Abstract
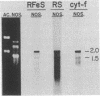
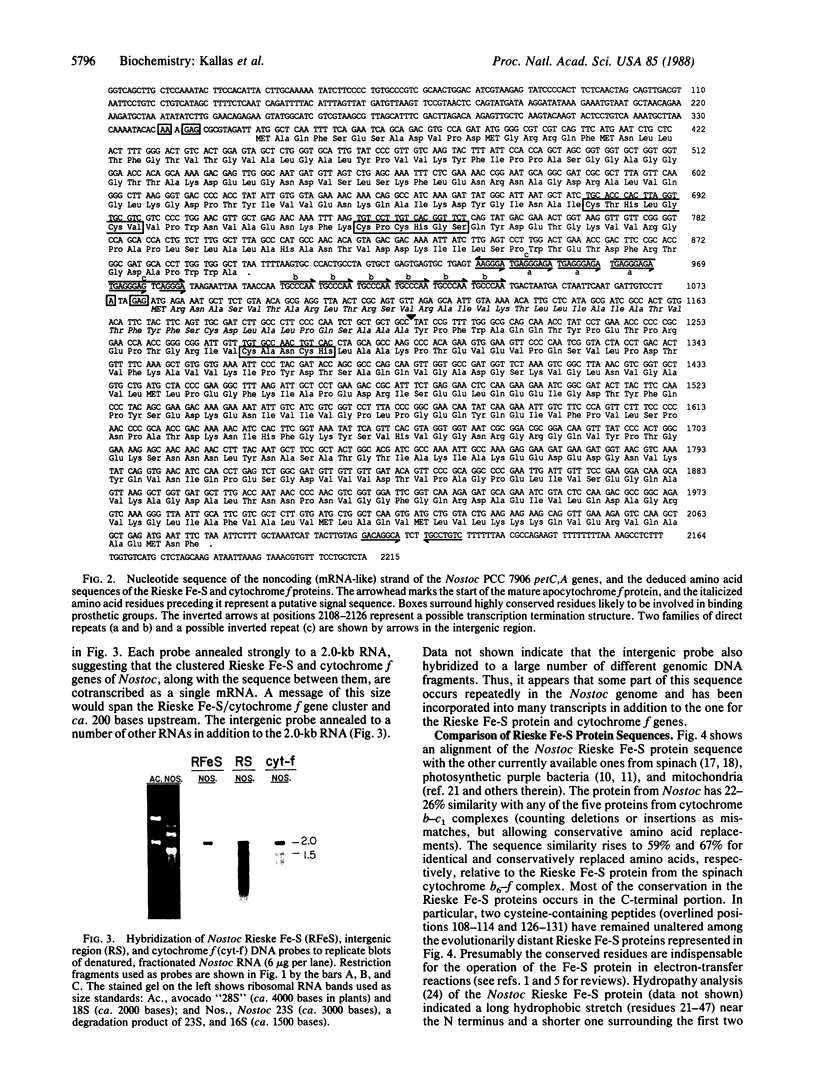
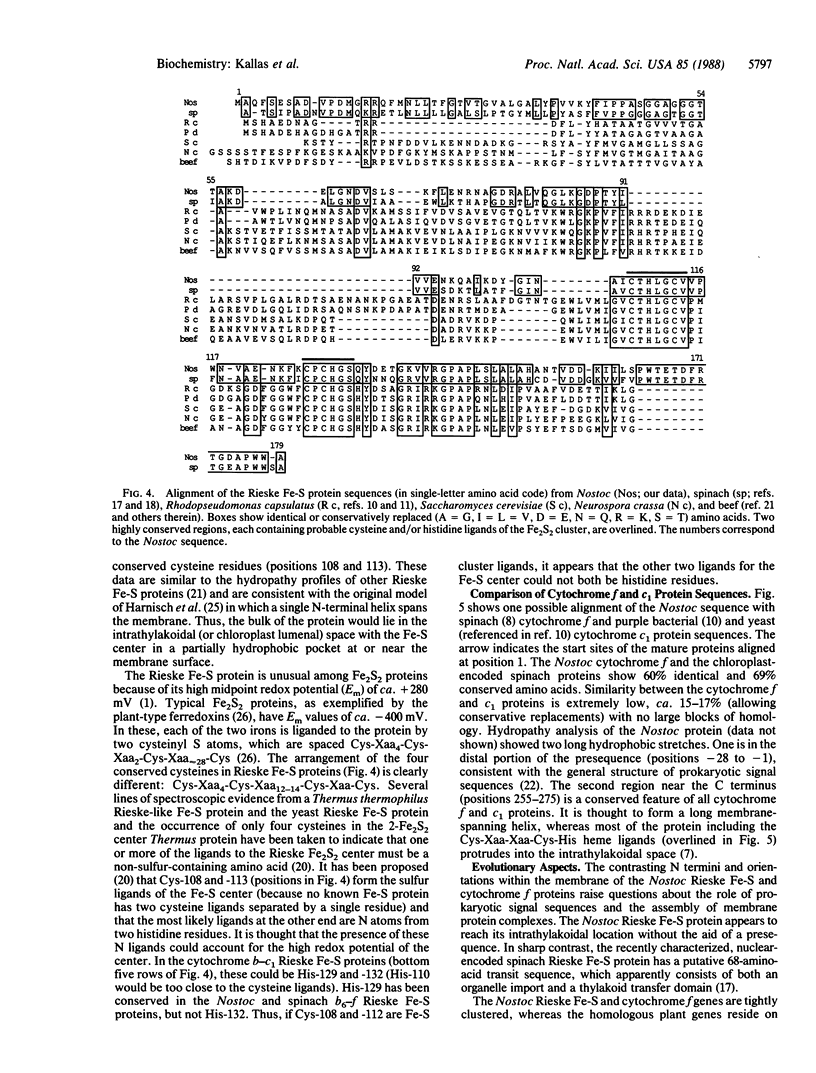
The thylakoid membrane cytochrome b6-f complex (plastoquinol:oxidized-plastocyanin oxidoreductase, EC 1.10.99.1) catalyzes electron-transfer and proton-translocation reactions essential for oxygenic photosynthesis. We have isolated and determined the nucleotide sequences of the petC and petA genes encoding the Rieske Fe-S and cytochrome f polypeptides from the filamentous cyanobacterium Nostoc PCC 7906. These genes occur as single genomic copies, are tightly linked, and, as indicated by hybridization of gene-specific probes to Nostoc RNA, are cotranscribed as a 2.0-kilobase message. The Rieske Fe-S/cytochrome f gene pair thus represents an example of clustering and cotranscription in cyanobacteria of functionally related genes that, in photosynthetic eukaryotes, reside on separate nuclear and plastid genomes. These data are consistent with the progressive degeneration of the modern chloroplast genome from the ancestral, cyanobacterial-like genome of an endosymbiont. The Rieske Fe-S and the mature cytochrome f apoproteins are encoded by 537 and 867 nucleotides and have molecular masses of 19.2 and 31.2 kDa, respectively. They show 59% and 60% protein sequence identity, respectively, relative to spinach. Forty-four amino acids (4.7 kDa) resembling a prokaryotic signal sequence precede apocytochrome f. In contrast, the Rieske Fe-S protein appears to be translated without a presequence. The 183 bases separating the Rieske Fe-S and preapocytochrome f genes contain two families of 7- to 9-base tandem repeats, and some part of this sequence is highly reiterated in the genome. The C terminus of the Rieske Fe-S protein contains cysteine and histidine residues (probable ligands for the Fe2S2 center) in two peptides, Cys-Thr-His-Leu-Gly-Cys-Val and Cys-Pro-Cys-His-Gly-Ser, which have been conserved in spinach and in the five available Rieske Fe-S sequences from the mitochondrial-type cytochrome b-c1 complexes. Cytochrome f shows the heme binding residues Cys-Xaa-Xaa-Cys-His near its N terminus. Single, long hydrophobic stretches occur near the N and C termini, respectively, of the Rieske Fe-S and cytochrome f proteins and may form membrane-spanning helices.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Davidson E., Daldal F. Primary structure of the bc1 complex of Rhodopseudomonas capsulata. Nucleotide sequence of the pet operon encoding the Rieske cytochrome b, and cytochrome c1 apoproteins. J Mol Biol. 1987 May 5;195(1):13–24. doi: 10.1016/0022-2836(87)90323-8. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Kuila D., Mather M. W., Yoshida T. Respiratory proteins from extremely thermophilic, aerobic bacteria. Biochim Biophys Acta. 1986;853(2):153–185. doi: 10.1016/0304-4173(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Sebald W. Nucleotide sequence and transcription of the fbc operon from Rhodopseudomonas sphaeroides. Evaluation of the deduced amino acid sequences of the FeS protein, cytochrome b and cytochrome c1. Eur J Biochem. 1986 Feb 3;154(3):569–579. doi: 10.1111/j.1432-1033.1986.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Harnisch U., Weiss H., Sebald W. The primary structure of the iron-sulfur subunit of ubiquinol-cytochrome c reductase from Neurospora, determined by cDNA and gene sequencing. Eur J Biochem. 1985 May 15;149(1):95–99. doi: 10.1111/j.1432-1033.1985.tb08898.x. [DOI] [PubMed] [Google Scholar]
- Hauska G., Hurt E., Gabellini N., Lockau W. Comparative aspects of quinol-cytochrome c/plastocyanin oxidoreductases. Biochim Biophys Acta. 1983 Jul 15;726(2):97–133. doi: 10.1016/0304-4173(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Ho K. K., Krogmann D. W. Cytochrome f from spinach and cyanobacteria. Purification and characterization. J Biol Chem. 1980 May 10;255(9):3855–3861. [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Malkin R. Interaction of photosynthetic electron transport inhibitors and the Rieske Iron-Sulfur center in chloroplasts and the cytochrome b6-f complex. Biochemistry. 1982 Jun 8;21(12):2945–2950. doi: 10.1021/bi00541a022. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIESKE J. S., ZAUGG W. S., HANSEN R. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LIX. DISTRIBUTION OF IRON AND OF THE COMPONENT GIVING AN ELECTRON PARAMAGNETIC RESONANCE SIGNAL AT G = 1.90 IN SUBFRACTIONS OF COMPLEX 3. J Biol Chem. 1964 Sep;239:3023–3030. [PubMed] [Google Scholar]
- Rich P. R. A perspective on Q-cycles. J Bioenerg Biomembr. 1986 Jun;18(3):145–156. doi: 10.1007/BF00743461. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., Borchart U., Machleidt W., Link T. A., Von Jagow G. Isolation and amino acid sequence of the 'Rieske' iron sulfur protein of beef heart ubiquinol:cytochrome c reductase. FEBS Lett. 1987 Jul 13;219(1):161–168. doi: 10.1016/0014-5793(87)81210-3. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]