Abstract
Prostate cancer is the most common non-cutaneous cancer in Western males. The majority of prostate cancer fatalities are caused by development of castration-resistant growth and metastatic spread of the primary tumor. The average duration of the response of primary prostate cancer to hormonal ablation is less than 3 years, and 75% of prostate cancers in the United States progress to hormone-refractory disease. The existing pharmacological therapies for metastatic and/or hormone-refractory prostate cancer do not provide significant survival benefit. This review summarizes the importance of transcription factor Stat5 signaling in the pathogenesis of prostate cancer and discusses the molecular basis why inhibition of Stat5a/b could be used as a therapeutic strategy for prostate cancer.
Keywords: Stat5a/b, prostate cancer, therapy development
1. Introduction
Organ-confined primary prostate cancer is typically treated by surgery, radiation, hormone therapy, or different combinations of these three treatment modalities, depending on the age and operability of the patient (Pestell and Nevalainen, 2008). For a significant fraction of prostate cancers, the existing therapies only provide a temporary relief of the symptoms and the cancer growth, while the hormone-refractory and/or metastatic forms of prostate cancer develop. Currently, there are no effective pharmacological therapies for castration-resistant and/or metastatic prostate cancer (Pestell and Nevalainen, 2008). Identification of the specific mechanisms underlying growth and survival of prostate cancer cells provides a rational basis for development of new therapies for organ-confined and advanced prostate cancer. Transcription factor Stat5a/b is critical for prostate cancer cell survival and for prostate tumor growth (Ahonen et al., 2003; Dagvadorj et al., 2007; Dagvadorj et al., 2008; Kazansky et al., 2003). Recent reports indicate that the Stat5a/b signaling pathway may contribute to the progression of organ-confined prostate cancer to castration-resistant and/or metastatic disease. Based on these findings, we propose that the Jak2-Stat5a/b signaling pathway provides molecular targets for development of pharmacological intervention for prostate cancer. In addition, Stat5a/b may be a prognostic marker of primary, organ-confined prostate cancers that are most likely to become aggressive disease.
2. Stat5a/b Transcription Factors in Prostate Cancer Pathogenesis
2.1. Protein structure of Stat5
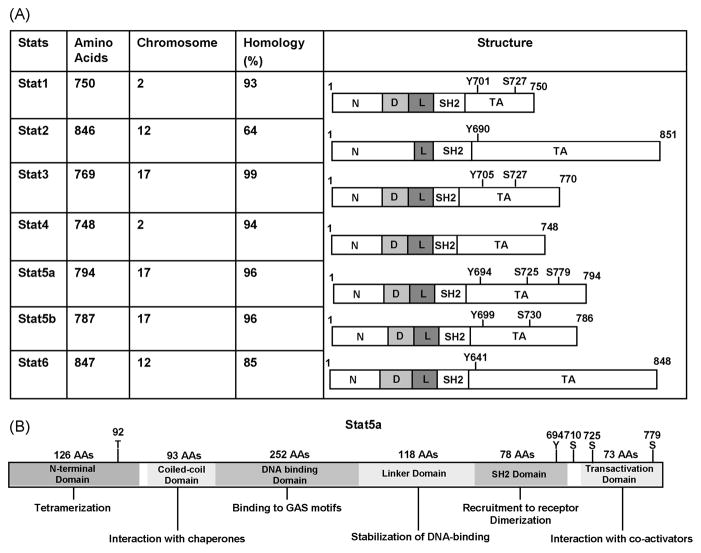
Stat5a and Stat5b belong to the Stat (Signal Transducer and Activator of Transcription) family of transcription factors which consists of seven members (Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6) (Hennighausen and Robinson, 2008; Tan and Nevalainen, 2008) (Fig. 1). Stat proteins have likely diverged from a single gene through several consecutive duplications into three separate genetic loci (Darnell et al., 1994). In humans, Stat1 and Stat4 map to chromosome 2 (bands q12 to q33) (Haddad et al., 1998; Yamamoto et al., 1997); Stat2 and Stat6 map to chromosome 12 (bands q13 to q14-1) (Goureau et al., 2001; Leek et al., 1997); and Stat3, Stat5a, and Stat5b map to chromosome 17 (bands q11-1 to q22) (Choi et al., 1996; Lin et al., 1996).
Figure 1. The members of Stat family of transcription factors.
A. Chromosomal location in human, sequence identity between human and mouse Stats and the major structural features and phosphorylation sites. B. Schematic Stat5a domains and specific amino acids mediating important functions. Glycosylation of threonine 92 (T92) is crucial for interaction with p300/CREB-binding protein. Phosphorylation of tyrosine 694 (Y694) is essential for Stat5a activation. Mutation of serine 710 to phenylalanine (S710F) confers constitutive activation to Stat5a (gain of fuction). Phosphorylation of serine 725 has an impact on signal duration.
Stat5a was originally identified as a mammary-gland specific transcription factor (MGF) that mediates the effects of prolactin (Prl) in mice (Wakao et al., 1992) and sheep (Gouilleux et al., 1994; Wakao et al., 1994). The Stat5b isoform was later discovered in the mouse mammary gland, and is encoded by a separate gene (Lin et al., 1996; Liu et al., 1996; Liu et al., 1995). While both have the structurally and functionally conserved domains of the other Stat proteins (Schindler and Darnell, 1995), Stat5a has 20 amino acids that are unique to it’s C-terminal sequence, while 8 amino acids in the C-terminus are specific to Stat5b. Both Stat5a and Stat5b are activated by Jak2 phosphorylation on a specific tyrosine residue (Liu et al., 1995). However, Stat5a transmits predominantly the signals initiated by the prolactin receptor (PrlR), while Stat5b mediates the biological effects of growth hormone (GH). In addition, Stat5a/b can also be activated by several other ligands including interleukin-2 (IL-2) (Hou et al., 1995), IL-3 (Mui et al., 1995a), IL-5 (Mui et al., 1995a), IL-7 (Foxwell et al., 1995), granulocyte-macrophage colony-stimulating factor (GM-CSF) (Mui et al., 1995b), insulin (Wartmann et al., 1996), erythropoietin (EPO) (Gouilleux et al., 1995; Wakao et al., 1994) and thrombopoietin (TPO) (Pallard et al., 1995).
Stat5a/b proteins contain six functional domains in their protein structure (Fig. 1). The N-terminal domain of Stat5a/b (NTD; aa 1-126) stabilizes interactions between two Stat dimers to form tetramers. The Stat5-DNA interaction is strengthened by tetramerization, particularly at tandemly linked non-consensus and consensus Stat5 binding sites, which augments the transcriptional activation of weak promoters (John et al., 1999; Meyer et al., 1997; Soldaini et al., 2000). Post-translational glycosylation of threonine 92 of Stat5 allows interaction with the CREB-binding protein, which is required for Stat5 – mediated gene transcription (Gewinner et al., 2004). The coiled-coil domain (CCD; aa 138-330) interacts with chaperones (Xu et al., 2004), recruits co-activators (Zhu et al., 1999) and co-repressors (Maurer et al., 2002), and facilitates multiple protein-protein interactions crucial for transcriptional regulation. The central DNA-binding domain (DBD; aa 332-583) permits binding of Stat5a/b to consensus gamma-interferon activation sequence (GAS) (TTC(C/T)N(G/A)GAA) motifs contained in the regulatory elements of target genes (Decker et al., 1991; Horvath et al., 1995; Soldaini et al., 2000).
The linker domain (LD; aa 475-592) of Stats stabilizes the DNA-binding. Specifically, site-directed mutagenesis of the Stat1 linker domain resulted in a mutant dimer that binds and dissociates from DNA more rapidly than the wild type protein, suggesting a time-dependent element in the linker domain that regulates gene transcription (Yang et al., 2002). The most highly conserved domain of Stat5a/b is the Src homology 2 domain (SH2; aa 593-670), which mediates receptor-specific recruitment and Stat dimerization (Stocklin et al., 1996) via the phosphorylated tyrosine residue of one Stat5 to the SH2 domain of another (Wakao et al., 1994). The most variable region is the transcriptional activation domain (TAD; Stat5a, aa 722-794;Stat5b, aa 727-787) in the C-terminus, which interacts with critical co-activators, such as the p300/CREB-binding protein (CBP)-associated co-activator NcoA-1 (Litterst et al., 2003), centrosomal P4.1-associated protein (CPAP) (Peng et al., 2002), P100 (Paukku et al., 2003) and Oct-1 (Magne et al., 2003).
2.2 The Canonical Prl-Jak2-Stat5a/b Signaling Pathway
The cytokine receptors that activate Stat5a/b generally lack tyrosine kinase activity. This kinase activity is provided by receptor-associated cytoplasmic Janus kinases (Jaks) (Hennighausen and Robinson, 2008). There are four members (Jak1, Jak2, Jak3 and Tyk2) in the Jak family. Jak3 is preferentially expressed in leukocytes. The other three Jak proteins (Jak1, Jak2, and Tyk2) are ubiquitously expressed in mammalian cells (Ghoreschi et al., 2009). The primary Jak protein that activates Stat5a/b is Jak2 (Gouilleux et al., 1994).
Prl binding induces a conformational change in the PrlR that brings the PrlR-associated Jak2 molecules into close proximity, allowing them to activate each other and phosphorylate specific tyrosine motifs of the PrlR (Fig. 2). Stat5a/b recognizes these phosphorylated tyrosine motifs through their SH2 domains, are recruited to the docking sites, and undergo rapid phosphorylation of a conserved tyrosine residue in the C-terminus by Jak2. The phosphorylation of tyrosine residues Y694 and Y699 activates Stat5a and Stat5b, respectively, leading them to homo- or heterodimerize through a phosphotyrosine–SH2 domain (Becker et al., 1998; Chen et al., 1998). A variety of protein kinases have been shown to phosphorylate Stats on serine residues, providing additional signaling pathways to potentiate the primary activating stimulus (Decker and Kovarik, 2000). However, the phosphorylation of the serine residues S726 of Stat5a (Yamashita et al., 2001), and S731 on Stat5b, may inhibit transcriptional activity (Yamashita et al., 1998).
Figure 2. The canonical Prolactin (Prl)-Jak2-Stat5a/b signaling pathway.
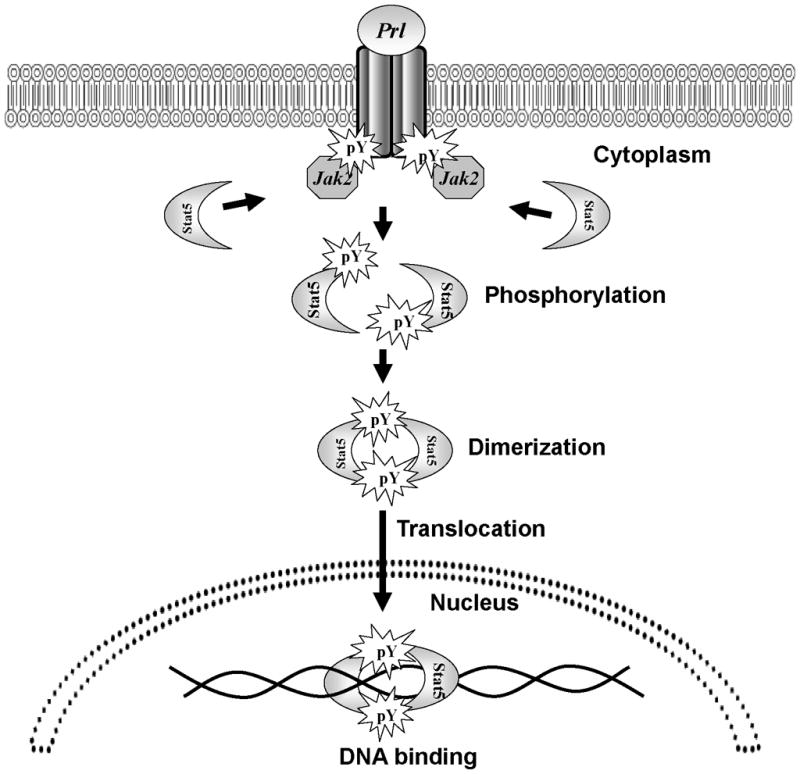
Prl binding brings the PrlR-associated Jak2 molecules into close proximity and subsequent activation. Cytoplasmic Stat5a/b proteins are recruited to the activated Prl-receptor-Jak2 complex, and Jak2 phosphorylates tyrosine residues Y694 and Y699 of Stat5a and Stat5b, respectively, leading them to homo- or heterodimerize through a phosphotyrosine SH2 domain interaction. Phosphorylated Stat5a/b dimers translocate from the cytoplasm into the nucleus, where they bind to DNA to regulate transcription.
Phosphorylated Stat5a/b dimers translocate from the cytoplasm into the nucleus, where they bind to an 8–10 base pair inverted repeat consensus sequence, TTC(C/T)N(G/A)GAA, referred to as the GAS element, and activate transcription of target genes (Horvath et al., 1995; Soldaini et al., 2000). Homodimers of Stat5a and Stat5b have equal binding specificities for palindromes that are spaced three base pairs apart (Ehret et al., 2001; Soldaini et al., 2000), while tetrameric binding occurs between tandem nonconsensus and consensus GAS motifs that are 6 base pairs apart (Soldaini et al., 2000). Therefore, the non-redundant functions of Stat5a and Stat5b during development are not a result of differences in DNA binding specificity, but could be attributed to their cell type-specific expression, or the interactions of their divergent C-terminus with different co-regulators (Ehret et al., 2001).
The exact molecular mechanisms of the nuclear import and export of Stat5a/b are still largely unclear. Nuclear and cytoplasmic pools of unphosphorylated Stat5a/b proteins may shuttle freely at high exchange rates in the absence of cytokine activation (Reich and Liu, 2006; Vinkemeier, 2004). In addition, recent reports demonstrate that the coiled coil domain is involved in constitutive nuclear import of latent and activated STAT5a (Iyer and Reich, 2008). While non-phosphorylated Stat5a/b proteins cycle between the cytoplasm and nucleus, the translocation of Stat5a/b dimers has been proposed to be an active, energy-dependent process (Reich and Liu, 2006; Vinkemeier, 2004) that utilizes components of Ran-dependent nuclear import machinery (Sekimoto et al., 1997; Sekimoto et al., 1996). MgcRacGAP, a chaperone protein that contains a nuclear localization signal, has been shown to be able to bind to the DNA-binding domain of the phosphorylated Stat5a/b dimer and, with GTP-bound Rac1, form a shuttling complex between the nucleus and cytoplasm (Kawashima, et al. 2009). The karyopherin importin-b (p97) binds to this complex, and was identified as the carrier that transports Stat5a/b into the nuclear compartment. Intriguing new evidence also has suggested that unphosphorylated Stats may be able to bind to DNA in association with other transcription factors (Chatterjee-Kishore et al., 2000; Yang et al., 2005; Yang et al., 2007).
2.3. Active Stat5a/b in clinical progression of prostate cancer
2.3.1. Stat5a/b is highly critical for the viability of human prostate cancer cells in vitro and in vivo
Stat5a/b is the key mediator of the Prl signaling in both normal and malignant prostate tissue (Ahonen et al., 2002; Li et al., 2004; Nevalainen et al., 1996; Xu et al., 2001). Autocrine Prl acts as a powerful autocrine mitogen and survival factor for prostate epithelium (Ahonen et al., 1999; Dagvadorj et al., 2007; Nevalainen et al., 1997b). The functional importance of Stat5 in prostate tissue has been determined through studies of human prostate cancer cells (in vitro and in vivo), the TRAMP mouse prostate cancer model and the phenotype analysis of the STAT5A knockout mice. Specifically, the prostate epithelium of STAT5A null mice was comprised of deformed or broken prostate acini that contained desquamated, granular epithelial cells in a dense, coagulated secretory material (Nevalainen et al., 2000). Importantly, the tissue architecture of STAT5A−/− mice did not display the morphological hallmarks of prostate epithelial hyperplasia. Rather, the prostate characteristics of STAT5A−/− mice suggested that Stat5a is involved in the maintenance of integrated prostate epithelial structures.
In human prostate cancer cells, transcription factor Stat5a/b is critical for cell viability and prostate tumor growth in vivo (Dagvadorj et al., 2008). The novel concept that Stat5a/b controls growth and survival of prostate cancer cells has been demonstrated by several different studies. First, Ahonen et al. showed that inhibition of Stat5a/b by adenoviral expression of a dominant-negative mutant of Stat5a/b (DNStat5a/b) in Stat5-positive human prostate cancer cells induced massive apoptotic death as determined by cell viability assays and biochemical analysis (Ahonen et al., 2003). Second, examination of the TRAMP mouse prostate cancer model confirmed the central role of Stat5a/b in regulating the viability of prostate cancer cells (Kazansky et al., 2003). Third, Dagvadorj et al. recently demonstrated that Stat5 inhibition, regardless of the methodological approach (Stat5a/b siRNA, antisense oligonucleotides or adenoviral expression of DNStat5a/b), results in massive cell death in a variety of Stat5-positive human prostate cancer cell lines (Dagvadorj et al., 2008). In addition, inhibition of Stat5a/b decreased both incidence and growth of human prostate subcutaneous xenograft tumors in nude mice (Dagvadorj et al., 2008). CYCLIN-D1 and BCL-xL are Stat5a/b target genes in human prostate cancer cells (Dagvadorj et al., 2008). The individual roles of Stat5a vs. Stat5b in the growth regulation of prostate cancer cells remain to be examined. Moreover, molecular mechanisms directing rapid apoptosis of prostate cancer cells as a result of Stat5a/b inhibition need to be understood since they may identify additional therapeutic target proteins for pharmaceutical intervention.
2.3.2. Active Stat5a/b as a prognostic factor for prostate cancer
Stat5a/b is persistently in the active state in human prostate cancer cells, but not in adjacent normal prostate acini (Ahonen et al., 2003). It has also been shown that activation of Stat5a/b is significantly more frequent in high grade human prostate cancer compared to intermediate or low grade prostate cancers (Li et al., 2004). The association of Stat5 activation with high histological grades of prostate cancer has been confirmed using tissue microarrays (Li et al., 2005). Furthermore, Stat5a/b is more likely to be active in the primary prostate cancer of a patient who had been treated with androgen ablation prior to radical prostatectomy vs. patients who have not received adjuvant androgen deprivation therapy (Tan et al., 2008). Moreover, active Stat5a/b in primary prostate cancer predicted early disease recurrence after initial treatment by radical prostatectomy (Li et al., 2005). Significantly, if only prostate cancers of intermediate Gleason grades were analyzed, active Stat5a/b still remained an independent prognostic marker of early recurrence of prostate cancer (Li et al., 2005). The distribution of active Stat5a vs. Stat5b in prostate cancers of different histological grades remains to be determined, as well as the individual prognostic value of Stat5a vs. Stat5b in prostate cancer.
2.3.3. Stat5a/b functionally interacts with androgen receptor in prostate cancer cells
Castration-resistant prostate cancer continues to express the androgen receptors (AR) and androgen-regulated genes, which implies that the AR signaling pathway remains active dispite low levels of circulatory androgens after androgen ablation therapy (Isaacs and Isaacs, 2004). Recently, Tan et al. demonstrated that active transcription factor Stat5a/b is expressed in 95% of castration-resistant clinical human prostate cancers (Tan et al., 2008). The study further showed that the activeated Stat5a/b signaling pathway increased the transcriptional activity of AR, and ligand-bound AR, in turn, increases transcriptional activity of Stat5a/b in prostate cancer cells (Tan et al., 2008) (Fig. 3). Furthermore, active Stat5a/b physically interacted with liganded AR, and both Stat5a/b and AR enhanced nuclear localization of each other when activated in prostate cancer cells (Tan et al., 2008). Recent findings indicate that recurrent prostate cancers develop the intracellular capacity to biosynthesize testicular androgens from adrenal androgens and cholesterol (Mohler et al., 2004; Penning and Byrns, 2009; Titus et al., 2005; Yuan and Balk, 2009). Given that Stat5a/b and AR are both anti-apoptotic and growth-promoting transcription factors in prostate cancer cells, promotion of AR transcriptional activity by Stat5a/b in the presence of low levels of androgens may therefore contribute to castration-resistant growth of prostate cancer. AR, in turn, by promoting transcriptional activity of Stat5a/b, may critically support viability of prostate cancer cells in growth conditions where prostate cancer cells would normally undergo apoptosis. Future work needs to identify the detailed molecular mechanisms underlying the co-action between Stat5a/b and AR, the effect of Stat5a/b-AR synergy on prostate cancer growth in vivo, and the Stat5 interaction with mutated AR liganded by adrenal androgens or anti-androgens.
Figure 3. Interaction of Stat5a/b and androgen receptor (AR) signaling pathways in prostate cancer cells.
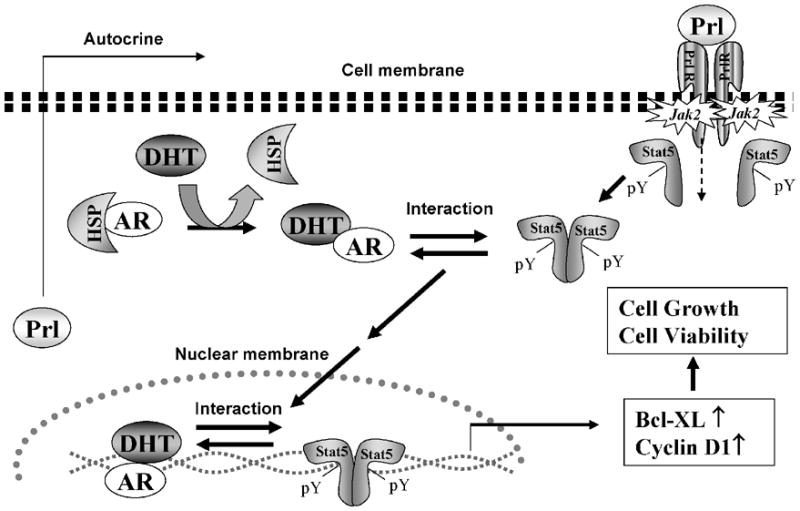
Binding of Prl leads to Prl-receptor (PrlR) dimerization and activation of Jak2 proteins pre-associated with the cytoplasmic domains of PrlR. Cytoplasmic Stat5a/b are recruited to the activated PrlR-Jak2 complex, Stat5a/b is phosphorylated on a conserved tyrosine residue in the C-terminus of Stat5a/b by Jak2 resulting in Stat5 dimerization. Ligand binding to the AR leads to its dissociation from the heat shock proteins (HSPs) and dimerization. Liganded AR physically interacts with Stat5a/b in prostate cancer cells. Liganded AR and active Stat5a/b promote nuclear translocation and transcriptional activity of each other. Both AR and Stat5 signaling pathways are critical regulators of growth, viability and apoptosis of prostate cancer cells.
2.3.4. Positive regulators of Stat5a/b in prostate cancer
The molecular mechanisms underlying high-level expression of active Stat5a/b in primary and castration resistant human prostate cancer are largely unclear. One mechanism may be autocrine Prl in prostate cancer cells responsible for the constitutive activation of Stat5a/b in human prostate cancer (Li et al., 2004; Nevalainen et al., 1997a; Nevalainen et al., 1997b). Prl is produced locally by normal prostate epithelium and prostate cancer, and is known to promote proliferation and survival of prostate cells (Ahonen et al., 1999; Dagvadorj et al., 2007; Kindblom et al., 2002; Kindblom et al., 2003; Nevalainen et al., 1997a; Nevalainen et al., 1996; Nevalainen et al., 1997b; Nevalainen et al., 1991; Wennbo et al., 1997). Prl is one of the predominant cytokines known to activate Jak2-Stat5a/b in normal and malignant prostate epithelium (Ahonen et al., 2002; Dagvadorj et al., 2007; Li et al., 2004), and Prl protein and PrlR expression are associated with high histological grades of human prostate cancer (Li et al., 2004). Activating mutations of Jak2 have been recently described in hematopoietic malignancies resulting in constitutive activation of Stat5 (Baxter et al., 2005). Since Jak2 is the predominant kinase that activates Stat5a/b (Li et al., 2004), such somatic gain-of-function Jak2-mutations may also occur in advanced prostate cancer. A third potential mechanism for the high abundance of Stat5a/b in prostate cancer is amplification of Stat5a/b genes. Importantly, the STAT5A/B genes are located on chromosome 17 (Clark et al., 2003), which is frequently altered in both incidental and hereditary prostate cancer (Gillanders et al., 2004). Stat5a/b might also be activated by tyrosine kinases such as Src (Yu and Jove, 2004) or fusion proteins containing tyrosine kinase activity such as Bcr-Abl (de Groot et al., 1999) or Tel-Jak (Schwaller et al., 2000). Although GH is a principal activator of Stat5b in a number of tissues (Halmos et al., 2002; Letsch et al., 2003; Wang et al., 2005; Weiss-Messer et al., 2004), there is currently no evidence to support of GH-Stat5-mediated stimulation of prostate cancer cell growth. Alternatively, reduced expression of Stat5a/b phosphatases or inhibitory proteins of Stat5a/b, such as PIAS, may result in active Stat5a/b in malignant prostate epithelium.
2.3.5. Negative Regulators of Stat5 Signaling in Prostate Cancer
Several different molecular mechanisms regulate the duration and magnitude of Stat5a/b activation in the cytoplasmic and nuclear compartments. These mechanisms involve: (1) protein inhibitors of activated Stat proteins (PIAS); (2) cytoplasmic and nuclear protein tyrosine phosphatases (PTP); and (3) suppressors of cytokine signaling (SOCS) proteins.
The PIAS family of proteins are localized within the nucleus and function as constitutive repressors of STAT activity by direct association (Schmidt and Muller, 2003; Shuai, 2006). The PIAS family members include PIAS1, PIAS3, PIASx, PIASy, and alternative splicing variants of PIASx (Palvimo, 2007). While PIASy acts as a co-repressor of AR (Gross et al., 2001; Junicho et al., 2000), other members PIAS1, PIAS3 (Wang and Banerjee, 2004) and PIAS-like proteins Zimp7 (Huang et al., 2005) and Zimp10 (Sharma et al., 2003) have been shown to function as co-activators of AR mediated transcription in human prostate epithelial cells. PIAS3 is the only member of the PIAS family that has been shown to directly interact with Stat5a/b and repress Stat5-mediated transcription in CHO and lymphoid NB2 cells (Rycyzyn and Clevenger, 2002). PIAS3 is expressed in prostate cancer tissues and cell lines (Wang and Banerjee, 2004), and PIAS1 expression has been shown to be 33% higher in primary prostate cancers compared to normal prostates, but this overexpression did not correlate with the Gleason score as determined by in situ hybridization of PIAS1 mRNA (Li et al., 2002). Furthermore, PIAS1 expression has been shown to be significantly lower in hormone-refractory prostate cancer than in untreated prostate tumors (Linja et al., 2004). However, interaction and the effects of PIAS1 on Stat5a/b activity in prostate cancer cells remain unclear. It will be important to determine the interaction of PIAS proteins with Stat5a/b and Stat5a/b-regulated gene transcription in prostate cancer cells to understand how PIAS protein expression contributes to prostate cancer progression.
PTPs are enzymes that regulate the dephosphorylation event of the Jak/Stat5 signaling components (Xu and Qu, 2008). One of the PTPs member, SHP-2 which contains two Src homology 2 (SH2) domains, directly interacts with Stats in the cytoplasm and translocates into the nucleus as a complex (Chen et al., 2003; Chughtai et al., 2002). Cytosolic PTP1B (Aoki and Matsuda, 2000) and the nuclear phosphatase TC-PTP (Aoki and Matsuda, 2002) are also known to dephosphorylate and inactivate Stat5a/b in mammary epithelial cells. However, to date there is no direct evidence of PTP regulation of Stat5a/b activity in prostate cancer cells.
A third mechanism for down-regulation of Stat5a/b signaling involves SOCS proteins family which comprises of eight members, including CIS (cytokine-inducible SH2 domain protein) and SOCS1–7 (Croker et al., 2008). SOCS proteins are rapidly induced by activated Stats and act to block the cytokine signaling by direct inhibition of the upstream activator Jaks and/or by competitive binding to tyrosine phosphorylated receptors (Alexander and Hilton, 2004). The expression levels of SOCS1, SOCS3, SOCS5, and CIS genes in PC-3 and DU145 human prostate cancer cells are significantly lower than in the normal RWPE-1 prostate cells (Evans et al., 2007). However, the expression of SOCS1, SOCS3, SOCS5 and CIS genes in LNCaP cells were at levels comparable to RWPE-1 cells indicating that SOCS proteins are not silenced in all human prostate cancer cell lines (Evans et al., 2007). Future studies need to evaluate whether SOCS proteins directly regulate the Jak2-Stat5a/b pathway in human prostate cancer cells in order to contribute to constitutive activation of Stat5a/b in clinical prostate cancer.
3. Therapeutic targeting of Stat5a/b in prostate cancer
Currently, there are no effective pharmacological therapies for castration-resistant and/or disseminated prostate cancer. We propose that Jak2-Stat5a/b signaling pathway provides several molecular targets for therapy development for prostate cancer. Stat5a/b activity and function in prostate cancer cells can be disrupted at multiple levels. First, activation of the PrlR can be blocked by specific PrlR antagonists, such as G129R-Prl (Llovera et al., 2000). The human Prl antagonist (Delta1-9G129R-hPrl) has been demonstrated to inhibit the Jak2/Stat5a/b signaling pathway by blocking the autocrine Prl activation of PrlR in prostate cancer (Dagvadorj et al., 2007). Second, small-molecule inhibitors for Jak2 are currently in active development (Gozgit et al., 2008) and may provide an effective therapeutic tool for those prostate cancers in which Jak2 is the primary kinase responsible for Stat5a/b activation. Third, direct blocking of the SH2 domain of Stat5a/b would theoretically result in the specific inhibition of Stat5a/b recruitment to an activated receptor (such as PrlR), in addition to inhibiting Stat5a/b phosphorylation, dimerization, nuclear translocation and DNA binding. Although SH2 domain binding phosphotyrosyl peptides or peptidomimetics have been developed to selectively inhibit Stat3 dimerization (Turkson et al., 2004; Turkson et al., 2001), no SH2-domain targeting compounds have been reported for Stat5a/b. In addition, peptide aptamers selected from combinatorial peptide libraries can potentially be used to block Stat5 dimerization or DNA binding (Nagel-Wolfrum et al., 2004). Another approach would be to design decoy oligonucleotides that mimic Stat5a/b binding sites, sequestering Stat5 from its target genes and inhibiting DNA binding (Guh et al., 2001). Transcriptional activation of Stat5a/b has been successfully disrupted by expression of Stat5 that lacks the C-terminal transactivation domain and therefore acts in a dominant-negative manner (Ahonen et al., 2003; Dagvadorj et al., 2008). Moreover, down-regulation of Stat5a/b gene expression by antisense oligonucleotides or RNA interference effectively induced apoptotic death of human prostate cancer cell lines (Dagvadorj et al., 2008). Finally, novel approaches for prostate cancer specific delivery of pharmacological agents are under development in a number of laboratories in order to overcome the adverse effects of systemic drugs (Isaacs, 2005). These delivery methods will eventually be available for pharmacological Stat5a/b inhibitors as a targeted treatment of prostate cancer.
Abbreviations
- Stat
Signal Transducer and Activator of Transcription
- Prl
prolactin
- PrlR
prolactin receptor
- AR
androgen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahonen TJ, Harkonen PL, Laine J, Rui H, Martikainen PM, Nevalainen MT. Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology. 1999;140:5412–21. doi: 10.1210/endo.140.11.7090. [DOI] [PubMed] [Google Scholar]
- Ahonen TJ, Harkonen PL, Rui H, Nevalainen MT. PRL signal transduction in the epithelial compartment of rat prostate maintained as long-term organ cultures in vitro. Endocrinology. 2002;143:228–38. doi: 10.1210/endo.143.1.8576. [DOI] [PubMed] [Google Scholar]
- Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–92. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem. 2000;275:39718–26. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- Aoki N, Matsuda T. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol Endocrinol. 2002;16:58–69. doi: 10.1210/mend.16.1.0761. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–51. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- Chatterjee-Kishore M, Wright KL, Ting JP, Stark GR. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. Embo J. 2000;19:4111–22. doi: 10.1093/emboj/19.15.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE, Jr, Kuriyan J. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–39. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wen R, Yang S, Schuman J, Zhang EE, Yi T, et al. Identification of Shp-2 as a Stat5A phosphatase. J Biol Chem. 2003;278:16520–7. doi: 10.1074/jbc.M210572200. [DOI] [PubMed] [Google Scholar]
- Choi JY, Li WL, Kouri RE, Yu J, Kao FT, Ruano G. Assignment of the acute phase response factor (APRF) gene to 17q21 by microdissection clone sequencing and fluorescence in situ hybridization of a P1 clone. Genomics. 1996;37:264–5. doi: 10.1006/geno.1996.0556. [DOI] [PubMed] [Google Scholar]
- Chughtai N, Schimchowitsch S, Lebrun JJ, Ali S. Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells. J Biol Chem. 2002;277:31107–14. doi: 10.1074/jbc.M200156200. [DOI] [PubMed] [Google Scholar]
- Clark J, Edwards S, Feber A, Flohr P, John M, Giddings I, et al. Genome-wide screening for complete genetic loss in prostate cancer by comparative hybridization onto cDNA microarrays. Oncogene. 2003;22:1247–52. doi: 10.1038/sj.onc.1206247. [DOI] [PubMed] [Google Scholar]
- Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–22. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–101. doi: 10.1210/en.2006-1761. [DOI] [PubMed] [Google Scholar]
- Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res. 2008;14:1317–24. doi: 10.1158/1078-0432.CCR-07-2024. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- de Groot RP, Raaijmakers JA, Lammers JW, Jove R, Koenderman L. STAT5 activation by BCR-Abl contributes to transformation of K562 leukemia cells. Blood. 1999;94:1108–12. [PubMed] [Google Scholar]
- Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- Decker T, Lew DJ, Mirkovitch J, Darnell JE., Jr Cytoplasmic activation of GAF, an IFN-gamma-regulated DNA-binding factor. Embo J. 1991;10:927–32. doi: 10.1002/j.1460-2075.1991.tb08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–88. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- Evans MK, Yu CR, Lohani A, Mahdi RM, Liu X, Trzeciak AR, et al. Expression of SOCS1 and SOCS3 genes is differentially regulated in breast cancer cells in response to proinflammatory cytokine and growth factor signals. Oncogene. 2007;26:1941–8. doi: 10.1038/sj.onc.1209993. [DOI] [PubMed] [Google Scholar]
- Foxwell BM, Beadling C, Guschin D, Kerr I, Cantrell D. Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur J Immunol. 1995;25:3041–6. doi: 10.1002/eji.1830251109. [DOI] [PubMed] [Google Scholar]
- Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem. 2004;279:3563–72. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillanders EM, Xu J, Chang BL, Lange EM, Wiklund F, Bailey-Wilson JE, et al. Combined genome-wide scan for prostate cancer susceptibility genes. J Natl Cancer Inst. 2004;96:1240–7. doi: 10.1093/jnci/djh228. [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen LA, Norstedt G, et al. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. Embo J. 1995;14:2005–13. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J. 1994;13:4361–9. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goureau A, Garrigues A, Tosser-Klopp G, Lahbib-Mansais Y, Chardon P, Yerle M. Conserved synteny and gene order difference between human chromosome 12 and pig chromosome 5. Cytogenet Cell Genet. 2001;94:49–54. doi: 10.1159/000048782. [DOI] [PubMed] [Google Scholar]
- Gozgit JM, Bebernitz G, Patil P, Ye M, Parmentier J, Wu J, et al. Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL-xL survival signaling in the human JAK2 V617F cell line SET-2. J Biol Chem. 2008;283:32334–43. doi: 10.1074/jbc.M803813200. [DOI] [PubMed] [Google Scholar]
- Gross M, Liu B, Tan J, French FS, Carey M, Shuai K. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene. 2001;20:3880–3887. doi: 10.1038/sj.onc.1204489. [DOI] [PubMed] [Google Scholar]
- Guh JY, Huang JS, Chen HC, Hung WC, Lai YH, Chuang LY. Advanced glycation end product-induced proliferation in NRK-49F cells is dependent on the JAK2/STAT5 pathway and cyclin D1. Am J Kidney Dis. 2001;38:1096–104. doi: 10.1053/ajkd.2001.28616. [DOI] [PubMed] [Google Scholar]
- Haddad B, Pabon-Pena CR, Young H, Sun WH. Assignment1 of STAT1 to human chromosome 2q32 by FISH and radiation hybrids. Cytogenet Cell Genet. 1998;83:58–9. doi: 10.1159/000015126. [DOI] [PubMed] [Google Scholar]
- Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z. Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer. J Clin Endocrinol Metab. 2002;87:4707–14. doi: 10.1210/jc.2002-020347. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–21. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–94. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–9. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- Huang CY, Beliakoff J, Li X, Lee J, Sharma M, Lim B, et al. hZimp7, a novel PIAS-like protein, enhances androgen receptor-mediated transcription and interacts with SWI/SNF-like BAF complexes. Mol Endocrinol. 2005;19:2915–29. doi: 10.1210/me.2005-0097. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU Int. 2005;96 (Suppl 2):35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Isaacs WB. Androgen receptor outwits prostate cancer drugs. Nat Med. 2004;10:26–7. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- Iyer J, Reich NC. Constitutive nuclear import of latent and activated STAT5a by its coiled coil domain. Faseb J. 2008;22:391–400. doi: 10.1096/fj.07-8965com. [DOI] [PubMed] [Google Scholar]
- John S, Vinkemeier U, Soldaini E, Darnell JE, Jr, Leonard WJ. The significance of tetramerization in promoter recruitment by Stat5. Mol Cell Biol. 1999;19:1910–8. doi: 10.1128/mcb.19.3.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junicho A, Matsuda T, Yamamoto T, Kishi H, Korkmaz K, Saatcioglu F, et al. Protein inhibitor of activated STAT3 regulates androgen receptor signaling in prostate carcinoma cells. Biochem Biophys Res Commun. 2000;278:9–13. doi: 10.1006/bbrc.2000.3753. [DOI] [PubMed] [Google Scholar]
- Kazansky AV, Spencer DM, Greenberg NM. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res. 2003;63:8757–62. [PubMed] [Google Scholar]
- Kindblom J, Dillner K, Ling C, Tornell J, Wennbo H. Progressive prostate hyperplasia in adult prolactin transgenic mice is not dependent on elevated serum androgen levels. Prostate. 2002;53:24–33. doi: 10.1002/pros.10113. [DOI] [PubMed] [Google Scholar]
- Kindblom J, Dillner K, Sahlin L, Robertson F, Ormandy C, Tornell J, et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology. 2003;144:2269–78. doi: 10.1210/en.2002-0187. [DOI] [PubMed] [Google Scholar]
- Leek JP, Hamlin PJ, Bell SM, Lench NJ. Assignment of the STAT6 gene (STAT6) to human chromosome band 12q13 by in situ hybridization. Cytogenet Cell Genet. 1997;79:208–9. doi: 10.1159/000134723. [DOI] [PubMed] [Google Scholar]
- Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci U S A. 2003;100:1250–5. doi: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–82. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863–8. doi: 10.1158/1078-0432.CCR-05-0562. [DOI] [PubMed] [Google Scholar]
- Li P, Yu X, Ge K, Melamed J, Roeder RG, Wang Z. Heterogenous expression and functions of androgen receptor co-factors in primary prostate cancer. Am J Pathol. 2002;161:1467–1474. doi: 10.1016/S0002-9440(10)64422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–44. [PubMed] [Google Scholar]
- Linja MJ, Porkka KP, Kang Z, Savinainen KJ, Janne OA, Tammela TL, et al. Expression of androgen receptor coregulators in prostate cancer. Clin Cancer Res. 2004;10:1032–1040. doi: 10.1158/1078-0432.ccr-0990-3. [DOI] [PubMed] [Google Scholar]
- Litterst CM, Kliem S, Marilley D, Pfitzner E. NCoA-1/SRC-1 is an essential coactivator of STAT5 that binds to the FDL motif in the alpha-helical region of the STAT5 transactivation domain. J Biol Chem. 2003;278:45340–51. doi: 10.1074/jbc.M303644200. [DOI] [PubMed] [Google Scholar]
- Liu JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–44. [PubMed] [Google Scholar]
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92:8831–5. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovera M, Pichard C, Bernichtein S, Jeay S, Touraine P, Kelly PA, et al. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation [In Process Citation] Oncogene. 2000;19:4695–705. doi: 10.1038/sj.onc.1203846. [DOI] [PubMed] [Google Scholar]
- Magne S, Caron S, Charon M, Rouyez MC, Dusanter-Fourt I. STAT5 and Oct-1 form a stable complex that modulates cyclin D1 expression. Mol Cell Biol. 2003;23:8934–45. doi: 10.1128/MCB.23.24.8934-8945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AB, Wichmann C, Gross A, Kunkel H, Heinzel T, Ruthardt M, et al. The Stat5-RARalpha fusion protein represses transcription and differentiation through interaction with a corepressor complex. Blood. 2002;99:2647–52. doi: 10.1182/blood.v99.8.2647. [DOI] [PubMed] [Google Scholar]
- Meyer WK, Reichenbach P, Schindler U, Soldaini E, Nabholz M. Interaction of STAT5 dimers on two low affinity binding sites mediates interleukin 2 (IL-2) stimulation of IL-2 receptor alpha gene transcription. J Biol Chem. 1997;272:31821–8. doi: 10.1074/jbc.272.50.31821. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Gregory CW, Ford OHr, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- Mui AL, Wakao H, Harada N, O’Farrell AM, Miyajima A. Interleukin-3, granulocyte-macrophage colony-stimulating factor, and interleukin-5 transduce signals through two forms of STAT5. J Leukoc Biol. 1995a;57:799–803. doi: 10.1002/jlb.57.5.799. [DOI] [PubMed] [Google Scholar]
- Mui AL, Wakao H, O’Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. Embo J. 1995b;14:1166–75. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel-Wolfrum K, Buerger C, Wittig I, Butz K, Hoppe-Seyler F, Groner B. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res. 2004;2:170–82. [PubMed] [Google Scholar]
- Nevalainen MT, Ahonen TJ, Yamashita H, Chandrashekar V, Bartke A, Grimley PM, et al. Epithelial defect in prostates of Stat5a-null mice. Lab Invest. 2000;80:993–1006. doi: 10.1038/labinvest.3780105. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ahonen T, Yagi A, Paranko J, Harkonen PL. Androgen-dependent expression of prolactin in rat prostate epithelium in vivo and in organ culture. Faseb J. 1997a;11:1297–307. doi: 10.1096/fasebj.11.14.9409549. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ingleton PM, Harkonen PL. Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate. Endocrinology. 1996;137:3078–88. doi: 10.1210/endo.137.7.8770934. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997b;99:618–27. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Makela SI, Blauer M, Tuohimaa PJ, Harkonen PL. Estrogen and prolactin regulation of rat dorsal and lateral prostate in organ culture. Endocrinology. 1991;129:612–22. doi: 10.1210/endo-129-2-612. [DOI] [PubMed] [Google Scholar]
- Pallard C, Gouilleux F, Benit L, Cocault L, Souyri M, Levy D, et al. Thrombopoietin activates a STAT5-like factor in hematopoietic cells. Embo J. 1995;14:2847–56. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palvimo JJ. PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochem Soc Trans. 2007;35:1405–8. doi: 10.1042/BST0351405. [DOI] [PubMed] [Google Scholar]
- Paukku K, Yang J, Silvennoinen O. Tudor and nuclease-like domains containing protein p100 function as coactivators for signal transducer and activator of transcription 5. Mol Endocrinol. 2003;17:1805–14. doi: 10.1210/me.2002-0256. [DOI] [PubMed] [Google Scholar]
- Peng B, Sutherland KD, Sum EY, Olayioye M, Wittlin S, Tang TK, et al. CPAP is a novel stat5-interacting cofactor that augments stat5-mediated transcriptional activity. Mol Endocrinol. 2002;16:2019–33. doi: 10.1210/me.2002-0108. [DOI] [PubMed] [Google Scholar]
- Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestell RG, Nevalainen MT, editors. Prostate Cancer: Signaling Networks, Genetics, and New Treatment Strategies. Humana Press; Totowa, NJ, USA: 2008. [Google Scholar]
- Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–12. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- Rycyzyn MA, Clevenger CV. The intranuclear prolactin/cyclophilin B complex as a transcriptional inducer. Proc Natl Acad Sci U S A. 2002;99:6790–5. doi: 10.1073/pnas.092160699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Muller S. PIAS/SUMO: new partners in transcriptional regulation. Cell Mol Life Sci. 2003;60:2561–74. doi: 10.1007/s00018-003-3129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. Embo J. 1997;16:7067–77. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y. Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996;271:31017–20. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- Sharma M, Li X, Wang Y, Zarnegar M, Huang CY, Palvimo JJ, et al. hZimp10 is an androgen receptor co-activator and forms a complex with SUMO-1 at replication foci. Embo J. 2003;22:6101–14. doi: 10.1093/emboj/cdg585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196–202. doi: 10.1038/sj.cr.7310027. [DOI] [PubMed] [Google Scholar]
- Soldaini E, John S, Moro S, Bollenbacher J, Schindler U, Leonard WJ. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20:389–401. doi: 10.1128/mcb.20.1.389-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–8. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- Tan SH, Dagvadorj A, Shen F, Gu L, Liao Z, Abdulghani J, et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68:236–48. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
- Tan SH, Nevalainen MT. Signal transducer and activator of transcription 5A/B in prostate and breast cancers. Endocr Relat Cancer. 2008;15:367–90. doi: 10.1677/ERC-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- Turkson J, Kim JS, Zhang S, Yuan J, Huang M, Glenn M, et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–9. [PubMed] [Google Scholar]
- Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- Vinkemeier U. Getting the message across, STAT! Design principles of a molecular signaling circuit. J Cell Biol. 2004;167:197–201. doi: 10.1083/jcb.200407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. Embo J. 1994;13:2182–91. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H, Schmitt-Ney M, Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992;267:16365–70. [PubMed] [Google Scholar]
- Wang L, Banerjee S. Differential PIAS3 expression in human malignancy. Oncol Rep. 2004;11:1319–24. [PubMed] [Google Scholar]
- Wang Z, Prins GS, Coschigano KT, Kopchick JJ, Green JE, Ray VH, et al. Endocrinology. 2005. Disruption of Growth Hormone Signaling Retards Early Stages of Prostate Carcinogenesis in the C3(1)/Tag Mouse. [DOI] [PubMed] [Google Scholar]
- Wartmann M, Cella N, Hofer P, Groner B, Liu X, Hennighausen L, et al. Lactogenic hormone activation of Stat5 and transcription of the beta- casein gene in mammary epithelial cells is independent of p42 ERK2 mitogen-activated protein kinase activity. J Biol Chem. 1996;271:31863–8. doi: 10.1074/jbc.271.50.31863. [DOI] [PubMed] [Google Scholar]
- Weiss-Messer E, Merom O, Adi A, Karry R, Bidosee M, Ber R, et al. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220:109–23. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Wennbo H, Kindblom J, Isaksson OG, Tornell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–5. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- Xu D, Qu CK. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–32. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yu F, Yan M, Lu L, Zou W, Sun L, et al. Geldanamycin, a heat shock protein 90-binding agent, disrupts Stat5 activation in IL-2-stimulated cells. J Cell Physiol. 2004;198:188–96. doi: 10.1002/jcp.10403. [DOI] [PubMed] [Google Scholar]
- Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when du145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61:6098–104. [PubMed] [Google Scholar]
- Yamamoto K, Kobayashi H, Arai A, Miura O, Hirosawa S, Miyasaka N. cDNA cloning, expression and chromosome mapping of the human STAT4 gene: both STAT4 and STAT1 genes are mapped to 2q32.2-->q32.3. Cytogenet Cell Genet. 1997;77:207–10. doi: 10.1159/000134578. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nevalainen MT, Xu J, LeBaron MJ, Wagner KU, Erwin RA, et al. Role of serine phosphorylation of Stat5a in prolactin-stimulated beta- casein gene expression. Mol Cell Endocrinol. 2001;183:151–63. doi: 10.1016/s0303-7207(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Xu J, Erwin RA, Farrar WL, Kirken RA, Rui H. Differential control of the phosphorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin- sensitive cells. J Biol Chem. 1998;273:30218–24. doi: 10.1074/jbc.273.46.30218. [DOI] [PubMed] [Google Scholar]
- Yang E, Henriksen MA, Schaefer O, Zakharova N, Darnell JE., Jr Dissociation time from DNA determines transcriptional function in a STAT1 linker mutant. J Biol Chem. 2002;277:13455–62. doi: 10.1074/jbc.M112038200. [DOI] [PubMed] [Google Scholar]
- Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–47. [PubMed] [Google Scholar]
- Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, John S, Berg M, Leonard WJ. Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell. 1999;96:121–30. doi: 10.1016/s0092-8674(00)80965-4. [DOI] [PubMed] [Google Scholar]