Summary
High mobility group N (HMGN) proteins are the only nuclear proteins known to specifically recognize the generic structure of the 147 base pair nucleosome core particle. Both in vitro and in vivo experiments demonstrate that HMGN proteins are involved in epigenetic regulation by modulating chromatin structure and levels of posttranslational modifications of nucleosomal histones. Expression of HMGN proteins is developmentally regulated, and the loss or overexpression of these proteins can lead to developmental abnormalities. This review will focus on the role and on the possible molecular mechanism whereby HMGN proteins affect cellular differentiation and development.
Keywords: HMGN protein, chromatin, embryogenesis, differentiation
Introduction
Proper cellular differentiation during embryonic development depends on the controlled regulation of tissue specific gene expression in a temporal and spatial fashion. The structure of the chromatin fiber plays a central role in regulating gene expression. The chromatin architectural proteins such as high mobility group (HMG) proteins are also involved in maintaining or altering the chromatin structure. There are three different subfamily of HMG proteins; HMGA, HMGB and HMGN, and each of these proteins have different role in cellular differentiation and development[1].
HMGN proteins specifically bind to nucleosome, influence epigenetic modifications and modulate chromatin structure and therefore may play a role in differentiation[2]. HMGN protein family is composed of five proteins, HMGN1 through 5, characterized by the presence of 3 distinct functional domains: a bipartite nuclear localization signal (NLS) a nucleosomal binding domain and an acidic C-terminal chromatin regulatory domain (Figure 1A). HMGN1 to 4 are about ~90 amino acids in length while HMGN5 (previously called NSBP1 or NBP45) is composed of ~400 amino acids (Figure 1B, [3, 4], Malicet and Rochman in this review). Hmgn genes are vertebrate specific and are located on different chromosomes. In humans, HMGN1 is located on chromosome 21 (21q22.3), HMGN2 is on chromosome 1 (1p36.1), HMGN3 and HMGN4 are on chromosome 6 (6q14.1 and 6p21.3 respectively) and HMGN5 is located on the X chromosome (Xp13.3) (Figure 1C). In addition, both mouse and human Hmgn genes have a large number of retropsudogenes [5].
Figure 1. Schematic diagram of the High Mobility Group N (HMGN) proteins.
(A) Representative HMGN protein is composed of three functional domains: a bipartite nuclear localization signal (NLS), a nucleosome binding domain and C-terminal chromatin regulatory domain. (B) Schematic diagram of mouse HMGN protein variant. Number of amino acids is indicated on right. While HMGN1, HMGN2, HMGN3a and HMGN4 show similar structure, HMGN3b lack C-terminal domain and HMGN5 possesses a long acidic tail which is composed of negatively charged amino acid repeats. (C) Chromosome locations of human HMGN genes. Each green bars represents chromosome and its number is written on the bottom. Yellow boxes indicate approximate HMGN gene location and exact locus of each HMGN variant is written on the right. Short arm and long arm is represented by separated bars.
The developmental roles of HMGN proteins are mainly based on in vitro cellular differentiation of mouse, chicken or human cells. Studies on expression patterns of these proteins, in vivo functional analysis of knockout mice, and in vitro experiments on cellular differentiation are beginning to reveal that the HMGN protein family may not only share overlapping functions but also have unique function.
In this review we will focus on the expression of HMGN during develop, on the possible role of these proteins in development and discuss possible molecular mechanisms whereby members of the HMGN protein family affect development and cellular differentiation. Additional information on the developmental role of HMGNs and of the other members of the HMG protein superfamily can be found in previous reviews [1].
HMGN1 and HMGN2
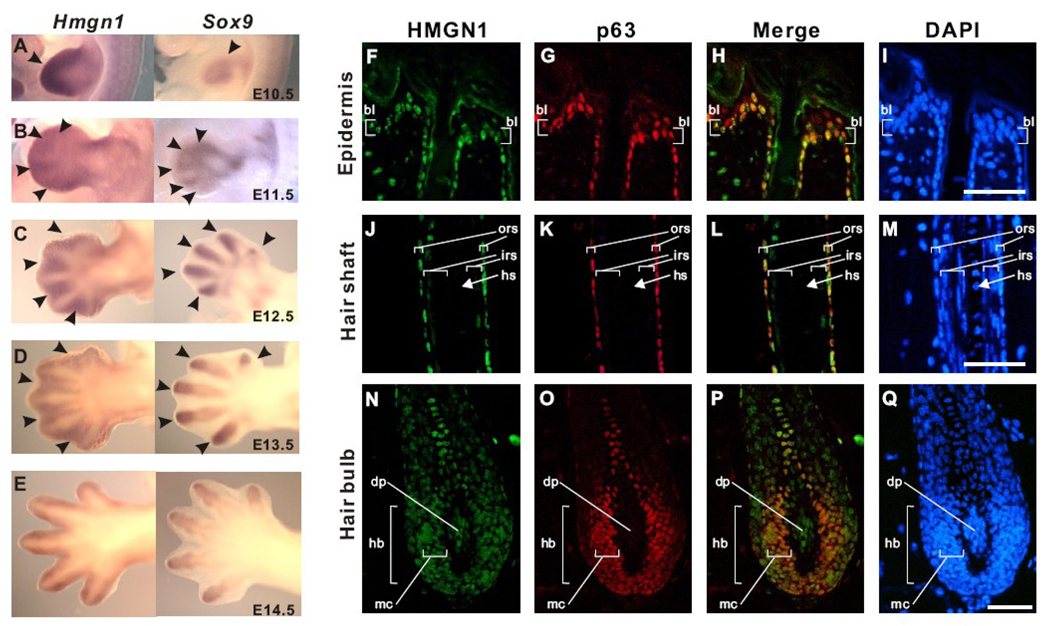
Hmgn1 and Hmgn2 are ubiquitously expressed in adult tissues however they are most strongly expressed during embryogenesis [6, 7]. The expression levels of these proteins are highly regulated in a developmental stage specific manner where HMGNs are highly expressed in the undifferentiated state and poorly expressed after differentiation (Figure 2A–E) [1]. HMGN1 and HMGN2 expression is downregulated during myogenesis, erythrogenesis, and chondrogenesis, as well as during embryonic organ formation, such as kidney development [6–9]. A recent study of the expression patterns of HMGN1 and HMGN2 in the hair follicle further demonstrates that the expression of these proteins is developmentally regulated [10]. Thus, in mature hair follicles, HMGN1 is expressed in the stem cell of hair follicle, or bulge cells. Both HMGN1 and HMGN2 are expressed in the less differentiated outer root sheath cells and basal layer cells, but not in the terminally differentiated inner root sheath cells, hair shaft, or supra-basal layer (Figure 2F–Q) [10]. These findings clearly demonstrate that the expression of these proteins is high in stem cells and stem cell derived transiently amplifying (TA) cells, and their expression is downregulated upon differentiation. Proper downregulation of HMGN proteins is required for normal cellular differentiation, an observation confirmed in studies that demonstrated that overexpression of either HMGN1 or HMGN2 inhibit in vitro myogenesis and chondrogenesis [6, 11].
Figure 2. HMGN1 expression is associated with developmental stage of mouse embryogenesis and hair follicle.
(A to E) The comparison of expression patterns of Hmgn1 (left) and Sox9 (right) in the developing limb bud. Hmgn1 expression is detected in the distal mesenchyme (filled arrowhead in A; left) and interdigit mesenchyme (filled arrowheads in B–E; left) while Sox9 is detected in the compensating mesenchyme (open arrow head in A; right) and digit mesenchyme (open arrowheads B–E; right). (F to Q) Expression of HMGN1 in the adult mouse hair follicle. HMGN1 (green) and p63 (red) protein are detected by immunofluorescence. Nuclear DNA is stained with DAPI (blue). Note that HMGN1 signal is detected in undifferentiated region and reduced in differentiated region. HMGN1 and p63 colocalize in most of the hair follicle except the dermal papilla. Scale bar: 50 µm. bl, basal layer; ors, outer root sheath; irs, inner root sheath; hs, hair shaft; hb, hair bulb; dp, dermal papilla; mc, matrix cells. (See details in [10])
The in vivo role of HMGN1 protein in development is studied using Hmgn1−/− mice. These mice appear normal during development but show various phenotypes when subjected to stress. For example, Hmgn1−/− mice and cells derived from these mice are hypersensitive to both UV and ionizing radiation [12, 13]. The hypersensitivity of Hmgn−/− cells to UV radiation might be due to the restricted accessibility of DNA repair machinery to the chromatin in the absence of HMGN1. In addition, HMGN1 may also have an important role in transcription-coupled repair as it is shown by the observation that Cockayne syndrome protein A recruits HMGN1 to the RNA polymerase stalled at the UV damaged sites [13, 14]. HMGN1 is also shown to play a crucial role in the repair of DNA damaged by ionizing radiation. In response to ionizing radiation, both Hmgn−/− mice and cells show an increased tumorigenic potential due to their impaired ability to activate G2-M checkpoint. Furthermore, HMGN1 affects the levels of ionizing radiation-induced activation of ATM, a key regulator of the cellular response to double stranded DNA damage. Significantly, HMGN1 affects ATM activation by modulating the levels of epigenetic modifications in the core histones [15].
Additional evidence for a possible role of HMGN in development was obtained in a study demonstrating that Hmgn1−/− mice had slight defects in the development of corneal epithelium [16]. The abnormal corneal epithelium in Hmgn−/− mice could be explained by the altered expression of cell adhesion molecules and of p63, major regulator of epithelial cell differentiation. The absence of severe phenotype in Hmgn−/− mice could be explained by the possible functional redundancy between HMGN1and HMGN2 [10], which may lower the deleterious effect due to loss of HMGN1 during embryogenesis. Supporting this in vivo finding, it was reported that in NIH3T3 cells, loss of HMGN1 does not affect in vitro myogenesis or in vitro adipogenesis, which are induced by MyoD or C/EBP, respectively [17]. This finding suggests that the downstream genes of tissue specific master regulators such as MyoD or C/EBP are not affected by loss of HMGN1. Similar to HMGN1, HMGN2 is also shown to play a role in chondrocyte differentiation. In chondrogenic ATDC5 cell line study, Hmgn2 was identified as one of the late response genes of parathyroid hormone (PTH) related peptide (PTHrP) treatment [18]. PTHrP plays a critical role in chondrocyte differentiation during endochondral bone formation by inhibiting hypertrophic chondrocyte differentiation and by keeping the chondrocytes in the proliferative state. In this study, Hmgn2 was downregulated by PTHrP treatment similar to early chondrocyte markers, suggesting that HMGN2 is involved in controlling the pace of terminal differentiation.
HMGN1 and HMGN2 were also shown to be involved in early stage embryogenesis. These proteins are detected throughout oogenesis, and embryonic transcripts of HMGN1 and HMGN2 accumulate beyond the two-cell stage. Transient depletion of HMGN1 and HMGN2 proteins by injecting antisense oligonucleotides into mouse oocytes delayed cell cleavage and the onset of the blastocyst stage [19]. During bovine embryo early stage development, HMGN1 and HMGN2 are expressed in oocytes but are downregulated after fertilization and almost disappear by the 8-cell stage [20]. An in vitro fertilization study of bovine embryos that are incapable of downregulating HMGN2 expression revealed that embryos with enhanced levels of HMGN2 failed to develop into blastocyst due to the altered chromatin remodeling which may be caused by hyperacetylation of Histone H3 lysine 14 (H3K14ac) in the embryo [21].
Further reports for the importance of the proper regulation of HMGN1 and HMGN2 expression is also clearly demonstrated in Xenopus development [22]. In Xenopus, both Hmgn1 and Hmgn2 are expressed in the three germinal layers [22]. Synthesis of HMGN1 and HMGN2 proteins start after mid blastula transition (MBT), and continues throughout the neurula and tadpole embryos [22]. Neither depletion nor overexpression of HMGN1 or HMGN2 affects the embryonic stages prior to MBT. After MBT, overexpression of either HMGN1 or HMGN2 causes imperfectly closed blastpore, distorted body axis and abnormal head structure in these embryos [22]. Interestingly, depletion of Hmgn1 or Hmgn2 resulted in similar developmental defects and reexpression of HMGN2 rescued these phenotypes, indicating that misregulation of HMGN levels perturb proper development. Furthermore, in Xenopus animal cap assay, injection of HMGN1 or HMGN2, but not of mutant HMGN, that does not bind do nucleosomes, prolonged the competence of mesoderm induction by Activin [22]. These experiments demonstrate that the binding of HMGN proteins to chromatin facilitate the competence of mesoderm specific gene expression in response to the Activin signals. Human HMGN1 gene is located on the chromosome 21 (Figure 1C), and its location is in a region associated with the pathogenesis of Down syndrome [23], the most common cause of mental retardation. Indeed, HMGN1 is overexpressed in the cells from Down syndrome patient [23] and this elevated level of HMGN1 is also observed in the Ts1Cje mouse, which is used as a mouse model for Down syndrome [24]. These studies suggest a possible role for HMGN1 in the etiology of Down Syndrome.
HMGN3
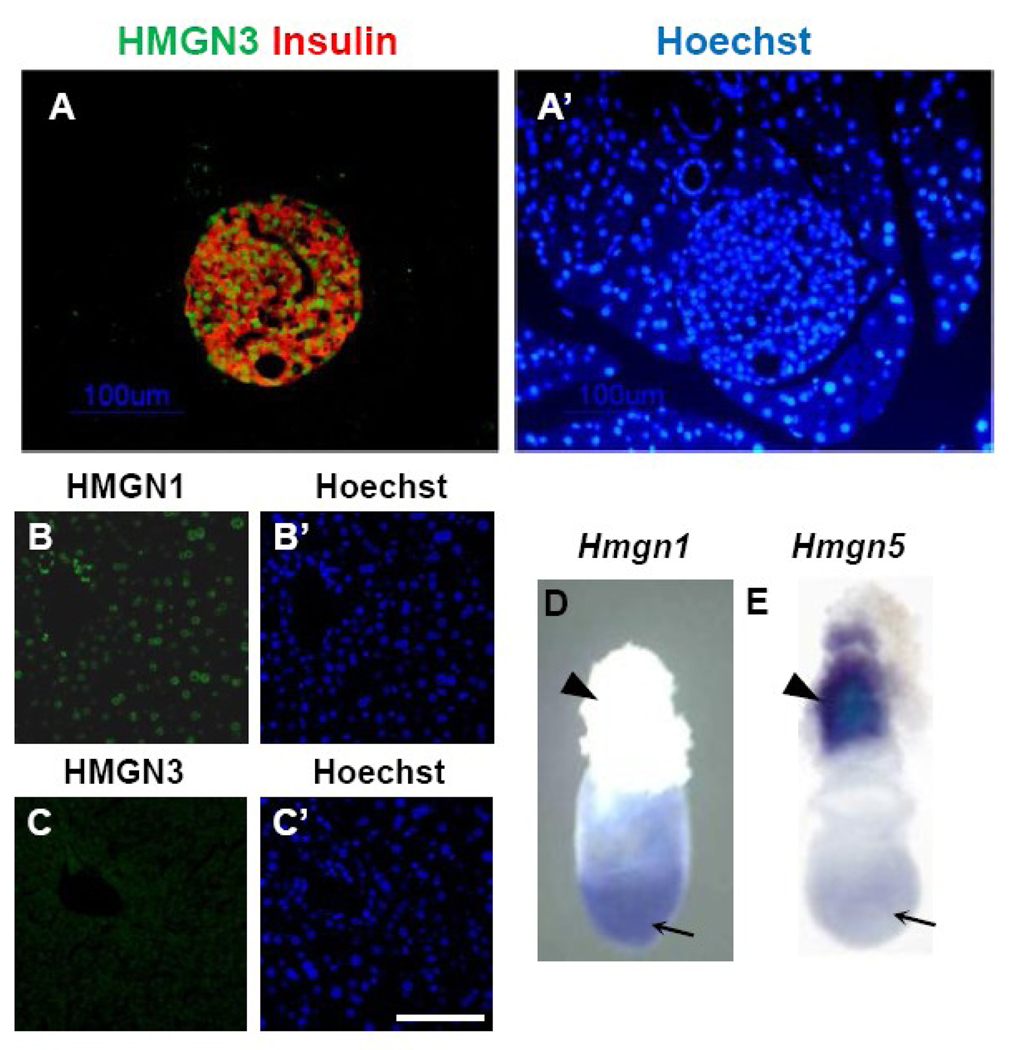
While HMGN1 and HMGN2 are ubiquitously expressed and are involved in the regulation of cellular differentiation, HMGN3 seems to be expressed in a tissue- and developmental stage-specific manner (Figure 3A–C). HMGN3 protein has two splice variants; HMGN3a and HMGN3b [25]. While the structure of HMGN3a is similar to HMGN1 and HMGN2, HMGN3b lacks C-terminal conserved domain (Figure 1B). To date, it has been shown that there is no difference in tissue distribution and function between HMGN3a and HMGN3b. Therefore in this review, HMGN3 will represent both HMGN3a and HMGN3b, unless specified otherwise. In mouse and human tissues, Hmgn3 is highly expressed in the eye and brain [25, 26]. HMGN3 expression is also detected in presumptive corneal epithelium and lens fiber during the embryonic eye development at 13.5 dpc [27]. In the adult eye, HMGN3 showed specific expression in the lens fiber and inner nuclear layer of retina [27]. Specific expression of HMGN3 is also observed in adult pancreatic islet cells (Figure 3, [28]).
Figure 3. Expression patterns of HMGN protein family in the mouse.
(A) Expression of HMGN3 proteins in the adult pancreas detected by immunofluorescence. HMGN3 protein is detected by green signal and pancreatic β-cells are detected by red signal with anti-Insulin. (A’) Nuclear DNA is stained with Hoechst. Note that HMGN3 expression is islet specific. (Refer details in [28]) (B and C) Expression of HMGN1 and HMGN3 in the adult liver. While HMGN1 is detected all over the liver, significant HMGN3 signal is not detected. (B’ and C’) Nuclear DNA is stained with Hoechst. (D and E) Expression of Hmgn1 and Hmgn5 in the mouse embryo at 7.5 days post coitus. Arrows indicate embryonic region and arrowheads indicate ectoplacental cone. Hmgn1 and Hmgn5 mRNA are detected by whole mount in situ hybridization. (Refer details in [31])
The in vivo role of HMNG3was studied using Hmgn3−/− mice which develop normally but are mildly diabetic that stems due to defective insulin secretion [28]. Molecular analysis of the mechanisms whereby HMGN3 affects beta function and insulin secretion revealed that HMGN3 is involved in transcriptional regulation of several genes in pancreatic β islet cells including glucose transporter 2 (Glut2) and Kir6, both genes play a key role in glucose-dependent insulin secretion [28].
HMGN4
HMGN4 was discovered by GenBank database search as a new HMGN2-like transcript… The human HMGN4 is located on the chromosome 6, in a region associated with schizophrenia [29]. Antibodies against this protein has been raised however the developmental role of this protein has not been studied [30]
HMGN5
HMGN5 has a unique molecular structure when compared to other HMGN family proteins ([4], (Figure 1, and see Rochman and Malicet in this issue). This protein contains a typical HMGN nucleosome binding domain but has a longer C-terminal domain which contains a unique repeat of acidic amino acid [4].
Initial study on Hmgn5 revealed that it is highly expressed in 7.5 day mouse embryo s [4]. Whole mount in situ hybridization of 7.5 day embryo revealed that Hmgn5 is not detected in the embryonic region but is present in ectoplacental cone (Figure 3D,E, [31]) which will form placenta in latter stages of embryo development. HMGN5 is also shown to be highly expressed in trophoblast giant cells, spongiotrophoblast, and trophoblast cells located in the placental labyrinth. The expression in other regions of the embryo remains relatively weak. These observations suggested that HMGN5 may play an important role in trophoblast development during embryogenesis. Indeed, Hmgn5 expression is upregulated during Rcho-1 cell differentiation, an in vitro model for trophoblast differentiation [31]. In this cell line, either depletion or overexpression of HMGN5 affected the differentiation-related expression of several prolactin related genes without altering the expression of transcriptional regulators such as GATA3 or Hand1 and without affecting the morphology of Rcho-1 cells [31]. Chromatin binding of HMGN5 to these prolactin-related genes is only observed in Rcho-1 cells but not in the H4IIE cell, a non-trophoblastic cell line, suggesting that HMGN5 modulates the expression of placental lactogens by inducing changes in chromatin structure.
In addition to placental development, HMGN5 is also involved in the development of the reproductive system. It is known that early life exposure to estrogenic chemicals such as diethylstilbestrol (DES) increases the risk for adenocarcinoma in aging animals [32]. Exposure of DES (or dietary phytoestrogens such as genistein, GEN) to neonatal mice caused aberrant overexpression of Hmgn5 in adult ovaries at 6 months and 18 months [32]. This overexpression correlated with hypomethylation of the promoter region of the Hmgn5 gene.
Perspective: HMGN proteins as regulators of cellular differentiation and development
HMGN proteins are chromatin architectural proteins that influence the levels of post-translational modifications of core histone proteins and modulate chromatin structure. During mouse embryogenesis and cellular differentiation the Hmgn genes are regulated in a developmental stage specific manner [6, 33] where their expression is gradually down regulated. This downregulation could be part of the mechanism that leads to changes in chromatin organization and in the cellular transcription potential that occur during differentiation. In several studies, experimental up or down regulation of HMGN expression results in marked changes in the developmental program, indicating that proper development and differentiation requires regulated levels of HMGN gene expression.
HMGN1 and HMGN2 are ubiquitously expressed and showed similar expression patterns [10, 28], and may share functional redundancy. However, Hmgn1−/− mice exhibited abnormalities in the development of corneal epithelium and have defective UV and IR induced DNA repair [16]. Thus, in this regard even though HMGN1 and HMGN2 showed similar expression patterns and molecular structure HMGN2 did not fully compensate for the loss of HMGN1. Experiments in progress suggest that loss of HMGN2 leads to embryonic lethality (S. Cherukuri, unpublished data). A possible explanation is the finding that s the proteins affect histone modifications in a different way [34]. These differences are governed by C-terminal region of HMGN1 and HMGN2. HMGN3b and HMGN5 exhibit tissue specific expression pattern and thus may have more specific roles in modulating chromatin structure and hence in development. The knockout mice of the other HMGN family proteins will reveal their specific roles in development.
Even though HMGN proteins are involved in global transcriptional regulation, HMGN proteins are also shown to be involved in the regulation of specific genes either directly [35, 36] or by modulating the binding of transcription factors at the target gene regulatory regions [28, 37, 38]. While in vitro studies revealed that HMGN may be involved in the regulation of specific genes, Hmgn1−/− or Hmgn3−/− mice did not show severe phenotype in terms of embryogenesis. This suggests that although HMGN proteins do bind to specific chromatin region of target gene, HMGN proteins work not as master regulators but as modulators of gene expression. Unlike Hmgn1 or Hmgn3 knockout mice, the loss of the other HMG superfamily of proteins such as HMGA or HMGB results in more severe phenotype during mouse development [1]. These observations reflect the functional specificity of HMG variants due to their tissue specific HMG-variant expression and different chromatin architectural effects induced by these HMG-variants.
HMGN proteins compete with linker Histone H1 for chromatin binding sites [39]. Histone H1 is composed of at least eight subtypes and the mice lacking one or two histone H1 genes develop normally [40]. However, compound knockout mouse which is depleted the histone H1c, H1d, and H1e dies around 11.5 days post coitus [40]. Hmgn1 knockout cells as well as triple histone H1 knockout cells exhibited altered chromatin structure and histone modifications [41, 42]. It is intriguing to investigate the effect of loss of histone H1 genes on HMGN function or the effect of loss of Hmgn1 gene on histone H1 function and these studies may reveal the in vivo relationship between these architectural proteins. In Xenopus animal cap assay, overexpression of histone H1 resulted in loss of mesoderm induction by Activin [43]. In contrast, depletion of histone H1 prolonged mesoderm induction by Activin signal [43], and this effect is similar to the overexpression of HMGN1 or HMGN2 [22], suggesting that these proteins may share functional competition in the regulation of Activin response genes during early stage embryonic development. It is noteworthy that while compound triple knockout mouse lacking histone H1c, H1d, and H1e had defects in embryogenesis, loss of the Hmgn2 by itself leads to embryonic lethality (S.C, unpublished data). Furthermore, single knockout mouse of either Hmgn1 or Hmgn3 showed severe phenotype in response to stress indicating that these HMGNs play important roles in regulating cellular functions. Direction of cellular fate is determined by the regulation of gene expression and epigenetic modifications. HMGN proteins play important roles in development by modulating the structure of the chromatin fiber. Further analysis of other Hmgn knockout mice or compound HMGN knockout mice will provide additional information on the developmental role of this chromatin-binding protein family.
Acknowledgments
We thank Dr. Liron Abuhatzira and Dr. Michael Bustin (Protein Section, Laboratory of Metabolism, Center for Cancer Research, National Cancer Institute, National Institutes of Health) and the NIH Fellows Editorial Board for constructive discussion of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–79. doi: 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bustin M. Chromatin unfolding and activation by HMGN(*) chromosomal proteins. TrendsBiochem Sci. 2001;26:431–437. doi: 10.1016/s0968-0004(01)01855-2. [DOI] [PubMed] [Google Scholar]
- 3.Rochman M, Postnikov Y, Correll S, Malicet C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA, Grigoryev S, Bustin M. The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Mol Cell. 2009;35:642–656. doi: 10.1016/j.molcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirakawa H, Landsman D, Postnikov YV, Bustin M. NBP-45, a novel nucleosomal binding protein with a tissue-specific and developmentally regulated expression. J Biol Chem. 2000;275:6368–6374. doi: 10.1074/jbc.275.9.6368. [DOI] [PubMed] [Google Scholar]
- 5.Strichman-Almashanu LZ, Bustin M, Landsman D. Retroposed copies of the HMG genes: a window to genome dynamics. Genome Res. 2003;13:800–812. doi: 10.1101/gr.893803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furusawa T, Lim JH, Catez F, Birger Y, Mackem S, Bustin M. Down-regulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol Cell Biol. 2006;26:592–604. doi: 10.1128/MCB.26.2.592-604.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehtonen S, Lehtonen E. HMG-17 is an early marker of inductive interactions in the developing mouse kidney. Differentiation. 2001;67:154–163. doi: 10.1046/j.1432-0436.2001.670407.x. [DOI] [PubMed] [Google Scholar]
- 8.Crippa MP, Nickol JM, Bustin M. Developmental changes in the expression of high mobility group chromosomal proteins. J Biol Chem. 1991;266:2712–2714. [PubMed] [Google Scholar]
- 9.Pash JM, Bhorjee JS, Patterson BM, Bustin M. Persistence of chromosomal proteins HMG-14/-17 in myotubes following differentiation-dependent reduction of HMG mRNA. J Biol Chem. 1990;265:4197–4199. [PubMed] [Google Scholar]
- 10.Furusawa T, Ko JH, Birger Y, Bustin M. Expression of nucleosomal protein HMGN1 in the cycling mouse hair follicle. Gene Expr Patterns. 2009;9:289–295. doi: 10.1016/j.gep.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pash JM, Alfonso PJ, Bustin M. Aberrant expression of high mobility group chromosomal protein 14 affects cellular differentiation. J Biol Chem. 1993;268:13632–13638. [PubMed] [Google Scholar]
- 12.Birger Y, Catez F, Furusawa T, Lim JH, Prymakowska-Bosak M, West KL, Postnikov YV, Haines DC, Bustin M. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 2005;65:6711–6718. doi: 10.1158/0008-5472.CAN-05-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birger Y, West KL, Postnikov YV, Lim JH, Furusawa T, Wagner JP, Laufer CS, Kraemer KH, Bustin M. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. Embo J. 2003;22:1665–1675. doi: 10.1093/emboj/cdg142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fousteri M, Vermeulen W, van Zeeland AA, Mullenders LH. Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol Cell. 2006;23:471–482. doi: 10.1016/j.molcel.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Kim YC, Gerlitz G, Furusawa T, Catez F, Nussenzweig A, Oh KS, Kraemer KH, Shiloh Y, Bustin M. Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat Cell Biol. 2009;11:92–96. doi: 10.1038/ncb1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birger Y, Davis J, Furusawa T, Rand E, Piatigorsky J, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006;74:19–29. doi: 10.1111/j.1432-0436.2006.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill DA, Imbalzano AN. HMGN1 is dispensable for myogenesis and adipogenesis. Gene. 2006;371:59–67. doi: 10.1016/j.gene.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Hoogendam J, Farih-Sips H, van Beek E, Lowik CW, Wit JM, Karperien M. Novel late response genes of PTHrP in chondrocytes. Horm Res. 2007;67:159–170. doi: 10.1159/000096586. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed OA, Bustin M, Clarke HJ. High-mobility group proteins 14 and 17 maintain the timing of early embryonic development in the mouse. Dev Biol. 2001;229:237–249. doi: 10.1006/dbio.2000.9942. [DOI] [PubMed] [Google Scholar]
- 20.Vigneault C, McGraw S, Massicotte L, Sirard MA. Transcription factor expression patterns in bovine in vitro-derived embryos prior to maternal-zygotic transition. Biol Reprod. 2004;70:1701–1709. doi: 10.1095/biolreprod.103.022970. [DOI] [PubMed] [Google Scholar]
- 21.Bastos GM, Goncalves PB, Bordignon V. Immunolocalization of the high-mobility group N2 protein and acetylated histone H3K14 in early developing parthenogenetic bovine embryos derived from oocytes of high and low developmental competence. Mol Reprod Dev. 2008;75:282–290. doi: 10.1002/mrd.20798. [DOI] [PubMed] [Google Scholar]
- 22.Korner U, Bustin M, Scheer U, Hock R. Developmental role of HMGN proteins in Xenopus laevis. Mech Dev. 2003;120:1177–1192. doi: 10.1016/j.mod.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Pash J, Popescu N, Matocha M, Rapoport S, Bustin M. Chromosomal protein HMG-14 gene maps to the Down syndrome region of human chromosome 21 and is overexpressed in mouse trisomy 16. Proc Natl Acad Sci U S A. 1990;87:3836–3840. doi: 10.1073/pnas.87.10.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potier MC, Rivals I, Mercier G, Ettwiller L, Moldrich RX, Laffaire J, Personnaz L, Rossier J, Dauphinot L. Transcriptional disruptions in Down syndrome: a case study in the Ts1Cje mouse cerebellum during post-natal development. J Neurochem. 2006;97 Suppl 1:104–109. doi: 10.1111/j.1471-4159.2005.03624.x. [DOI] [PubMed] [Google Scholar]
- 25.West KL, Ito Y, Birger Y, Postnikov Y, Shirakawa H, Bustin M. HMGN3a and HMGN3b, two protein isoforms with a tissue-specific expression pattern, expand the cellular repertoire of nucleosome-binding proteins. J Biol Chem. 2001;276:25959–25969. doi: 10.1074/jbc.M101692200. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Bustin M. Immunohistochemical localization of the nucleosome-binding protein HMGN3 in mouse brain. J Histochem Cytochem. 2002;50:1273–1275. doi: 10.1177/002215540205000914. [DOI] [PubMed] [Google Scholar]
- 27.Lucey MM, Wang Y, Bustin M, Duncan MK. Differential expression of the HMGN family of chromatin proteins during ocular development. Gene Expr Patterns. 2008;8:433–437. doi: 10.1016/j.gep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda T, Furusawa T, Kurahashi T, Tessarollo L, Bustin M. The Nucleosome Binding Protein HMGN3 Modulates The Transcription Profile Of Pancreatic {beta}-Cells And Affects Insulin Secretion. Mol Cell Biol. 2009 doi: 10.1128/MCB.00526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birger Y, Ito Y, West KL, Landsman D, Bustin M. HMGN4, a newly discovered nucleosomebinding protein encoded by an intronless gene. DNA Cell Biol. 2001;20:257–264. doi: 10.1089/104454901750232454. [DOI] [PubMed] [Google Scholar]
- 31.Shirakawa H, Rochman M, Furusawa T, Kuehn MR, Horigome S, Haketa K, Sugita Y, Inada T, Komai M, Bustin M. The nucleosomal binding protein NSBP1 is highly expressed in the placenta and modulates the expression of differentiation markers in placental Rcho-1 cells. J Cell Biochem. 2009;106:651–658. doi: 10.1002/jcb.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehtonen S, Olkkonen VM, Stapleton M, Zerial M, Lehtonen E. HMG-17, a chromosomal non-histone protein, shows developmental regulation during organogenesis. Int J Dev Biol. 1998;42:775–782. [PubMed] [Google Scholar]
- 34.Ueda T, Postnikov YV, Bustin M. Distinct domains in high mobility group N variants modulate specific chromatin modifications. J Biol Chem. 2006;281:10182–10187. doi: 10.1074/jbc.M600821200. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein YR, Furusawa T, Lim JH, Postnikov YV, West KL, Birger Y, Lee S, Nguyen P, Trepel JB, Bustin M. Chromosomal protein HMGN1 modulates the expression of N-cadherin. Febs J. 2005;272:5853–5863. doi: 10.1111/j.1742-4658.2005.04980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West KL, Castellini MA, Duncan MK, Bustin M. Chromosomal proteins HMGN3a and HMGN3b regulate the expression of glycine transporter 1. Mol Cell Biol. 2004;24:3747–3756. doi: 10.1128/MCB.24.9.3747-3756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amen M, Espinoza HM, Cox C, Liang X, Wang J, Link TM, Brennan RG, Martin JF, Amendt BA. Chromatin-associated HMG-17 is a major regulator of homeodomain transcription factor activity modulated by Wnt/beta-catenin signaling. Nucleic Acids Res. 2008;36:462–476. doi: 10.1093/nar/gkm1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N, Hansen U. HMGN1 modulates estrogen-mediated transcriptional activation through interactions with specific DNA-binding transcription factors. Mol Cell Biol. 2007;27:8859–8873. doi: 10.1128/MCB.01724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, Bustin M. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol Cell Biol. 2004;24:4321–4328. doi: 10.1128/MCB.24.10.4321-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Sirotkin A, Russell RG, Ayala J, Skoultchi AI. Individual somatic H1 subtypes are dispensable for mouse development even in mice lacking the H1(0) replacement subtype. Mol Cell Biol. 2001;21:7933–7943. doi: 10.1128/MCB.21.23.7933-7943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y, Nikitina T, Zhao J, Fleury TJ, Bhattacharyya R, Bouhassira EE, Stein A, Woodcock CL, Skoultchi AI. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005;123:1199–1212. doi: 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Lim JH, Catez F, Birger Y, West KL, Prymakowska-Bosak M, Postnikov YV, Bustin M. Chromosomal protein HMGN1 modulates histone H3 phosphorylation. Mol Cell. 2004;15:573–584. doi: 10.1016/j.molcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Steinbach OC, Wolffe AP, Rupp RA. Somatic linker histones cause loss of mesodermal competence in Xenopus. Nature. 1997;389:395–399. doi: 10.1038/38755. [DOI] [PubMed] [Google Scholar]