurotransmission (Cameron et al., 1995), and it suggests multiple mechanisms by which neurogenesis in the adolescent brain may be differentially affected by alcohol than in the adult.
Inflammation
Extensive research has demonstrated an intimate relationship between inflammatory responses and the regulation of neurogenesis (Whitney et al., 2009). The level of inflammation not only affects traditional neurodegenerative processes such as cell death (Block and Hong, 2005), but recent work has shown that the level of inflammation also determines its role on the components of neurogenesis. In the brain, inflammation is mediated by glia and specifically, microglia have been shown to affect the neurogenic niche and neurogenesis (Whitney et al., 2009). Specifically, phagocytic microglia characteristic of high levels of inflammation, appear to reduce neurogenesis (Monje et al., 2003) whereas low level activation of microglia promote neural stem cell proliferation and neurogenesis (Battista et al., 2006; Butovsky et al., 2006; Ziv et al., 2006). Thus, developmental differences in microglial activation, macrophage recruitment, or the balance of pro-inflammatory to anti-inflammatory cytokine release in neurodegeneration might underlie the sensitivity of the adolescent hippocampus to alcohol-induced damage. Indeed, multiple animal models of CNS injury including inflammatory challenges, hypoxia-ischemia and excitotoxicity reveal developmental differences in blood brain barrier integrity and leukocyte/macrophage recruitment in response to insult (Bolton and Perry, 1998; Campbell et al., 2007). These studies and others suggest that there is a juvenile window of susceptibility that makes this developmental period particularly vulnerable to neuroinflammation (Campbell et al., 2007). However, it is not known if this window in juveniles extends to adolescence. Regardless, the susceptibility of the adolescent brain to inflammation will impact both cell death and cell birth mechanisms in alcohol-induced neurodegeneration.
Unfortunately, alcohol neurotoxicity is only tenuously linked to microglial activation through alcohol production of oxidative stress (Bondy, 1992), excitotoxicity (Lovinger, 1993), cell death (Crews and Nixon, 2009), and/or the overlap between microglial signaling cascades and alcohol effects on cell signaling (Crews et al., 2006b; Davis and Syapin, 2005). Although many have hypothesized that microglia have a significant role in alcohol-induced brain damage, there are only limited reports of activation (Crews et al., 2006b; Nixon et al., 2008). And, there are no reports to date of phagocytic activation in vivo in either adolescents or adults. There is evidence of inflammation in both adult and adolescent models of alcohol exposure (Pascual et al., 2007; Qin et al., 2008), but the ties to microglia are indirect, which is consistent with the historical suggestion that the damage in alcoholism is “too chronic” or too low level to result in significant effects on microglia (Kalehua et al., 1992). However, if the adolescent brain has greater susceptibility to inflammation, it suggests that pro-inflammatory events, such as excessive alcohol intake (e.g. Pascual et al., 2007), may differentially impact neurogenesis events in the adolescent brain.
Neurotrophic Factors
Growth and neurotrophic factors play pivotal roles in the regulation of adult neurogenesis (Balu and Lucki, 2009; Cameron et al., 1998). As well, neurotrophic signaling has many proposed roles in the AUDs ranging from influencing alcohol preference to promoting alcohol dependence, besides its role in alcohol-induced brain damage (Pandey et al., 2005). Brain-derived neurotrophic factor (BDNF), part of the nerve growth factor family of neurotrophic factors, is commonly discussed with regard to its role in cell differentiation and survival, but it is also developmentally regulated and altered in models of AUDs (Pandey et al., 2005). Indeed, neurogenesis plays a role in alcohol-induced brain damage such that one might correlate BDNF expression in the differences in susceptibility to alcoholic neurodegeneration between adults and adolescents. However, no reports have explored this relationship and the data on BDNF levels and its receptor, TrkB, vary wildly, due to differences in alcohol exposure, methods, timing and the like (Davis, 2008). An in-depth synthesis of BDNF in alcohol-related disorders report that in general, long term alcohol exposure decreases BDNF mRNA expression, an effect that may correlate with alcohol-induced neurodegeneration (reviewed in Davis, 2008). Although BDNF-related signaling may underlie developmental (pup) brain susceptibility to alcohol neurotoxicity (Chandler and Sutton, 2005), the needed adolescent-adult comparison has not yet been made. Notably, BDNF mRNA in the dentate gyrus appears to peak during adolescence (Webster et al., 2006). Dentate gyrus BDNF mRNA, specifically, was 29% higher (though not significantly) than both neonate and adult levels whereas other regions of the hippocampus did not show developmental changes (Webster et al., 2006). This potential developmental difference in the dentate gyrus is very intriguing for adolescent versus adult alcohol comparisons, especially when combined with studies investigating the role of growth factors on rodent embryonic neural stem cells. For example, neural stem cell differentiation was significantly inhibited by alcohol concentrations as low as 20mM (∼0.09 mg%), an effect that reversed in the presence of BDNF (Tateno et al., 2004). Further as long-term alcohol exposures appear to reduce BDNF and also result in decreased newborn cell survival (e.g. Herrera et al., 2003; Nixon and Crews, 2002), it supports that the known role of cell survival-promoting effects of BDNF should be investigated in alcohol effects on newborn cell survival. However, whether BDNF expression can be linked to adolescent brain vulnerability to alcohol-induced damage is not clear.
Conclusions
In summary, neural stem cells contribute to hippocampal structure and function such that events or agents, such as excessive alcohol intake, may alter hippocampal integrity by its action on the process of adult neurogenesis. Alcohol intoxication reduces adult neurogenesis, though the effect and the mechanism of inhibition vary due to alcohol dose, pattern and duration of exposure (a point derived from the fetal alcohol spectrum disorders literature; Goodlett et al., 2005). The rate of inhibition of adult neurogenesis is similar between adults and adolescents but as adolescents produce more new neurons overall (e.g. He and Crews, 2007), alcohol results in a greater net loss in neuron number in adolescents. It is possible that this greater neuron loss could underlie the known susceptibility of the adolescent hippocampus to alcohol-induced damage. However, it is not yet known how alcohol effects the neurogenic niche or neurogenesis in the long term; thus far only a single time point, during intoxication, has been investigated in models of AUDs (e.g. Morris et al., 2009b). Several potential mechanisms by which alcohol may differentially affect the neurogenic niche in the adolescent were suggested, which highlights the need for additional research in this area. The data and discussion above contribute to a growing body of work on the role of neural stem cells and adult neurogenesis in neurodegenerative and psychiatric disease and specifically within AUDs. Specifically, any form of damage to the hippocampus, and in this case alcohol effects on neural stem cells or the neurogenic niche, may be a critical step in the downward spiral from alcohol experimentation to alcohol addiction (Crews, 1999). Hopefully, applying this newly considered mechanism of structural plasticity will reveal new mechanisms that underlie the vulnerability of the adolescent to developing an AUD and lead to new approaches to reduce and prevent the development of AUDs in adolescents.
Acknowledgments
The authors thank the funding agencies that have supported the work reviewed therein: NIAAA R21AA16307 (KN), R01AA16959 (KN), NIDA T32DA16176 (SAM; DJL), the Alcoholic Beverage Medical Research Foundation (KN) and the University of Kentucky Chandler Medical Center (KN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg E, Hofstetter CP, Olson L, Brene S. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int J Neuropsychopharmacol. 2005;8:557–567. doi: 10.1017/S1461145705005286. [DOI] [PubMed] [Google Scholar]
- Acheson SK, Richardson R, Swartzwelder HS. Developmental changes in seizure susceptibility during ethanol withdrawal. Alcohol. 1999;18:23–26. doi: 10.1016/s0741-8329(98)00063-9. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Armstrong RJ, Barker RA. Neurodegeneration: a failure of neuroregeneration? Lancet. 2001;358:1174–1176. doi: 10.1016/S0140-6736(01)06260-2. [DOI] [PubMed] [Google Scholar]
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE. 2007;2:e388. doi: 10.1371/journal.pone.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol. 1990;5:349–357. [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Perry VH. Differential blood-brain barrier breakdown and leucocyte recruitment following excitotoxic lesions in juvenile and adult rats. Exp Neurol. 1998;154:231–240. doi: 10.1006/exnr.1998.6927. [DOI] [PubMed] [Google Scholar]
- Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Bukstein OG, Glancy LJ, Kaminer Y. Patterns of affective comorbidity in a clinical population of dually diagnosed adolescent substance abusers. JAACP. 1992;31:1041–1045. doi: 10.1097/00004583-199211000-00007. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Uylings HBM, Paula-Barbosa MM. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Res. 1988;473:1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Carare-Nnadi RO, Losey PH, Anthony DC. Loss of the atypical inflammatory response in juvenile and aged rats. Neuropathol Appl Neurobiol. 2007;33:108–120. doi: 10.1111/j.1365-2990.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci. 2007;257:261–70. doi: 10.1007/s00406-007-0730-6. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharm Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Structural and functional modifications in glutamatergic synapses following prolonged ethanol exposure. Alcohol Clin Exp Res. 2006;30:368–76. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–68. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–58. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3′:5′-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcohol Clin Exp Res. 2005;29:672–682. doi: 10.1097/01.alc.0000158935.53360.5f. [DOI] [PubMed] [Google Scholar]
- Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269:9304–9309. [PubMed] [Google Scholar]
- Chipperfield H, Cool S, Bedi K, Nurcombe V. Adult CNS explants as a source of neural progenitors. Brain Res Protoc. 2005;14:146–53. doi: 10.1016/j.brainresprot.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res. 2008;1228:14–19. doi: 10.1016/j.brainres.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharm Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Reviews. 1999;5:379–394. [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006b;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim DH, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006c;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Miller MW, Ma W, Nixon K, Zawada WM, Zakhari S. Neural stem cells and alcohol. Alcohol Clin Exp Res. 2003;27:324–335. doi: 10.1097/01.ALC.0000052581.46630.C5. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health. 2003;27:197–204. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008;118:36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res Brain Res Rev. 2005;49:494–504. doi: 10.1016/j.brainresrev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: Developmental considerations in alcohol users. Alcohol Clin Exp Res. 2000;24:232–237. [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–2. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Abrous DN. Stem Cell Review Series: Role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Saint-Cyr JA, Gurevich N, Carlen PL. Ethanol-induced dendritic alterations in hippocampal granule cells. Brain Res. 1989;477:373–337. doi: 10.1016/0006-8993(89)91430-3. [DOI] [PubMed] [Google Scholar]
- Eisch AJ. Adult neurogenesis: implications for psychiatry. Prog Brain Res. 2002;138:315–342. doi: 10.1016/S0079-6123(02)38085-3. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200:438–459. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti Z. Chronic ethanol consumption: From neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Stem cells: potency, plasticity and public perception. J Anat. 2002;200:277–282. doi: 10.1046/j.1469-7580.2002.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nature Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hall W, Zador D. The alcohol withdrawal syndrome. Lancet. 1997;349:1897–1900. doi: 10.1016/S0140-6736(97)04572-8. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Persistent suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. 2009 doi: 10.1017/S1461145710000246. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves GA, Quinn H, Kashem MA, Matsumoto I, McGregor IS. Proteomic analysis demonstrates adolescent vulnerability to lasting hippocampal changes following chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:86–94. doi: 10.1111/j.1530-0277.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly J. Are we drinking our neurons away? Br Med J. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharm Biochem Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex. 2008;18:2560–2573. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. PNAS. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez J, Herrero MT, Insausti R, Belzunegui T, Tunon T, Garcia-Bragado F, Gonzalo LM. Chronic alcoholism decreases neuronal nuclear size in the human entorhinal cortex. Neurosci Lett. 1995;183:71–74. doi: 10.1016/0304-3940(94)11117-2. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Kim EH, Kim CJ. Acute alcohol intoxication decreases cell proliferation and nitric oxide synthase expression in dentate gyrus of rats. Toxicol Lett. 2002;133:255–262. doi: 10.1016/s0378-4274(02)00129-7. [DOI] [PubMed] [Google Scholar]
- Jarrard L. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–4. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2006: Volume I, Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2007. [Google Scholar]
- Kalehua AN, Streit WJ, Walker DW, Hunter BE. Chronic ethanol treatment promotes aberrant microglial morphology in area CA1 of the rat hippocampus. Alcohol Clin Exp Res. 1992;16:401. [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. TINS. 2004a;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Op Neurobiol. 2004b;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? TINS. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. PNAS. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Herper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. 1989;79:200–4. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Rao MS, Mattson MP, French-Constant C. The microenvironment of the embryonic neural stem cell: lessons from adult niches? Dev Dyn. 2007;236:3267–3282. doi: 10.1002/dvdy.21319. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. PNAS. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01105.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Spuhler-Phillips K, Randall PK, Leslie SW. Effects of prenatal ethanol exposure on N-methyl-D-aspartate-mediated calcium entry into dissociated neurons. JPET. 1994;271:1291–1298. [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. PNAS. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J Neurosci. 2006;26:13437–42. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PD. Kinetics of cell proliferation in the postnatal rat dentate gyrus. Neuropathol Appl Neurobiol. 1978;4:191–5. doi: 10.1111/j.1365-2990.1978.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Lin H, Zhu Y, Wang M, Luo J. Disruption of cell cycle kinetics and cyclin-dependent kinase system by ethanol in cultured cerebellar granule progenitors. Brain Res. 2001;132:47–58. doi: 10.1016/s0165-3806(01)00294-2. [DOI] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Madeira MD, Paula-Barbosa MM. Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiol Behav. 1999;66:337–346. doi: 10.1016/s0031-9384(98)00301-1. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Brandão F, Cadete-Leite A, Madeira MD, Paula-Barbosa MM. Synaptic reorganization in the hippocampal formation of alcohol-fed rats may compensate for functional deficits related to neuronal loss. Alcohol. 2000;20:139–48. doi: 10.1016/s0741-8329(99)00069-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Luo J, West JR, Cook RT, Pantazis NJ. Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol Clin Exp Res. 1999;23:644–656. [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2009a doi: 10.1016/j.alcohol.2009.10.017. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Smith AR, Eaves DW, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2009b doi: 10.1002/hipo.20665. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ. The thorny side of addiction: adaptive plasticity and dendritic spines. Scientific World Journal. 2007;7:9–21. doi: 10.1100/tsw.2007.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Hughes PD, Amsel A, Leslie SW. NMDA receptor subunit expression following early postnatal exposure to ethanol. Brain Res Dev Brain Res. 2002;139:295–299. doi: 10.1016/s0165-3806(02)00515-1. [DOI] [PubMed] [Google Scholar]
- Nixon K, Hughes PD, Amsel A, Leslie SW. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alcohol Clin Exp Res. 2004;28:105–112. doi: 10.1097/01.ALC.0000106311.88523.7B. [DOI] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Packard DS, Jr, Menzies RA, Skalko RG. Incorportaiton of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1:397–404. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chandler LJ, Nixon K, Crews FT, Hensler JG, Ukai W, Saito T. Neurotrophic factor signaling in alcoholism. Alcohol Clin Exp Res. 2005;29:1098–1105. [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage (NIAAA Research Monograph No 22) Rockville, MD: National Institutes of Health; 1993. pp. 173–194. [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Brandao F, Madeira MD, Cadete-Leite A. Structural changes in the hippocampal formation after long-term alcohol consumption and withdrawal in the rat. Addiction. 1993;88:237–247. doi: 10.1111/j.1360-0443.1993.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. PNAS. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. TIPS. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Powrozek TA, Sari Y, Singh RP, Zhou FC. Neurotransmitters and substances of abuse: effects on adult neurogenesis. Curr Neurovasc Res. 2004;1:251–260. doi: 10.2174/1567202043362225. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharm Biochem Behav. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Matsumoto I, Wilce PA. Expression of the gene coding for the NR1 subunit on the NMDA receptor during rat brain development. Neurosci Lett. 1993;162:10993–11006. doi: 10.1016/0304-3940(93)90561-x. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–67. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rice AC, Bullock MR, Shelton KL. Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Res. 2004;1011:94–98. doi: 10.1016/j.brainres.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JN, Walker DW. Morphological alterations in hippocampus after long-term alcohol consumption in mice. Science. 1978;201:646–648. doi: 10.1126/science.566953. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. PNAS. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004;32:145–155. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Alcohol's effects on adolescents. Alcohol Res Health. 2002;26:287–291. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol sensitivity, tolerance and intake. Alcohol Clin Exp Res. 2006;30:245A. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharm. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57:768–76. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Tateno M, Ukai W, Ozawa H, Yamamoto M, Toki S, Ikeda H, Saito T. Ethanol inhibition of neural stem cell differentiation is reduced by neurotrophic factors. Alcohol Clin Exp Res. 2004;28:134S–138S. doi: 10.1097/01.alc.0000133538.40841.36. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharm Biochem Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- White AM, Kraus CL, McCracken LA, Swartzwelder HS. Do college students drink more than they think? Use of a free-pour paradigm to determine how college students define standard drinks. Alcohol Clin Exp Res. 2003;27:1750–1756. doi: 10.1097/01.ALC.0000095866.17973.AF. [DOI] [PubMed] [Google Scholar]
- White AM, Kraus CL, Swartzwelder H. Many college freshmen drink at levels far beyond the binge threshold. Alcohol Clin Exp Res. 2006;30:1006–1010. doi: 10.1111/j.1530-0277.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, World Health Organization. Highlights: Child and Adolescent Health and Development Progress Report 2006-2007. WHO Press; 2008. p. 20. [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zou JY, Martinez DB, Neafsey EJ, Collins MA. Binge ethanol-induced brain damage in rats: Effects of inhibitors of nitric oxide synthase. Alcohol Clin Exp Res. 1996;20:1406–1411. doi: 10.1111/j.1530-0277.1996.tb01141.x. [DOI] [PubMed] [Google Scholar]


