Abstract
The extracellular signal-regulated kinases 1/2 (ERK1/2) are serine/threonine-selective protein kinases involved in proliferation and differentiation of cells, including thymocytes. The requirement of ERK1/2 for thymocyte differentiation and maturation has been well established; however, their role in regulating thymocyte survival and apoptosis has not been resolved.
Here, we asked whether ERK1/2 affected thymocyte survival in vitro in response to apoptotic stimuli. The results show that phorbol 12-myristate 13-acetate (PMA) treatment (with or without ionomycin) and serum starvation (s/s) induced sustained ERK1/2 activation in murine thymocytes. Importantly, pharmacological treatment of thymocytes with the MEK inhibitor UO126 revealed that PMA-induced ERK1/2 activation was pro-apoptotic, whereas serum starvation-induced ERK1/2 activation inhibited apoptosis and promoted cell survival. While basal MEK activity was required for both s/s and PMA-induced ERK1/2 activation, MEK activity increased only in response to PMA. The results show that the suppression of ERK1/2 phosphatases was responsible for s/s-induced sustained ERK1/2 activation. Unexpectedly, neither s/s-induced pro-apoptotic nor PMA-induced anti-apoptotic functions of ERK1/2 depended on the Bcl-2 family phosphoprotein BimEL, which was previously implicated in thymocyte apoptosis. Lastly, etoposide treatment of immature thymocytes induced both p53 and ERK1/2 activation, but ERK1/2 activity did not affect the phosphorylation and stabilization of p53. Thus, ERK1/2 has a dual role in promoting cell survival and cell death in thymocytes in the context of different stimuli.
Keywords: Thymocyte, apoptosis, rodent, ERK, MAPK
Introduction
ERK1 and 2 are well known to be involved in cell survival (1, 2). However, recent evidence suggests that activation of ERK1/2 can promote apoptosis as well (3). The cell type, the nature of the stimulus, and the duration of ERK1/2 activation may therefore determine its proapoptotic or prosurvival function.
ERKs are expressed in all nucleated mammalian cells and their role in thymocytes has been extensively studied. ERK1/2 signaling has been shown to be critical for thymocyte maturation and development. ERK1/2 regulate positive and negative selection of thymocytes, as well as their lineage commitment (4, 5, 6, 7), with negative selection and lineage commitment realized through ERK-dependent apoptosis. Apoptotic cell death of thymocytes is induced in response to a variety of stimuli and it is fundamental to the maintenance of self-tolerance (8, 9, 10).
In mature T cells, ERK1/2 are primarily thought to promote cell survival, differentiation, and proliferation, but a pro-apoptotic role for ERKs has also been described. Studies with Jurkat cells showed that ERK1/2 activation can abrogate signals from death receptors and promote cell survival by suppressing the caspase effector machinery (11, 12, 13). It was shown also that ERK1/2 has a dominant protective effect over apoptotic signaling from the glucocorticoid receptor in immortalized T cell and thymocytes lines, and in primary T cells (14). However, there is evidence that ERK-mediated signals are required for activation–induced cell death (AICD) of T cells (15).
Bcl-2 family members are major components of the apoptotic mechanism. The Bcl-2 family consists of two classes of molecules: anti-apoptotic family members, that protect cells from apoptosis, and pro-apoptotic family members that trigger or sensitize for apoptosis. Bim, the pro-apoptotic member of the Bcl-2 family, appears to have an important role for many hematopoietic cell types (16, 17). In this respect, deletion of Bim indicates that it plays a major role in the apoptotic response of thymocytes and T cells (16). Indeed, Bim knockout mice exhibit defects in the deletion of autoreactive thymocytes and in the response to stress (17). Three Bim proteins, namely Bims, BimL and BimEL are synthesized from the same transcript (18). BimEL is a phosphoprotein and it was shown that activation of the ERK1/2 pathway promotes BimEL phosphorylation on serine 69 in humans (65 in mouse), thereby targeting it for ubiquitination and degradation by the proteasome (19). Surprisingly, phosphorylation of serine 69 has been found to both increase and decrease the proapoptotic activity of BimEL (19, 20). This suggests that the phosphorylation of BimEL can have different effects depending on the context. Previously, it was shown that apoptosis of thymocytes could be p53-dependent and p53 independent (21, 22). In general, p53-dependent apoptosis is connected to DNA damage. It is also known that DNA damage can lead to ERK1/2 activation (23). ERK1/2 activation was observed in response to different modes of DNA damage in different cell lines (24). Furthermore, ERK1/2 was shown to phosphorylate p53 (23). Meanwhile, Tang and colleagues showed that ERK1/2 activation is independent from p53 in inducing DNA-damage apoptosis and cell cycle arrest (24).
In the present study, we report that serum starvation and PMA treatment induced sustained ERK1/2 activation in thymocytes in vitro. ERK1/2 activation was proapoptotic in PMA stimulated cells, whereas it promoted survival in serum starved cells. Thus, ERK1/2 has a dual role in promoting cell survival and cell death in thymocytes in the context of different stimuli.
Materials and Methods
Mice and preparation of thymocytes
C57BL/6 mice (6 – 10 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions and all animal procedures were conducted according to guidelines of the Institutional Care and Use Committee (IACUC) at the University of Texas at San Antonio. Thymocytes were prepared by excising thymuses from the mice and disrupt the tissue between plastic slides to obtain single cell suspensions. Cells were suspended in RPMI 1640 medium supplemented with 10% FCS, 0.2mM L-glutamine and 50μg/ml Gentamicin. The cell suspensions were then filtered through nylon cell strainers to remove debris and plated at a concentration of 5×106 cells per well on 24 wells plates.
Reagents
The following reagents were used for inhibition and apoptosis assays: UO126 (Cell Signaling Technology, Beverly, MA), etoposide (MP Biomedicals. Inc), Sodium orthovanadate, PMA and ionomycin from Sigma-Aldrich, St.Louis, MO.
Fluorescence staining and flow cytometry assay
An apoptosis detection kit (BD Biosciences, San Jose, CA) was used to determine apoptotic cells. Cells were exposed to treatment as indicated in the experiments and were stained with Annexin V-FITC and propidium iodide following the manufacturer's instructions and analyzed by flow cytometry on a BD Biosciences LSR II or FACSAria using BD FACS Diva software.
Statistical analysis
Student's t-test was used to compare mean values where appropriate using SigmaStat 3.5 software. P values <0.05 were considered significant. All data are expressed as means ±SD for a series of experiments.
DNA fragmentation assay
Detection of DNA fragmentation into oligonucleosomal DNA fragments by agarose gel electrophoresis was performed according to the manufacturer's instructions (BioVision, Mountain View, CA).
Western blot analysis
For Western blot analysis, cells or tissues were rinsed in ice-cold PBS twice and lysed in cell lysis buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Nonidet P-40, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 50 mM NaF, 2 mM Na3 VO4, 2 mM EGTA, 2 mM EDTA, and 0.25 mM PMSF) for 30 min. Samples were sonicated for 30 s and centrifuged for 20 min at 12,000 × g at 4°C. Samples were electrophoresed on a 7–12% SDS-polyacrylamide gel and electrophoretically blotted on nitrocellulose membrane. Membranes were blocked in TBS/Tween-20 with 5% milk and incubated with primary Abs diluted in TBS/Tween-20/BSA overnight at 4°C. The following primary antibodies were used (all from Cell Signaling Technology, Beverly, MA): phospho-ERK1/2 (# 9101), ERK1/2 (#4695), phospho-MEK1/2 (#9121), MEK1/2 (#9122), phospho-p53 (# 9284), p53 (# 9211), Bim (#2819) and MKP-1 (M-18), MKP-2 (S-18) from Santa Cruz biotechnology, Ca. All blots were incubated with secondary Abs conjugated to HRP (1/2000; Amersham Biosciences, Piscataway, NJ) and developed using the ECL method (Pierce, Rockford, IL). Protein concentration was determined using bicinchoninic acid assay (Pierce, Rockford, IL).
Results
Serum starvation and PMA treatment promote apoptosis in thymocytes
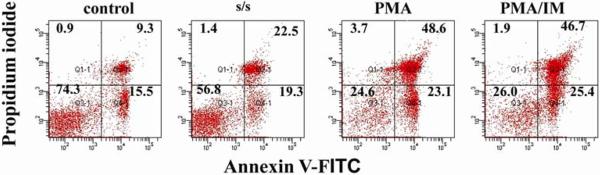
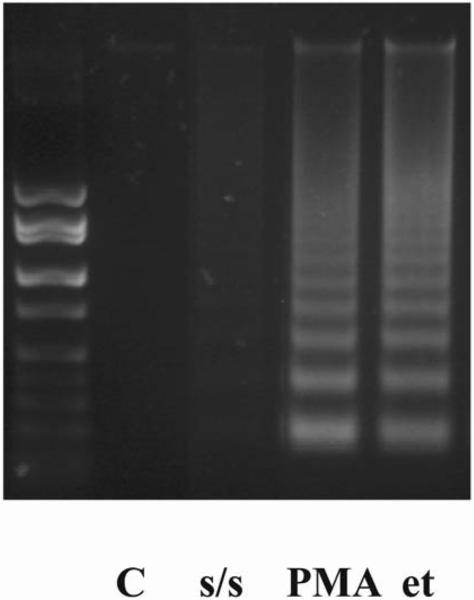
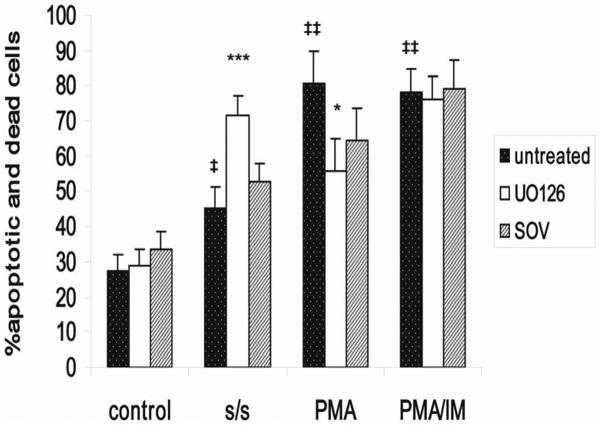
To determine whether s/s and PMA treatment influenced the survival of thymocytes in vitro we analyzed the characteristic signs of apoptosis, i.e. phosphatidylserine expression on the cell membrane by Annexin V staining in combination with propidium iodide (PI), as well as DNA fragmentation using DNA laddering. The results show that both PMA and s/s substantially increased apoptosis, but that cell death was more pronounced with PMA. The percentage of Annexin V and PI stained cells reached 48.6 % after 4 hours of PMA treatment and 22.5 % after 24 hours of s/s treatment in comparison with 9.3% in the control (Fig. 1). DNA fragmentation was already much more pronounced after 4 hours of PMA treatment as compared with s/s (Fig. 2).
Figure 1. Serum starvation, PMA and PMA/IM promote thymocyte apoptosis.
Apoptosis of thymocytes was determined after PMA, PMA+ ionomycin and serum starvation treatment. Cells were kept in the presence of PMA (1μM) with or without ionomycin (0.5μM) for 4 hours and serum starved for 24 hours. After the different treatments cells were stained with Annexin V-FITC and propidium iodide following the manufactures instructions and analyzed by flow cytometry on a BD LSR II or FACSAria using BD FACS Diva software (BD Bioscience). Data represent one of five different experiments with similar results. Cells exposed to vehicle (DMSO) did not differ from untreated cells.
Figure 2. DNA fragmentation in thymocytes after PMA, serum starvation and etoposide treatment.
Cells were kept under different conditions (1 μM PMA or 100 μM etoposide), including serum starvation, for 4 hours. DNA fragmentation was promoted in PMA and etoposide treated thymocytes in comparison with serum starved cells. Cells exposed to vehicle (DMSO) did not differ from untreated cells.
Serum starvation and PMA treatment induce sustained ERK1/2 activation
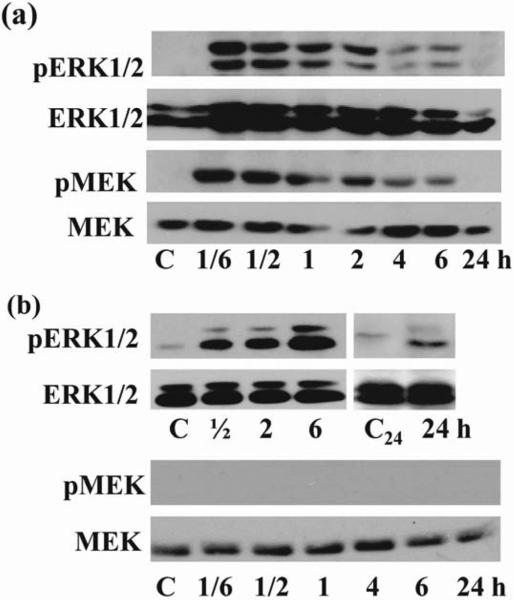
To begin to address the role of ERK1/2 in PMA and s/s induced thymocyte apoptosis we determined the kinetics of ERK activation. An elevation of phosphorylated ERK1/2 was observed as early as 10 minutes after PMA treatment (Fig. 3a) and 30 minutes after serum withdrawal (Fig. 3b). ERK1/2 remained increased up to 6 hours of PMA treatment (Fig. 3a) and for 24 hours upon serum starvation (Fig. 3b). The earlier decrease in phosphorylated ERK1/2 in PMA treated thymocytes as compared with s/s could be due to the cells undergoing apoptosis, consistent with the substantial DNA fragmentation observed already after 4 hours in PMA treated cells (Fig. 2). Interestingly, PMA induced more pERK1, whereas serum starvation favored induction of pERK2. In contrast to the activation of ERK1/2, we did not observe activation of the MAPK p38 and JNK (data not shown). The addition of ionomycin to PMA had no significant effect on the kinetics of ERK1/2 activation (data not shown).
Figure 3. Kinetic of ERK1/2 and MEK1/2 activation after PMA treatment and after serum starvation.
(a) Addition of PMA (1 μM) induced rapid and sustained ERK1/2 activation for up to 6 hours that correlated with MEK activation. Thymocytes exposed to vehicle (DMSO) were used as control. (b). Serum withdrawal induced rapid and sustained ERK1/2 activation for up to 24 hours independent of MEK activation. As control, thymocytes incubated 6(C) or 24(C24 ) hours in complete medium were used. Western blots of thymocytes from mice C57BL/6 were probed with anti-phospho-ERK1/2 Ab and anti-phospho-MEK Ab. Blots were probed with anti-ERK1/2 Ab and anti-MEK Ab to control equal loading of protein. The representative blots from 3 experiments are shown in each (a), (b).
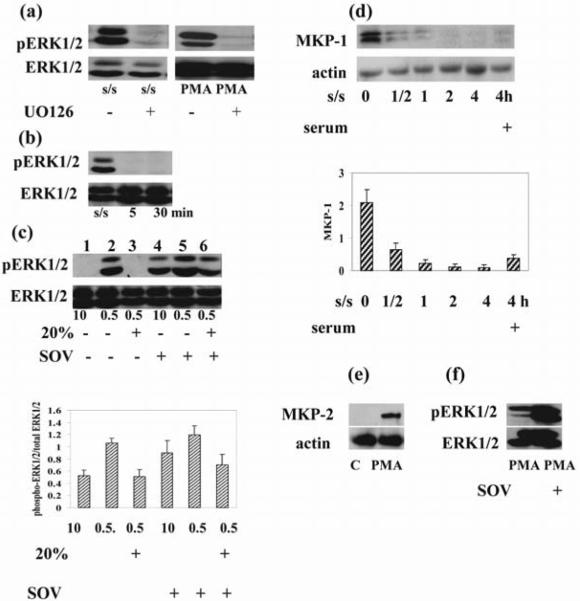
The enzymatic activity of ERK isoforms is positively regulated by the protein kinases MEK1/MEK2 (25) and negatively regulated by phosphatases (26). Therefore, we attempted to evaluate the respective contribution of these mechanisms to the regulation of ERK1/2. First, we investigated the role of MEK in PMA and s/s induced ERK activation. As shown in Fig. 3 a & b, PMA stimulation induced phosphorylation of MEK, whereas s/s did not show a substantial increase in pMEK. While we could not detect significant pMEK in these studies, this could be due to the lower levels of MEK protein after s/s as compared with PMA stimulation. Importantly, pretreatment of thymocytes with 10μM UO126, a specific MEK inhibitor, effectively blocked both PMA and s/s stimulated ERK activity (Fig. 4a), supporting a role for MEK in ERK activation in both models. Interestingly, the addition of serum to serum starved cells also neutralized the ERK1/2 activity (Fig. 4b). Thus, an alternative, but not mutually exclusive explanation for our results was that growth factors contained in serum induced the activation of phosphatases. MAP kinase phosphatases are encoded by immediate early genes, so the effect of addition of serum would be expected immediately, consistent with our results (Fig. 4b). This view is further supported by the results received with sodium orthovanadate, a broad-acting protein tyrosine phosphatase inhibitor. Pretreatment of cells with sodium orthovanadate resulted in enhanced basal activity of ERK in the presence of 10% serum (Fig. 4c, lane 4 versus lane 1), and in the inhibition of ERK downregulation induced by addition of 20% serum (Fig. 4c, lane 6 versus lane 3). We conclude that suppression of MAP phosphatases at low serum concentrations (i.e. serum starvation) could account for the observed activation of ERK1/2. Along these lines, we found that the phosphatase MKP-1 disappeared as early as 30 minutes after withdrawal of serum and became detectable after adding serum (Fig. 4d). This observation suggested that down-regulation of MKP-1 contributed to s/s-induced ERK activation. In contrast, it is known that PMA induces the activation of MKP-2, which is a phosphatase that dephosphorylates and thereby neutralizes ERK activity (27), as supported by the enhanced activation of MKP-2 by PMA in our studies (Fig. 4e). Consistent with this view is the observed increase in PMA-induced ERK activation upon treatment with sodium orthovanadate (Fig. 4f). However, despite the MKP-2 activation, the PMA-induced balance between phosphorylation and dephosphorylation of kinases ultimately resulted in sustained (6 hours) duration of ERK1/2 activation in thymocytes (Fig.3a).
Figure 4. Pharmacological inhibition of MEK1/2 and phosphatases result in opposite outcomes.
(a) Thymocytes were cultured in medium in the presence or absence of UO126 (10 μM) for 1 hour and exposed to PMA (1 μM) for 30 minutes. Thymocytes were serum-starved for 6 hours in the presence or absence of UO126 (10 μM). (b) Addition of 20% serum to serum-starved (24 hours) cells suppressed ERK1/2 activation at 5 and 30 minutes. (c). Thymocytes were cultured in the presence or absence of sodium orthovanadate in medium with 0.5%, 10% serum and in medium with 0.5% stimulated with 20% serum. Addition of sodium orthovanadate to cells cultured in 10% serum as well as to serum starved cells after serum stimulation increased ERK1/2 activation. Densitometric quantification from (c) is shown. The phospho ERK1/2 bands were densitized and normalized to ERK1/2 immunoblotted from the same membrane to demonstrate the phosphorylation levels of ERKs. (d) Serum withdrawal suppressed, but serum addition activated MKP-1 as early as 30 minutes after its addition. Actin was used as a control for equal loading of protein. The histogram shows the MKP-1expression level relative to actin by densitometry. (e) PMA (1 μM) treatment induced MKP-2 activation. Actin was used as a control for equal loading of protein. (f) Thymocytes were cultured in medium in the presence or absence of sodium orthovanadate (100 μM) for 1 hour and exposed to PMA (1 μM) for 30 minutes. Sodium orthovanadate pretreatment increased ERK1/2 activation. Total and phosphorylated ERK1/2 as well as MKP-1, MKP-2 and actin was determined by immunoblotting. Representative blots from 3 experiments are shown in each (a)–(f).
Altogether, these results indicate that while basal MEK activity is required for both s/s and PMA-triggered ERK activity, MEK activity increased substantially only in response to PMA in thymocytes. In contrast, the suppression of phosphatases seemed primarily responsible for s/s-induced sustained ERK1/2 activation.
Different roles of ERK1/2 activation in s/s and PMA-induced apoptosis
To study how activation of ERK affects s/s and PMA-mediated apoptosis we treated thymocytes with the MEK-specific inhibitor UO126. Unexpectedly, incubation with UO126 reduced the amount of apoptotic thymocytes by 15 % for PMA, and increased the percent apoptotic cells by 31 % for s/s (Fig. 5). Analysis of early (Annexin V+, PI−) versus late apoptotic events (Annexin V+ PI+) showed that UO126 treatment was more effective in decreasing late apoptotic events (not shown). Overall, these findings suggested that ERK1/2 had two opposing effects: a proapoptotic function for PMA and an antiapoptotic effect for s/s. Sodium orthovanadate also differentially affected PMA and s/s induced apoptosis. In the presence of the phosphatase inhibitor the amount of dead cells after PMA treatment decreased, whereas the SOV treatment increased cell death for serum starved cells, albeit not as prominently as UO126 (Figs. 2 & 5). PMA/IM treated cells appeared less sensitive to UO126 and SOV treatment in comparison with only PMA treated cells (Fig. 5).
Figure 5. Role of s/s and PMA-induced ERK activation for apoptosis.
Apoptosis of thymocytes was determined after PMA, PMA + ionomycin and serum starvation treatment in the presence or absence of UO126 or sodium orthovanadate. UO126 (10μM) and sodium orthovanadate (100μM) were added for 1 hour before adding PMA (1μM) and PMA (1μM) +ionomycin (0.5μM) and simultaneously with serum withdrawal. Cells were kept in the presence of PMA or PMA + ionomycin for 4 hours and serum starved for 24 hours. Cells after different treatments were stained with Annexin V-FITC and propidium iodide following the manufactures instructions and analyzed by flow cytometry on a LSR II or FACSAria using BD FACS Diva software (BD Biosciences). Data are expressed as % of Annexin V-FITC + and PI -negative cells (early state of apoptosis) + % of Annexin V-FITC + and PI-positive cells (late state of apoptosis and necrosis). Cells exposed to vehicle (DMSO) did not differ from untreated cells. Shown are the means and SD of four independent experiments. Symbol ‡ denotes a value that differs significantly from the corresponding value in control cells. ‡P < 0.05; ‡‡P < 0.001 by Student's t-test. Asterisks denote responses to UO126 that differ significantly from those in cells treated only with PMA or s/s. *P < 0.05 and ***P <0.001 by Student's t-test.
The pro-apoptotic and anti-apoptotic functions of ERK1/2 do not involve BimEL
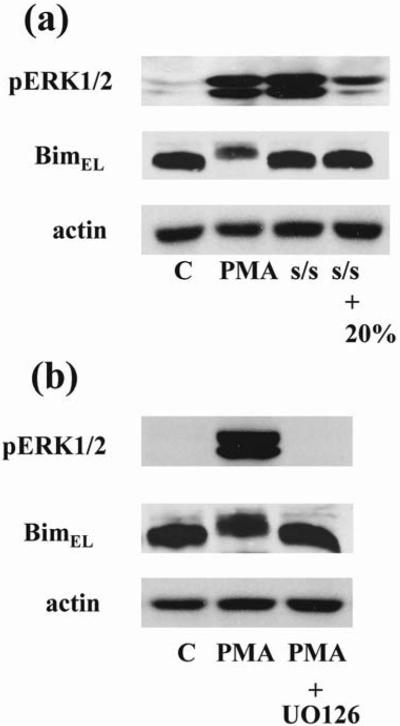
Members of the Bcl-2 protein family are essential regulators of apoptosis. Initial studies in fibroblasts showed that ERK1/2 can phosphorylate BimEL, one of the pro-apoptotic members of Bcl-2 family, thereby targeting it for ubiquitination and degradation (28). Proteasome-dependent turnover of BimEL has been confirmed in other cell types, in particular in thymocytes (19). Therefore we determined the phosphorylation status of BimEL in thymocytes after PMA and serum withdrawal treatment. Contrary to our expectations of a prominent role for BimEL, PMA-induced pro-apoptotic ERK activation resulted in a marked mobility shift and reduced the protein level of BimEL, consistent with its phosphorylation and subsequent degradation (Fig. 6a). The fact that the MEK inhibitor UO126 prevented the mobility shift indicated phosphorylation and subsequent BimEL degradation and supported that ERK1/2 activity mediated this effect (Fig. 6b). At the same time serum starvation-induced pro-survival ERK activation in thymocytes did not lead to BimEL phosphorylation and degradation and the phosphorylation status of BimEL did not change after addition of serum (Fig. 6a).
Figure 6. Phosphorylation of BimEL by ERK activated by PMA treatment but not by s/s.
(a) Thymocytes were treated with PMA (1μM) for 30 minutes or serum-starved for 24 hours. Serum-starved cells were left untreated or stimulated with serum. (b) A one hour pretreatment with UO126 (10 μM) prevented the PMA-induced mobility shift of BimEL indicative of its phosphorylation. Cell lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Actin was used for control equal loading of protein. The representative blots from 3 experiments are shown in each (a) and (b).
Taken together, the results show that ERK1/2 activation induced by PMA and serum starvation exerted different effects on the activity of BimEL.
DNA-damage induces proapoptotic ERK1/2 activation in thymocytes
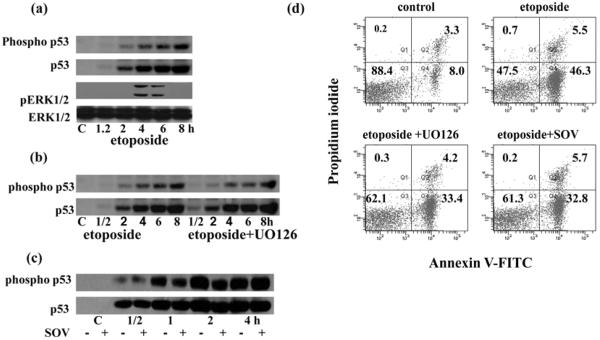
To study whether DNA damage affects ERK activation and how this activation influences apoptosis, we treated thymocytes with etoposide, an inhibitor of the enzyme topoisomerase II. Treatment with etoposide results in DNA strand breaks and leads to cell death. Because p53 is a major effector in the DNA damage response it seemed logical to determine the relation between p53 and ERK activation after DNA damage. Evidently, etoposide induced the phosphorylation and stabilization of p53 (Fig. 7a). At the same time, etoposide resulted in the activation of ERK1/2 (Fig. 7a). The kinetic of these two processes did not coincide. ERK activation lagged behind phosphorylation and corresponding stabilization of p53 (Fig. 7a). Pre-treatment of thymocytes with UO126 did not influence p53 stabilization (Fig. 7b), but suppressed etoposide-induced apoptosis by 30% (Fig.7d). Sodium orthovanadate also significantly increased the survival of etoposide treated cells (Fig. 2, 7d), but like UO126, did not affect the accumulation and phosphorylation of Ser15 of p53 (Fig. 7c).
Figure 7. DNA damage induced both p53 and ERK1/2 activation as well as apoptosis.
(a) Thymocytes were treated with 100μM etoposide. Total and phosphorylated p53 and total and phosphorylated ERK1/2 were determined by immunoblotting. Phosphorylation and stabilization of p53 was also determined in etoposide treated thymocytes with or without 1 hour UO126 (10μM) pretreatment (b) and with or without 1 hour sodium orthovanadate (100μM) pretreatment (c). Representative blots from three experiments are shown in each (a), (b) and (c).
(d) Apoptosis of thymocytes was determined after etoposide treatment in the presence or absence of UO126 or sodium orthovanadate. UO126 (10μM) and sodium orthovanadate (100μM) were added for 1 hour before addition of etoposide (100 μM). Cells were maintained in the presence of etoposide for 4 hours. Cells were stained after the different treatments with Annexin V-FITC and propidium iodide following the manufactures instructions and analyzed by flow cytometry on a LSR II or FACSAria using BD FACS Diva software (BD Bioscience). Data represent one of five different experiments with similar results.
Collectively, the results suggest that in vitro DNA damage-induced apoptosis in thymocytes is mediated via both the p53 and MEK-ERK1/2 pathways, but that p53 is independently regulated of the MEK-ERK1/2 pathway.
Discussion
The focus of this study was to explore how the activation of ERK1/2 affects apoptosis induced by different pro-apoptotic stimuli in immature thymocytes. Apoptosis of murine thymocytes was assayed after serum withdrawal, treatment with PMA, PMA with ionomycin, and treatment with etoposide.
It is known that ERK1/2 can have pro-survival and pro-apoptotic functions in cells depending on the context and stimulus (1, 2, 3). Our results show that this also applied to ERK in the context of apoptosis of murine thymocytes in vitro. We show that PMA alone or in combination with ionomycin, as well as serum starvation, induced sustained activation of ERK1/2. However, ERK activation of thymocytes resulted in different outcomes and was pro-apoptotic for PMA, but inhibited apoptosis in the context of serum starvation.
Because of the requirement for self-tolerance, immature thymocytes are programmed to die in response to a variety of stimuli (9, 10, 29). However, the same stimuli do not necessarily induce cell death of mature T cells, as is for example the case for PMA, a well known T cell mitogen (30). Various studies have suggested that PMA activates the Raf/MEK/ERK pathway in many cell lines (19, 31). In some cells, such as Jurkat T lymphocytes, PMA-induced ERK activation protects cells from apoptosis (31). However, PMA stimulation of immature thymocytes promotes apoptosis, as was shown (32) and confirmed by our data. Recently it was shown using different cell types that PMA-mediated activation of ERK1/2 can promote the phosphorylation and subsequent degradation of the pro-apoptotic molecule BimEL (19). Thus, it was thought that the pro-survival function of ERK was due to BimEL degradation (19). We also observed substantial degradation of BimEL in immature thymocytes after PMA treatment. However, UO126-mediated suppression of ERK1/2, as well as the corresponding suppression of BimEL degradation did not enhance cell death in our model in thymocytes. To the contrary, ERK1/2 suppression promoted the survival of PMA-treated thymocytes. The phosphorylation of serine 69 in human BimEL (serine 65 in mouse) by ERK1/2 can have dual function and either increase or decrease the pro-apoptotic activity of BimEL (18, 19). It was shown that Bim-deficient thymocytes were essentially as sensitive as Wt cells to some apoptotic stimuli, in particular to PMA treatment (33). Our results with murine thymocytes support this view of a context dependent effect of BimEL phosphorylation on apoptosis. In addition, PMA-induced apoptosis may be mediated via other, still unknown, but ERK-dependent mechanisms. Furthermore, additional signals, including pro-survival costimulatory signals may allow mature T cells to avoid apoptosis. A combination of ionomycin and PMA has been reported to mimic some signals required for positive selection (36) as well as have anti-apoptotic effects in thymocytes (35). While we did not observe enhanced survival mediated by ionomycin in our studies, this could be due to differences in experimental conditions in our hands. We propose that the higher dose of PMA and ionomycin used in our experiments could explain why pretreatment with UO126 as well as with SOV did not have a substantial effect on the survival of PMA/IM treated thymocytes, whereas the same treatment enhanced the survival of PMA treated cells in the absence of ionomycin.
It is known that PMA activates ERK1/2 along with the phosphatase MKP-2, which subsequently serves to inactivate ERK (27). MKP-2 activation limits the duration of ERK activation; however the balance between ERK1/2 kinases and MKP-2 resulted in a prolonged activation of ERK1/2 in our studies.
Serum starvation has been shown to affect ERK activity. In a number of cell lines, serum withdrawal resulted in decreased ERK activity (36), whereas in some circumstances ERK activity was enhanced (37). Our studies with thymocytes showed that serum starvation resulted in substantially enhanced ERK1/2 kinase activity. Furthermore, our results show that the s/s induced increase in ERK1/2 kinase activity contributed to thymocyte survival as evidenced by enhanced cell death in the presence of MEK inhibitors.
Because of the role of BimEL for thymocyte apoptosis we expected that the prosurvival function of ERK1/2 in our experiments could be mediated via the phosphorylation and degradation of this protein. However, we did not detect changes in mobility shift and protein levels of BimEL in serum starved cells or upon addition of 20% serum. This observation suggested that the pro-survival activity of ERK1/2 in serum starved thymocytes did not depend on BimEL inactivation.
It is known that the kinetics and duration of ERK activation may play an important role in its effects on cell fate (38). It has been suggested that prolonged ERK activation results in pro-apoptotic activity (39), whereas transient ERK activation protects cells from death (40). Nevertheless a pro-survival function of sustained ERK activation has also been reported (41). Our experiments show that PMA treatment and serum starvation induced apoptosis and sustained ERK activation in immature thymocytes. PMA treatment resulted in fast and extensive cell death, whereas cell death induced by serum starvation was more protracted. PMA-induced ERK activation was mediated by MEK1/2 and was detected for up to 6 hours after treatment. In contrast, the prolonged ERK activation observed after serum starvation was due to down-regulation of MAP kinase phosphatases, such as MKP-1. It is not inconceivable that pERK1/2 decreased as cells died. The different character of cell death could explain the difference in duration of PMA and s/s-induced apoptosis. Nevertheless, the net outcome of both PMA and s/s treatment resulted in sustained ERK activation. Thus, it is noteworthy that PMA-induced ERK activation promoted cell death, whereas serum starvation-induced ERK activation inhibited cell death. The mechanism underlying the differential effect of PMA- or s/s-induced ERK activation on cell survival is currently unresolved. Conceivably, the proliferative (PMA) versus anti-proliferative (s/s) character of the stimuli determined the outcome in the face of sustained ERK activation. Irrespective, the data show that sustained ERK activation can have different functional outcomes in thymocytes.
An unexpected observation in our studies was that sodium orthovanadate treatment in combination with PMA decreased apoptosis, whereas it enhanced cell death in combination with serum starvation. This is in contrast to what would be expected since sodium orthovanadate is a phosphatase inhibitor and increases ERK activity and signal duration, and treatment of thymocytes with this inhibitor should therefore have enhanced the effects of ERK in this model, i.e. promote PMA-induced apoptosis and s/s-mediated ERK-dependent cell survival. The interpretation of sodium orthovanadate treatment is difficult, however, since it not only inhibits phosphatase activity, but it can also affect other cellular processes. Consistent with our results, Morita and colleagues found that sodium orthovanadate inhibits apoptosis in T lymphoblast (42). In their studies, inhibition of apoptosis was mediated via the suppression of caspase activation, as well as alteration of mitochondrial membrane potential and conformational changes of Bax and p53 transactivation. Nevertheless, investigating the role of sodium orthovanadate on ERK-mediated apoptosis and survival of thymocytes may provide a valuable tool for the understanding of the underlying molecular pathways.
The role of ERK signaling in response to DNA damage is not well understood. For example, ERK signaling does not appear to play a role in radiation-induced DNA damage in a number of cell lines (43). However ERK activation has been shown following cisplatin treatment of ovarian cancer cells (23). ERK activation was also shown in response to DNA damage induced by treatment with etoposide (24). Along these lines, it is known that ERK1/2 can phosphorylate p53 (23). Furthermore, ERK1/2 activation can contribute to cell cycle arrest and apoptosis independently of p53 (24). In addition, data from several groups suggested a dual-function positive and negative nature of ERK signaling in the control of cell survival in response to different DNA-damaging agents (43).
Our studies in thymocytes showed that etoposide treatment induced ERK1/2 activation. Inhibition of etoposide-induced ERK activation by UO126 decreased thymocyte apoptosis, which was independent of the phosphorylation and stabilization of p53. The latter observation strongly suggested that p53 stabilization does not depend on ERK activation, whereas apoptosis does. This interpretation is consistent with previous studies that thymocytes can undergo apoptosis by p53 dependent and p53 independent pathways (21, 22). Thus, our data show that etoposide-induced cell death is dependent on ERK and p53, but independent of the phosphorylation of p53 by ERK. It is currently unresolved whether ERK activation in this context is p53 dependent. Consistent with this view, inhibition of p53 transactivation by sodium orthovanadate also inhibited apoptosis, but it remains unresolved whether this was dependent on ERK1/2.
Finally, our studies were conducted on unfractionated thymocyte populations composed of CD4+CD8+ double positive immature thymocytes and single positive thymocytes, and we cannot rule out that this may have affected the interpretation of our results. We expect that future studies with isolated double positive thymocytes may provide additional information to help understand the role of ERKs in thymocytes.
In conclusion, our studies showed the dual function of ERK1/2 in promoting PMA and etoposide mediated apoptosis, and, on the other hand, inhibiting serum starvation induced cell death of immature thymocytes. The PMA-induced pro-apoptotic and s/s-induced anti-apoptotic functions were paralleled by prolonged ERK activation and were independent of BimEL.
Acknowledgments
We thank Cara Westbrook and Robert Day for excellent technical assistance.
This work was supported by grant NS-52177 from the National Institute of Health, and grants RG3499 and RG3701 from the National Multiple Sclerosis Society (T.G.F.).
Abbreviations
- s/s
serum starvation
- PMA
phorbol 12-myristate 13-acetate
- IM
ionomycin
- SOV
sodium orthovanadate
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr125 by ERK MAPK. Nat. Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 2.Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouyssegur J, Van Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol. Biol. Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- 4.Alberola-Ila J, Hernández-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Bommhardt U, Scheuring Y, Bickel C, Zamoyska R, Hünig T. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J. Immunol. 2000;164:2326–2337. doi: 10.4049/jimmunol.164.5.2326. [DOI] [PubMed] [Google Scholar]
- 6.Adachi S, Iwata M. Duration of calcineurin and Erk signals regulates CD4/CD8 lineage commitment of thymocytes. Cell Immunol. 2002;215:45–53. doi: 10.1016/s0008-8749(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca (2+)-ATPase inhibitor thapsigargin. Exp. Cell Res. 1994;212:84–92. doi: 10.1006/excr.1994.1121. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J. Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 10.Cohen GM, Sun XM, Snowden RT, Ormerod MG, Dinsdale D. Identification of a transitional preapoptotic population of thymocytes. J. Immunol. 1993;151:566–574. [PubMed] [Google Scholar]
- 11.Holmström TH, Schmitz I, Söderström TS, Poukkula M, Johnson VL, Chow SC, Krammer PH, Eriksson JE. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrant M, Luciano F, Loubat A, Auberger P. The protective effect of phorbol esters on Fas-mediated apoptosis in T cells. Transcriptional and postranscriptional regulation. Oncogene. 2002;21:4957–4968. doi: 10.1038/sj.onc.1205689. [DOI] [PubMed] [Google Scholar]
- 13.Engedal N, Blomhoff HK. Combined action of ERK and NF kappa B mediates the protective effect of phorbol ester on Fas-induced apoptosis in Jurkat cells. J. Biol. Chem. 2003;278:10934–10941. doi: 10.1074/jbc.M211556200. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson CAM, Yamamoto KR. Crosstalk pathway for inhibition of glucocorticoid-induced apoptosis by T cell receptor signaling. Proc. Natl. Acad. Sci. USA. 2000;97:7319–7324. doi: 10.1073/pnas.97.13.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Brink MR, Kapeller R, Pratt JC, Chang JH JH, Burakoff SJ. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J. Biol. Chem. 1999;274:11178–11185. doi: 10.1074/jbc.274.16.11178. [DOI] [PubMed] [Google Scholar]
- 16.Strasser A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 17.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 20.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 21.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–52. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane M, Jones NA, Dive C, Cohen GM. DNA-damaging agents induce both p53-dependent and p53-independent apoptosis in immature thymocytes. Mol. Pharmacol. 1996;50:900–911. [PubMed] [Google Scholar]
- 23.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J. Biol. Chem. 2000;275:35778–35785. doi: 10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 24.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. Erk activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 25.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim. Biophys. Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Choy M, Jo M, Roberson MS. Structural organization of the rat mitogen-activated protein kinase phosphatase 2 gene. Gene. 2001;273:71–79. doi: 10.1016/s0378-1119(01)00574-1. [DOI] [PubMed] [Google Scholar]
- 28.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca (2+)-ATPase inhibitor thapsigargin. Exp. Cell Res. 1994;212:84–92. doi: 10.1006/excr.1994.1121. [DOI] [PubMed] [Google Scholar]
- 30.Smith Ch.A., Williams GT, Kingstone R, Jenkinson EJ, Owen JJT. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 31.Takagi Y, Du J, Ma XY, Nakashima I, Nagase F. Phorbol 12-myristate 13-acetate protects Jurkat cells from methylglyoxal-induced apoptosis by preventing c-Jun N-terminal kinase-mediated leakage of cytochrome c in an extracellular signal-regulated kinase-dependent manner. Mol. Pharmacol. 2004;65:778–787. doi: 10.1124/mol.65.3.778. [DOI] [PubMed] [Google Scholar]
- 32.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, Okumura K. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur. J. Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 33.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 34.Ohoka Y, Kuwata T, Tozawa Y, Zhao Y, Mukai M, Motegi Y, Suzuki R, Yokoyama M, Iwata M. In vitro differentiation and commitment of CD4+ CD8+ thymocytes to the CD4 lineage, without TCR engagement. Int. Immunol. 1996;8:297–306. doi: 10.1093/intimm/8.3.297. [DOI] [PubMed] [Google Scholar]
- 35.Asada A, Zhao Y, Kondo S, Iwata M. Induction of thymocyte apoptosis by Ca2+-independent protein kinase C (nPKC) activation and its regulation by calcineurin activation. J. Biol. Chem. 1998;273:28392–28398. doi: 10.1074/jbc.273.43.28392. [DOI] [PubMed] [Google Scholar]
- 36.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J. Biol. Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 37.Sinha D, Bannergee S, Schwartz JH, Lieberthal W, Levine JS. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. J. Biol. Chem. 2004;279:10962–10972. doi: 10.1074/jbc.M312048200. [DOI] [PubMed] [Google Scholar]
- 38.Ebisuy M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J. Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 39.Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, Kim JM. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Appl. Toxicol. 2005;25:374–382. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
- 40.Al-Ayoubi A, Tarcsafalvi A, Zheng H, Sakati W, Eblen ST. ERK activation and nuclear signaling induced by the loss of cell/matrix adhesion stimulates anchorage-independent growth of ovarian cancer cells. J. Cell Biochem. 2008;105:875–884. doi: 10.1002/jcb.21889. [DOI] [PubMed] [Google Scholar]
- 41.Nyunoya T, Monick MM, Powers LS, Yarovinsky TO, Hunninghake GW. Macrophages survive hyperoxia via prolonged ERK activation due to phosphatase down-regulation. J. Biol. Chem. 2005;280:26295–26302. doi: 10.1074/jbc.M500185200. [DOI] [PubMed] [Google Scholar]
- 42.Morita A, Zhu J, Suzuki N, Enomoto A, Matsumoto Y, Tomita M, Suzuki T, Ohtomo K, Hosoi Y. Sodium orthovanadate suppresses DNA damage-induced caspase activation and apoptosis by inactivating p53. Cell Death Differ. 2006;13:499–511. doi: 10.1038/sj.cdd.4401768. [DOI] [PubMed] [Google Scholar]
- 43.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]