Abstract
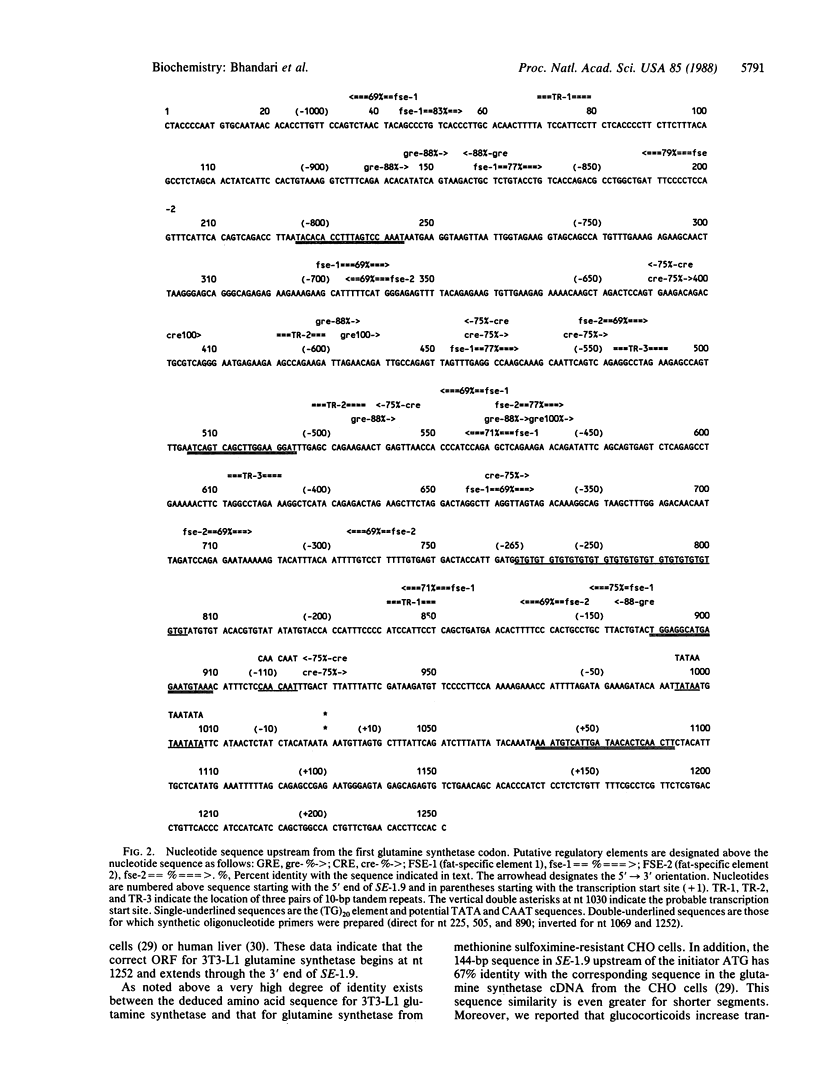
Glutamine synthetase [L-glutamate:ammonia ligase (ADP-forming); EC 6.3.1.2] specific activity, cellular content, mRNA abundance, and gene transcription rate increase by greater than 100-fold during adipocyte differentiation of 3T3-L1 cells. In 3T3-L1 adipocytes dexamethasone increases, whereas insulin as well as N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate decrease, glutamine synthetase gene expression. We analyzed the nucleotide sequence of a 1.9-kilobase Sal I-EcoRI restriction fragment from a 3T3-L1 glutamine synthetase genomic clone. This genomic fragment is composed of 1851 base pairs (bp) and includes the first exon and 1029 bp of the 5' flanking sequence. The 600 bp at the 3' end of the 1.9-kb Sal I-EcoRI restriction fragment constitute an open reading frame. We identified the transcription start site at a location 222 bp upstream of the glutamine synthetase coding sequences. The 5' flanking region of the gene encompasses several potential regulatory elements including TATA and CAAT sequences and a 40-bp poly(dT-dG).poly(dC-dA) putative enhancer element. Potential hormone and fat-specific regulatory elements are also located upstream of the transcription start site; they include glucocorticoid and cAMP response elements and fat-specific elements. These potential regulatory elements could account for the differentiation-associated changes and hormone-mediated changes seen in glutamine synthetase gene transcription and mRNA abundance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bhandari B., Burns D. M., Hoffman R. C., Miller R. E. Glutamine synthetase mRNA in cultured 3T3-L1 adipocytes. Complexity, content and hormonal regulation. Mol Cell Endocrinol. 1986 Sep;47(1-2):49–57. doi: 10.1016/0303-7207(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Bhandari B., Miller R. E. Glutamine synthetase gene transcription in cultured 3T3-L1 adipocytes: regulation by dexamethasone, insulin and dibutyryl cyclic AMP. Mol Cell Endocrinol. 1987 May;51(1-2):7–11. doi: 10.1016/0303-7207(87)90112-2. [DOI] [PubMed] [Google Scholar]
- Bhandari B., Wilson R. H., Miller R. E. Insulin and dexamethasone stimulate transcription of an amplified glutamine synthetase gene in Chinese hamster ovary cells. Mol Endocrinol. 1987 Jun;1(6):403–407. doi: 10.1210/mend-1-6-403. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Bhandari B., Short J. M., Sanders P. G., Wilson R. H., Miller R. E. Selection of a rat glutamine synthetase cDNA clone. Biochem Biophys Res Commun. 1986 Jan 14;134(1):146–151. doi: 10.1016/0006-291x(86)90539-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. S., Campbell K. E., Wilson R. H. Sequence of a human glutamine synthetase cDNA. Nucleic Acids Res. 1987 Aug 11;15(15):6293–6293. doi: 10.1093/nar/15.15.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T., Seidman M., Stollar B. D. Characterization of genomic poly(dT-dG).poly(dC-dA) sequences: structure, organization, and conformation. Mol Cell Biol. 1984 Dec;4(12):2610–2621. doi: 10.1128/mcb.4.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Miller S. M., Samols D. Cloning and characterization of the gene for rabbit C-reactive protein. Biochemistry. 1986 Dec 2;25(24):7834–7839. doi: 10.1021/bi00372a008. [DOI] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Piskiewicz D. Primary structure of peptides from bovine brain glutamine synthetase. Comparison with sequences of glutamine synthetases from other organisms. Biochim Biophys Acta. 1985 Mar 1;827(3):439–446. doi: 10.1016/0167-4838(85)90230-4. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Rhee S. G. Sequence of peptides from Saccharomyces cerevisiae glutamine synthetase. N-terminal peptide and ATP-binding domain. J Biol Chem. 1988 Jan 15;263(2):833–838. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor virus. VI. Processive mode of action of avian myeloblastosis virus polymerase. J Virol. 1976 Sep;19(3):932–939. doi: 10.1128/jvi.19.3.932-939.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
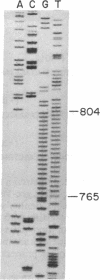
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Carrino D. A. Dibutyryl cyclic AMP decreases glutamine synthetase in cultured 3T3-L1 adipocytes. J Biol Chem. 1980 Jun 10;255(11):5490–5500. [PubMed] [Google Scholar]
- Miller R. E., Hackenberg R., Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Pope S. R., DeWille J. W., Burns D. M. Insulin decreases and hydrocortisone increases the synthesis of glutamine synthetase in cultured 3T3-L1 adipocytes. J Biol Chem. 1983 May 10;258(9):5405–5413. [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. G., Wilson R. H. Amplification and cloning of the Chinese hamster glutamine synthetase gene. EMBO J. 1984 Jan;3(1):65–71. doi: 10.1002/j.1460-2075.1984.tb01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]