Abstract
Astrocytes modulate the formation and function of glutamatergic synapses in the CNS, but whether astrocytes modulate GABAergic synaptogenesis is unknown. We demonstrate that media conditioned by astrocytes, but not other cells, enhanced GABAergic but not glutamatergic axon length and branching, and increased the number and density of presynaptically active GABAergic synapses in dissociated hippocampal cultures. Candidate mechanisms and factors, such as activity, neurotrophins, and cholesterol were excluded as mediating these effects. While thrombospondins secreted by astrocytes are necessary and sufficient to increase hippocampal glutamatergic synaptogenesis, they do not mediate astrocyte effects on GABAergic synaptogenesis. We show that the factors in astrocyte conditioned media that selectively affect GABAergic neurons are proteins. Taken together, our results show that astrocytes increase glutamatergic and GABAergic synaptogenesis via different mechanisms and release one or more proteins with the novel functions of increasing GABAergic axon length, branching and synaptogenesis.
Keywords: Astrocyte, inhibitory neuron, GABA, axon, synaptogenesis, hippocampus
Introduction
The development of CNS synapses requires not only the exchange of signals between pre- and postsynaptic neurons but also communication with adjacent glia. Astrocytes up-regulate the formation of functional glutamatergic synapses through a variety of signaling mechanisms; both pre- and postsynaptic effects have been identified (Christopherson et al., 2005; Hama et al., 2004; Mauch et al., 2001). In purified retinal ganglion cell cultures, astrocyte-conditioned medium (ACM) dramatically increases the number of presynaptic contacts made between neurons, the quantal size and efficacy of neurotransmitter release, and the number of postsynaptic AMPA receptor clusters (Nagler et al., 2001; Ullian et al., 2004; Ullian et al., 2001). Some of the factors released by astrocytes that mediate these effects have been identified. Astrocyte-derived cholesterol complexed to apolipoprotein E increases the number of functional glutamatergic presynaptic terminals in retinal ganglion cell autaptic cultures (Mauch et al., 2001). In the cerebral cortex, immature astrocytes express thrombospondins (TSPs), which increase the number of ultrastructually normal glutamatergic synapses that are presynaptically active but postsynaptically silent (Christopherson et al., 2005). Thus soluble factors, including proteins and lipids, released by astrocytes enhance glutamatergic synaptogenesis.
By contrast, little is known about whether and how astrocytes modulate the development of GABAergic synapses. In earlier work, astrocytes increase GABA-mediated currents in neurons prior to synaptogenesis (Liu et al., 1997; Liu et al., 1996), but the basis of these effects was not shown. Here we demonstrate that astrocytes increase glutamatergic and GABAergic synaptogenesis via distinct mechanisms and release one or more proteins that specifically increase GABAergic axon length, branching and synaptogenesis.
Results
Astrocytes selectively increase GABAergic neuron axon length and branching
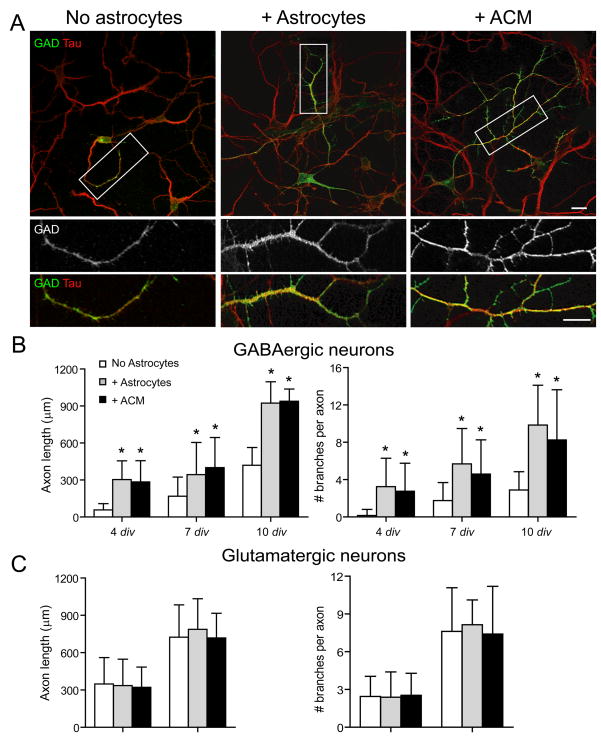
GABAergic neuron morphology and neurite outgrowth was examined in hippocampal neurons cultured alone or with astrocytes. At all ages examined neuron co-culture with astrocytes or ACM had a profound effect on the length and branching of GABAergic axons assayed after tau immunostaining. In both culture conditions, GABAergic neurons underwent significant growth and maturation during the first two weeks in vitro (Fig. 1; Supp. Table 1). In contrast, GAD-negative glutamatergic axons were not significantly longer when neurons were cultured with astrocytes or ACM relative to neurons cultured alone (Fig. 1; Supp. Table 1). Cell survival, density, soma size and number and length of primary dendrites of GABAergic neurons or glutamatergic neurons were similar among all culture conditions (Supp. Table 1; Elmariah et al., 2005)). Thus, while neither glutamatergic nor GABAergic axons require the presence of astrocytes for growth, astrocyte-derived cues specifically enhance the outgrowth of GABAergic axons.
Figure 1. Astrocytes selectively increase GABAergic axon length and branching.
Hippocampal neurons were cultured alone, with astrocytes or ACM and were immunostained at 4, 7, and 10 div with antibodies against tau (red) and glutamic acid decarboxylase (GAD; green). (A), GABAergic axon length and branching were significantly increased in neurons cultured with astrocytes (middle) or ACM (right) compared to neurons cultured alone (left) at 4 div (Supp. Table 1). Areas within white boxes are shown below at higher magnification. Note that GAD expression is dimmer in neuron-only cultures compared to neurons cultured with astrocytes or with ACM, as represented in these representative figure panels, and confirmed by Western blot analyses (data not shown). Scale bar = 25 (top), 10 (bottom) μm. (B, C), Quantification of the effect of astrocytes or ACM on GABAergic axon length (B, left) and branching (B, right) or on glutamatergic axon length (C, left) and branching (C, right). All values are shown as mean ± s.d. (n = 129 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
We compared the effects of acutely isolated and cultured astrocytes (14–21 div) in neuron-astrocyte co-cultures. No differences were observed in the effects of acutely isolated and cultured astrocytes on GABAergic neuron axon length or branching at 4 div (length: acute 303.9 ± 16.5 (N = 85 neurons), cultured 291.7 ± 12.9 (24); number of secondary branches: acute 3.24 ± 0.33 (85), cultured 3.79 ± 0.41 (24); not significantly different, Student’s t-test). Thus, acutely isolated astrocytes were used for experiments.
GABAergic neurons co-cultured with astrocytes or ACM showed significantly increased axon branching compared to neurons cultured alone (Fig. 1, Supp. Table 1). In the absence of astrocytes, most GABAergic axons remained unbranched at 4 div and exhibited relatively few secondary or tertiary branches at 7 div (Fig. 1; Supp. Table 1). In contrast, GABAergic neurons cultured with astrocytes or ACM had more complex axonal arbors than neurons cultured alone during the first 2 weeks in vitro, exhibiting 2 to 3 secondary branches as early as 4 div (Supp. Table 1). These effects on branching were not seen for glutamatergic neurons (Fig. 1; Supp. Table 1). These results demonstrate that astrocytes release soluble factors that increase the length and branching of GABAergic but not glutamatergic axons.
Astrocytes increase GABAergic synaptogenesis
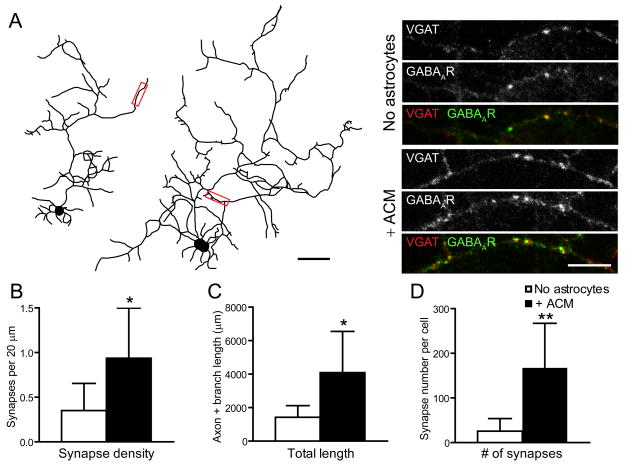
We next asked whether astrocytes or ACM caused an increase in GABAergic synaptogenesis by assaying the number of GABAergic synapses per length of GABAergic axon. Neurons cultured with ACM or astrocytes had ~2-fold higher density of GABAergic synapses than did neurons cultured in the absence of astrocytes (Fig. 2A). ACM increased total GABAergic axon length (sum of the length of primary, secondary, and tertiary axon branches) by ~3-fold (Fig. 2B) and the total number of synapses by ~7-fold (Fig. 2C). Thus astrocytes secrete factors that increase GABAergic synapse density, independent of increasing GABAergic axon length and branching.
Figure 2. Astrocyte soluble factors increase GABAergic synapse density.
(A), GABAergic axon length and branching was significantly increased in neurons cultured alone (left) compared to neurons cultured with ACM (right) at 7 div (see also Fig. 1, Supp. Table 1). Scale bar = 10 μm. Areas within red boxes, shown to the right at higher magnification, show presynaptic VGAT (top), postsynaptic GABAARs (middle) and their colocalization (bottom). Neurons cultured with ACM (right) have a significantly greater synaptic density than neurons cultured alone (left). Scale bar = 10 μm. (B, C, D), Neurons cultured with ACM have significantly increased GABAergic synapse density (B), axon and branch length (C) and total number of GABAergic synapses (D) compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 7 cells, 3 independent expts.; Student’s t test, Single asterisk indicates p < 0.05, ** indicates p < 0.01).
Soluble factors specifically released by astrocytes increase GABAergic axon length, branching and synaptogenesis
To determine whether these soluble factors are released specifically from astrocytes, we examined the ability of media conditioned by another cell type - primary meningeal fibroblasts - to increase GABAergic axon length, branching, and synapse density. We found that 3 days of treatment with fibroblast conditioned media (FCM) did not significantly affect GABAergic axon length or branching at 4 or 7 div compared to neurons cultured alone (Supp. Fig. 1 A, B). Immunostaining for synaptic markers showed that FCM treatment did not significantly increase GABAergic synapse density at 4 or 7 div compared to neurons cultured alone (see Elmariah et al., 2005)), assayed by the colocalization of presynaptic synaptophysin and postsynaptic GABAAR clusters. Taken together, these results suggest that astrocytes, but not fibroblasts, release factors that increase GABAergic axon length, branching and synaptogenesis.
ACM increases the number of presynaptically active GABAergic synapses
To determine whether astrocytes also promote the function of newly formed hippocampal synapses, we examined the number of synapses which were labeled with an antibody directed to the luminal domain of the vesicular GABA transporter (VGAT), which can be used to mark the sites of synaptic vesicle release and recycling (Martens et al., 2008). In neuron-only cultures, there are few GABAergic synapses and both neuron-astrocyte co-culture and ACM significantly increase the number of GABAergic synapses (Fig. 3A, B; see also Fig. 2). The number of GABAergic synapses labeled with the anti-VGAT-C luminal antibody (clusters triple stained with VGAT-N, VGAT-C and gephyrin antibodies were identified as presynaptically active GABAergic synapses) also was significantly increased in neuron-astrocyte co-cultures and ACM, compared to neuron only cultures (Fig. 3C). To examine whether astrocytes increase the proportion of presynaptically active GABAergic synapses in addition to increasing GABAergic synapse number, we plotted the number of presynaptically active GABAergic synapses against total GABAergic synapse number per neuron. Although there are fewer GABAergic synapses in neuron only cultures, our findings suggest that astrocytes do not affect presynaptic activity, because the proportion of synapses that are presynaptically active are equal in neuron only, neuron-astrocytes and ACM conditions. (Fig. 3D). These data show that astrocytes increase the number but not the proportion of synapses that are presynaptically active. Taken together, these results suggest that soluble factors released by astrocytes increase the number of GABAergic synapses, and these newly formed synapses are presynaptically active.
Figure 3. Astrocyte soluble factors increase the number of presynaptically active GABAergic synapses.
(A), Neurons cultured with astrocytes of ACM have a significantly greater number of GABAergic synapses (colocalization of blue and green clusters) labeled by the VGAT-C luminal antibody (red) after stimulation with a Krebs-Ringer solution containing 55 mM KCl compared to neurons cultured alone. Scale bar = 10 μm. (B, C), Quantification of the effect of astrocytes or ACM on GABAergic synapse density (B) and presynaptically active GABAergic synapse density (C). All values are shown as mean ± s.d. (n = 37 cells, 3 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (D), Quantification of the number of GABAergic synapses plotted against the number of presynaptically active synapses per cell. The proportion of synapses that are presynaptically active are equal in neuron only, neuron-astrocytes and ACM conditions. (linear regression analysis; neurons only, R2 = 0.99; + Astrocytes, R2 = 0.98; + ACM, R2 = 0.99)
The effects of ACM on GABAergic neurons do not require neurotrophin signaling, action potential activity, or cholesterol
We evaluated several candidate mechanisms mediated by astrocyte soluble factors that increase GABAergic axon length, branching and synaptogenesis. First, we blocked action potential activity using TTX which had no effect on GABAergic axon length, branching, or presynaptic terminal density in neuron-only cultures, and did not alter the effect of astrocytes or ACM on GABAergic axon length, branching, presynaptic terminal density (Supp. Fig. 2A). To examine whether neurotrophin signaling mediates the effects of astrocytes, we scavenged endogenous BDNF or NT3 using TrkB- or TrkC-IgG fusion protein, which did not prevent the increase in GABAergic axon length, branching, or presynaptic terminal density in neurons cultured with astrocytes or with ACM compared to neurons cultured alone at 7 div (Supp. Fig. 2B, see also Elmariah et al., 2005). Finally, we examined the role of cholesterol. We found that cholesterol did not increase GABAergic axon length, branching or GABAergic synapse density when compared to untreated and vehicle treated cultures (Supp. Fig. 2C; see also Elmariah et al., 2005). Parallel cultures of neurons grown on astrocytes showed the expected increase in axon length, branching, and synapse density. Taken together, these results suggest that the effects of ACM on GABAergic neurons do not require neuronal activity, are neurotrophin-independent, and cannot be mimicked by addition of exogenous cholesterol.
Effects of astrocytes on GABAergic neurons are not mediated by TSPs
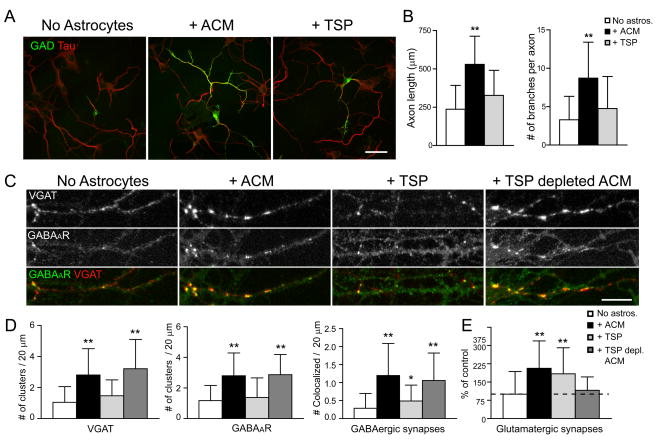
Recent work has shown that TSPs, in particular TSP-1 and -2, are released by astrocytes and mediate the increase in glutamatergic synaptogenesis in retinal ganglion cells in vitro and in vivo (Christopherson et al., 2005). TSPs have also been previously shown to increase retinal neurite outgrowth (DeFreitas et al., 1995; Neugebauer et al., 1991; but see Christopherson et al., 2005). To examine the role of TSPs on GABAergic axon length, branching and synaptogenesis, neuron-only cultures were treated with purified TSP-1. TSP-1 treatment did not significantly increase GABAergic axon length or branching at 7 div compared to neuron-only cultures, whereas parallel cultures of neurons and ACM showed the expected increases (Fig. 4A, B). This result shows that TSPs do not mediate the effects of astrocytes on GABAergic axon length or branching.
Figure 4. TSPs do not increase GABAergic axon length, branching, or synaptogenesis.
Hippocampal neurons were cultured alone, with ACM, with TSP-1, or with TSP-depleted ACM, and were immunostained at 7 div with antibodies against GAD (green) and tau (red) to label GABAergic axons. (A), GABAergic axon length and branching were increased in neurons cultured with ACM (middle) but not when neurons were treated with TSP-1 (right) compared to neurons cultured alone (left) at 4 div. Scale bar = 25 μm. (B), Quantification shows that GABAergic axon length and branching were significantly increased in neurons cultured with ACM compared to neurons treated with TSP-1 and neurons cultured alone. All values are shown as mean ± s.d. (n = 133 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (C), Hippocampal neurons were cultured alone, with ACM, with TSP-1, or with TSP-depleted ACM, and were immunostained at 7 div with antibodies against VGAT (red), and GABAAR β3 subunit (green) to label GABAergic synapses. An increase in presynaptic VGAT clusters, postsynaptic GABAAR clusters and GABAergic synapse density were observed at 7 div in neurons cultured with ACM (middle left) or with TSP-depleted ACM (right) but not when neurons were treated with TSP-1 (middle right) compared to neurons cultured alone (left). Scale bar = 10 μm. (D), Quantification of increase in presynaptic VGAT clusters (left), postsynaptic GABAAR clusters (middle) and GABAergic synapse density (right) per length dendrite in neurons treated with ACM, TSP-1, or TSP-depleted ACM. All values are shown as mean ± s.d. (n = 75 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, Single asterisk indicates p < 0.05, ** indicates p < 0.001). (E), Quantification of increase in glutamatergic synapse number per dendrite length (clusters double stained with VGlut and PSD-95 antibodies) in neurons treated with ACM, TSP-1 but not TSP-depleted ACM. TSPs increase glutamatergic synapse number (see also Christopherson et al., 2005). All values are shown as mean ± s.d. (n = 59 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
We next determined whether TSPs could account for the effects of astrocytes on GABAergic synapse density. TSP-1 treatment of neuron-only cultures from 1 to 7 div did not affect the density of VGAT+ presynaptic terminals or postsynaptic GABAAR clusters per dendrite length, whereas cultures treated with ACM showed the expected increases (Fig. 4C, D). TSP treatment of neuron-only cultures did slightly increase GABAergic synapse density. This increase, however, was significantly less than that observed in neurons treated with ACM (Fig. 4C, D). Thus, TSPs may increase the density of GABAergic synapses, but this does not account for the complete activity in ACM that increases GABAergic synapse density by ~4-fold.
As an additional test of whether TSPs released by astrocytes increase GABAergic synapse density, endogenous TSPs were immunodepleted from ACM using a combination of antisera against TSP-1 and TSP-2 (Supp. Fig. 3). The density of GABAergic synapses in neuron-only cultures at 7 div that were treated from 1 to 7 div with TSP-depleted ACM was significantly higher than in untreated neuron-only cultures, but not significantly different in neuron-only cultures treated with ACM (Fig. 4C, D).
As a positive control, we examined the ability of TSP-depleted media to increase glutamatergic synaptogenesis in hippocampal neuron cultures. First, we found that by treating neuron-only cultures with purified TSP-1 the number of glutamatergic synapses (clusters double stained with VGlut and PSD-95 antibodies were identified as excitatory synapses) was increased by 2-fold at 4 div, mimicking the effects of ACM on glutamatergic synaptogenesis (Fig. 4E). TSP-depleted ACM failed to increase glutamatergic synapse density compared to control neuron-only cultures (Fig. 4E). These results are consistent with recent work by Christopherson et al. (2005) that shows that TSPs are necessary and sufficient to promote glutamatergic synapse formation.
Thus, in contrast to glutamatergic synaptogenesis, TSPs do not appear to be necessary or sufficient to increase GABAergic synaptogenesis, suggesting that another factor(s) released from astrocytes increase GABAergic axon length, branching, and synaptogenesis.
Soluble factors released by astrocytes that increase GABAergic axon length, branching and synaptogenesis are proteins
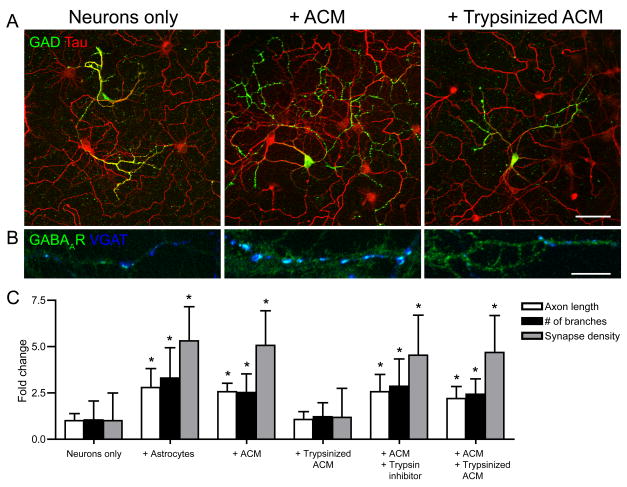
We next tested whether the biological activity of ACM was sensitive to trypsin. Astrocytes, ACM, ACM + trypsin inhibitor and ACM + trypsinized ACM all significantly increased GABAergic axon length, branching, and synapse density, compared to neuron-only cultures (Fig. 5). In contrast, trypsinized ACM did not change GABAergic axon length, branching, and the density of GABAergic synapses compared to neuron only cultures at 7 div. Since ACM + trypsin inhibitor increased GABAergic axon length, branching, and synapse density this shows that the soybean trypsin inhibitor does not interfere with the ability of ACM to affect GABAergic neurons. Furthermore, the ACM + trypsinized ACM condition also increased GABAergic axon length, branching, and synapse density, ruling out any secondary effects of trypsin on neurons that may affect GABAergic axon length, branching, and synapse density. Together, these results show that factors released by astrocytes that increase GABAergic axon length, branching and synapse density are proteins.
Figure 5. Astrocyte soluble factors are trypsin-sensitive.
Hippocampal neurons were cultured alone, with ACM or with trypsinized ACM and were immunostained at 4 div with antibodies against GAD and tau to label GABAergic axons and VGAT to label GABAergic presynaptic terminals. (A, B), GABAergic axon length and branching (A) and GABAergic synapse density (B) were significantly increased in neurons cultured with astrocytes (middle) but not with trypsinized ACM compared to neurons cultured alone (left). Scale bar = 25 (top), 10 (bottom) μm. (C), Quantification of GABAergic axon length, branching and synapse density in neuron-only cultures cultured with astrocytes, treated with ACM, trypsinized ACM, ACM + trypsin inhibitor or ACM + trypsinized ACM. All values are shown as mean ± s.d. (n = 76 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
Discussion
Here we demonstrate for the first time that astrocytes release proteins that selectively increase GABAergic axon length, branching and synapse density. Neurotrophins, neuronal activity, cholesterol and TSPs were eliminated as candidate mechanisms underlying these effects. These results show that astrocytes utilize different mechanisms to affect glutamatergic compared to GABAergic neuron maturation and synaptogenesis. Thus, astrocytes release proteins that selectively affect GABAergic neurons, expanding the repertoire of glial function in the development of neural circuits.
Astrocyte released proteins increase GABAergic neuron axon length, branching, synapse formation and function
We report for the first time that astrocytes release proteins that selectively enhance the development of hippocampal GABAergic neurons by increasing GABAergic axon length, branching, synapse density and synapse function. In the context of previous work, our results highlight several important features of astrocyte-neuron signaling during development. First, astrocytes release one or more proteins that increase GABAergic axon but not dendrite length and branching. Astrocytes express and release numerous factors, including many proteins, some of which stimulate neurite outgrowth, including laminin, fibronectin and N-cadherins, among others (Aoyagi et al., 1994; Matthiessen et al., 1989; Tomaselli et al., 1988). Recent work suggests that cortical astrocytes also release trophic factors, such as fibroblast growth factor, that stimulate both axonal and dendritic growth in postnatal mouse cortical neurons (Le and Esquenazi, 2002). Previously identified proteins have not been shown to differentially increase axon compared to dendrite length and branching in GABAergic neurons. The specific effects of astrocytes on GABAergic axons compared to dendrites, or neurites generally, suggest that astrocyte soluble proteins not only modulate signaling pathways important for axon growth and branching, but may also play important roles in axon guidance and synaptic targeting, as has been shown for glia in invertebrate systems (Colon-Ramos et al., 2007; Shen et al., 2004).
Second, astrocytes secrete proteins that differentially modulate glutamatergic compared to GABAergic neurons. We found that astrocyte secreted proteins that increased GABAergic but not glutamatergic axon outgrowth, and furthermore, that astrocyte secreted TSPs promote glutamatergic but not GABAergic synaptogenesis. Our finding that astrocytes did not affect glutmatergic axon length or branching is consistent with previous work that showed that retinal ganglion cell neurite length and branching are not affected by astrocytes or ACM (Ullian et al., 2001). These differential effects of astrocytes on GABAergic compared to glutamatergic neurons suggest that astrocytes secrete different proteins that have specific effects on different types of neurons. Thus, while astrocytes may have some similar affects on glutamatergic and GABAergic neurons, they may use distinct signaling pathways to differentially affect these cells which may differentially shape the maturation and connectivity of these two types of neurons.
Third, astrocyte secreted proteins increased not only GABAergic axon length and branching, but also synaptogenesis. Previous studies that examined the effects of astrocytes on glutamatergic synaptogenesis found that astrocyte soluble factors increase glutamatergic synapse formation without affecting the growth or complexity of glutamatergic axonal arbors, suggesting that astrocytes directly promote synaptogenesis (Christopherson et al., 2005; Mauch et al., 2001). We show that astrocyte released proteins increased not only GABAergic axon length and branching but also the number of GABAergic synapses, raising the possibility that the increase in axon length and branching accounts for the increase in synapse formation. To assess this possibility, we assayed GABAergic synapse density, i.e., the number of GABAergic synapses per length of axon, normalizing the effects of astrocytes on axon length and branching. This analysis showed that the astrocyte promotion of axon length and branching does not solely account for the observed increase in GABAergic synapse number. Furthermore, we found that the 2-fold increase in GABAergic synapse density, times the 3-fold increase in total axon length, comes very close to the total 7-fold increase in total GABAergic synapse number that we observed in cultures treated with ACM. These data suggest that astrocyte proteins influence synaptic connectivity in multiple ways, promoting not only GABAergic axon length and branching, but also GABAergic synapse formation.
Finally, the generation of astrocytes in the developing brain overlaps with the formation of synaptic networks. Although the formation of both glutamatergic and GABAergic synapses peaks during postnatal stages of brain development (Aghajanian and Bloom, 1967), recent work has shown that GABAergic synapses become functional before glutamatergic synapses (Ben-Ari, 2002). In the hippocampus, first GABAergic then glutamatergic synapses are the formed among interneurons in late embryonic stages, followed by GABAergic then glutatmatergic synapses onto pyramidal neurons somewhat later, during early postnatal development (Hennou et al., 2002; Tyzio et al., 1999). GABAergic synapse formation continues from late embryonic into postnatal stages in a protracted process that is regulated by neural activity and experience (Huang and Scheiffele, 2008; Jiao et al., 2006; Miller, 1986). The generation of astrocytes also begins during late embryonic stages, and continues during postnatal development (Wang and Bordey, 2008), reaching a peak in cortex and hippocampus between P0 – P2 (Sauvageot and Stiles, 2002). The timing of GABAergic synaptogenesis and astrocytogenesis positions astrocytes, via the proteins they secrete, to influence GABAergic synapse formation over a prolonged period of postnatal maturation in the developing brain in vivo.
We show that astrocyte secreted proteins dramatically increase not only the number but also the density of synapses on GABAergic axons of embryonic hippocampal neurons. This result confirms previous work that showed that astrocytes increase the number of GABAergic synapses impinging on dendrites, and increase the frequency of miniature inhibitory synaptic potentials (Elmariah et al., 2005). This result, however, contrasts with recent reports which showed that astrocytes do not affect hippocampal glutamatergic or GABAergic synapse number or function (Steinmetz et al., 2006). This difference may be explained by the different age of animals from which the cultures were prepared, as well as to different neuron and astrocyte culture conditions. Steinmetz and colleagues prepared hippocampal neurons and astrocytes from postnatal mice using immunoisolation to purify hippocampal neurons. Postnatal neurons may not be affected in the same fashion as embryonic neurons by astrocyte secreted proteins. Alternatively, the expression and/or secretion of astrocyte synaptogenic and other proteins maybe developmentally regulated. Comparison of neurons and astrocytes isolated from different developmental stages in mix and match experiments, and identification and characterization of astrocyte proteins that influence neuron maturation and synaptogenesis, will be necessary to differentiate between these possibilities.
The effects of astrocyte released proteins on GABAergic neurons are not mediated by action potential activity, neurotrophins, cholesterol or TSPs
Since astrocyte soluble factors are known to enhance synaptic efficacy and function (Pfrieger and Barres, 1997; Ullian et al., 2001), we examined the role of neural activity in mediating the effect of astrocytes on GABAergic neurons. Treatment with TTX during early stages of synapse formation (from 1 to 7 div) did not affect GABAergic synapse number in neuron-only cultures, nor did it alter the ability of ACM to increase GABAergic axon length, branching, and synaptogenesis. In contrast to our results, previous studies have reported a decrease in GABAergic synaptogenesis after chronic blockade of synaptic transmission. However, these experiments involved activity blockade at later stages of synapse formation and maturation (TTX treatment from 5 div onward; Hartman et al., 2006; Kilman et al., 2002) suggesting that activity may have different effects on synapse formation at different developmental stages. Our work suggests that astrocytes secrete proteins that increase GABAergic axon length, branching and synaptogenesis via mechanism(s) that are independent of neuronal activity during early stages of development.
Recent work has demonstrated that TSP-1 and -2 expressed by immature astrocytes increase the number of glutamatergic synapses between retinal ganglion cells in vitro and in vivo (Christopherson et al., 2005). While our work confirmed that TSPs are required for hippocampal glutamatergic synaptogenesis, we showed that addition of exogenous TSPs slightly increased GABAergic synaptogenesis but did not mimic the effects of ACM on GABAergic neurons. The small effect of exogenous TSPs on GABAergic synaptogenesis may be indirect, secondary to the large increase in glutamatergic synaptogenesis mediated by TSPs. We also found that depletion of TSPs from ACM did not abrogate the effect of ACM on GABAergic synaptogenesis. These results demonstrate that while TSPs are required for hippocampal glutamatergic synaptogenesis, other astrocyte proteins modulate GABAergic axon length, branching and synaptogenesis. Importantly, these experiments show for the first time that hippocampal glutamatergic and GABAergic synaptogenesis are regulated by different mechanisms. Furthermore, ACM depleted of TSPs failed to increase glutamatergic synapses, but still robustly increases the number of GABAergic synapses, suggesting that astrocytes do not increase GABAergic synaptogenesis through homeostatic mechanisms. The work we report here establishes new roles for astrocytes in mediating GABAergic synaptogenesis, highlighting the importance of this glial cell type in the development and maintenance of neural circuits.
Identification of astrocyte proteins that regulate synapse formation has been limited by the lack of characterization of the genes and proteins expressed by astrocytes. Recently, using different purification strategies approaches, two groups have recently profiled the gene expression of astrocytes in vitro and in vivo, or the astrocyte transcriptome (Cahoy et al., 2008; Lovatt et al., 2007). Several other groups have used proteomic approaches to determine the repertoire of proteins released by astrocytes, or the astrocyte secretome (Dowell et al., 2009; Keene et al., 2009; Moore et al., 2009). These analyses of the astrocyte transcriptome and secretome reveal many interesting candidates and pathways that can now be examined for their roles in synaptogenesis and other processes. Using these and other strategies to identify the proteins that selectively increase GABAergic axon length, branching, synapse formation and function, and the relevant downstream signaling pathways, is the focus of ongoing work.
Experimental Methods
Cell cultures
Primary neurons co-cultured with astrocytes were prepared as described previously (Elmariah et al., 2005), with minor modifications. Briefly, hippocampi were dissected from embryonic day (E) 18 rats, dissociated in HBSS (Earls Buffered Saline Solution (EBSS) with MgCl2 and HEPES) containing 1% papain for 20 min., triturated in BME (Basal Medium Eagle), and plated at 75,000–100,000 cells/ml in Neurobasal medium (Invitrogen, Grand Island, NY) on poly-L-lysine (1 mg/ml; Sigma, St. Louis, MO) coated coverslips in 24-well plates. Cells were grown at 37°C, 5% CO2, 95% humidity in Neurobasal medium plus B27 (Invitrogen) that was changed weekly.
Approximately 15% of the neurons in these cultures have small soma size, less branched dendritic arbors, and express GAD. We have previously shown that, in our culture system, activation of GABAARs is depolarizing until 4–5 div; after this time, activation of GABAARs is hyperpolarizing (Elmariah et al., 2004). Since the experiments reported here were performed at 4 div and older, for simplicity’s sake, cells and synapses that express GABA-related markers are referred to as inhibitory.
Primary astrocyte cultures were prepared as described previously (Duan et al., 2003; Zhang et al., 2003). Briefly, hippocampi were dissected from E18 rats, dissociated in HBSS containing 0.25% trypsin for 20 min, triturated in BME, and plated in T25 flasks in MMEM (Modified Minimum Essential Medium; Invitrogen) supplemented with 10% heat-inactivated FBS (HyClone, Logan, UT), 2 mM L-glutamine, 14 mM sodium bicarbonate, 40 mM D-glucose, 1% sodium pyruvate and 1% penicillin and streptomycin. Astrocytes proliferated for 10–14 days; after reaching confluence, they were rinsed in cold EBSS and shaken at 260 rpm for 18–20 hours in MMEM to remove neurons and other cells. Purified astrocytes were then plated onto poly D-lysine coated coverslips at 400,000 cells/ml in MMEM. Medium was changed to Neurobasal medium and coverslips used for ACM experiments within 1–3 days. Coverslips were immunostained with an antibody against GFAP (Chemicon, Temecula, CA) to assess purity prior to use; in the experiments described here, > 98% of the cells were GFAP+.
For neuron-only cultures, cytosine arabinoside (Ara-C; 10μM; Sigma) was added to cultures 18–20 hours after plating to prevent glial proliferation. For neurons cultured with acutely dissociated astrocytes, neurons were prepared in Neurobasal plus B27 supplemented with 5% FBS, 1% L-glutamine, and 1% penicillin and streptomycin as described above without the addition Ara-C, allowing astrocyte proliferation. At 4 div, when astrocytes composed approximately 75–80% of cells (N = 35 coverslips), culture media was changed to Neurobasal medium with B27 and co-cultures were maintained up to 21 div. For neurons cultured with astrocytes maintained for 14–21 div, neurons were plated onto confluent monolayers of astrocytes grown on coverslips and maintained up to 21 div in Neurobasal medium.
For neurons cultured with ACM, neurons were plated in Neurobasal medium that had been conditioned by astrocytes (14–21 days old) during the previous 72 hours. Sterile inserts with 3 m high pore density polyethylene terephthalate membranes (Becton Dickinson Labware, Franklin Lakes, NJ) were placed into each well, and coverslips with astrocyte monolayers were inverted 0.9 mm above neurons. Inserts remained in place for the duration of culture. Neuron-only cultures treated with astrocyte inserts or ACM both had increased GABAergic axon length, branching and synaptogenesis that were not significantly different from each other (data not shown); depending on the experiment, these conditions are interchangeably referred to as ACM. Cell survival and density were similar among neurons co-cultured with astrocytes or ACM or cultured alone (data not shown, see (Elmariah et al., 2005)). In addition, neuron co-culture with astrocytes or ACM produced no change in the number or length of primary dendrites or in the soma size of GABAergic neurons or glutamatergic neurons compared to neurons cultured alone (Supp. Table 1).
Primary meningeal fibroblast cultures were prepared from E18 rat meninges, which were dissociated in 0.25% trypsin for 20 min. and plated in T25 flasks in modified MMEM supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 14 mM sodium bicarbonate, 40 mM D-glucose, 1% sodium pyruvate and 1% penicillin and streptomycin for 4–7 days before use. For neurons cultured with fibroblast conditioned media (FCM), neuron-only cultures were plated in Neurobasal medium that had been conditioned by fibroblasts for 48 hours. Sterile inserts with 3μm high pore density polyethylene terephthalate membranes (Becton Dickinson Labware, Franklin Lakes, NJ) were placed into each well, and coverslips with fibroblast monolayers were inverted 0.9 mm above neurons. Inserts remained in place for the duration of culture. At 1 and 4 div, neuron cultures were treated with FCM for 3 days, and immunostained at 4 and 7 div.
Immunostaining and confocal microscopy
Coverslips were fixed in 4% paraformaldehyde and 4% sucrose for 15 min., permeabilized with cold 0.25% Triton X-100 for 5 min., and blocked in 5% normal goat serum (Invitrogen) for 1 hour at RT. Immunostaining was performed with combinations of primary antibodies: A2B5 (1:5 dilution, polyclonal; gift of Dr. J. Grinspan), GAD-6 (1:10, monoclonal; Developmental Studies Hybridoma Bank, Iowa), GalC (1:500, monoclonal, gift of Dr. Judy Grinspan), GFAP (1:1000, rabbit antiserum; Sigma), MAP2 (1:1000, rabbit antiserum; gift of Dr. V. Lee), synaptophysin (1:200, rabbit antiserum; NeoMarkers, Fremont, CA), tau (1:1000, rabbit antiserum; gift of Dr. V. Lee), VGAT-N (1:200, guinea pig antiserum; Synaptic Systems, Goettingen, Germany), VGAT-C (1:200, rabbit antiserum; Synaptic Systems, Goettingen, Germany), gephryin (1:200, mouse antiserum; Synaptic Systems, Goettingen, Germany), VGlut (1:1000, guinea pig antiserum; Chemicon), and PSD-95 (1:500, rabbit antiserum; Bioaffinity Reagents). Antibodies were visualized after staining with the appropriate FITC-, TRITC- or CY5-conjugated secondary antibodies (all used at 1:200, Jackson ImmunoResearch, Inc., West Grove, PA). Immunostaining was performed at 4, 7 and 10 div or later ages in vitro with antibodies against glutamic acid decarboxylase (GAD), the synthetic enzyme for GABA, to identify GABAergic neurons, and tau, to visualize axons. Because synaptophysin labels both glutamatergic and GABAergic synapses, and because GABAARs can be transiently clustered beneath both types of terminals during development (Rao et al., 2000), immunostaining was performed at 7 div with antibodies against the presynaptic vesicular GABA transporter (VGAT) and postsynaptic GABAARs or gephyrin to label GABAergic synapses. Images were obtained using a confocal microscope (Leica TCS SP2). In each image, laser light levels and detector gain and offset were adjusted so that no pixel values were saturated in regions analyzed.
VGAT-C luminal antibody labeling
Labeling was performed as described previously (Martens et al., 2008). Briefly, primary hippocampal neurons were incubated in Krebs-Ringer solution containing 55 mM KCl and rabbit polyclonal anti-VGAT-C antibody (5 μg/ml) at 37°C for 5 min. Cells were repeatedly washed in Krebs-Ringer buffer and subsequently in PBS then immunostained as described above. Specific labeling was confirmed by co-staining with a guinea pig polycolonal anti-VGAT-N antibody.
GABAergic synapses were identified as clusters which were double stained with VGAT-N terminus and gephyrin immunostaining. Presynaptically active GABAergic synapses were identified as clusters which were triple stained with VGAT-C, VGAT-N and gephyrin immunostaining and thus were GABAergic synapses that underwent vesicle recycling during stimulation. All synapse counts were obtained from cultures that were tripled stained with VGAT-N, VGAT-C, and gephyrin antibodies.
Neurotrophin scavenging and TTX, cholesterol and TSP treatment
Cultures were treated with 2 μg/ml TrkB-IgG, TrkC-IgG or control IgG (gift of Regeneron Pharmaceuticals, Inc., Tarrytown, NY) to scavenge neurotrophins. This scavenger concentration blocked the neurotrophin mediated increase in postsynaptic GABAAR clusters (Elmariah et al., 2004, 2005). 1 μM tetrodotoxin (TTX, Sigma) was used to block action potentials (Elmariah et al., 2004, 2005). Treatments began after the first day in culture and were replenished every 24 hours until immunostaining was performed at 4 or 7 div. For experiments investigating the role of cholesterol, neuron-only cultures were treated with 10 μg/ml cholesterol (a concentration that increased the number of glutamatergic presynaptic terminals in retinal ganglion cell autaptic cultures (Mauch et al., 2001); Sigma) or vehicle (EtOH) from 4–7 div. For experiments investigating the role of TSPs, neuron-only cultures were treated with 5 μg/ml purified TSP-1 (Christopherson et al., 2005); Haematological Tech., Essex Junction, VT) from 1 to 7 div.
TSP immunodepletion
Confluent purified astrocyte monolayers were washed with EBSS and treated with serum-free medium (Neurobasal plus B27 supplemented with 1% L-Glutamine, and 1% penicillin and streptomycin) for 5–7 days. ACM was filtered to remove cell debris and was concentrated 5-10x on Amicon Ultra columns (3–5 kDa; Millipore, Wilmington, DE). Fresh ACM was maintained at 4 °C for no longer than 7 days prior to use in the bioassays and other samples were stored at −80 °C until use. Concentrated ACM was incubated with 50 μl protein A beads (Pierce, Rockford, IL) and 50 μl protein G beads (Amersham, Piscataway, NJ) for 1 hour at RT. Beads were washed 1x with PBS followed by incubation with a cocktail of the following anti-TSP-1 and -2 antibodies: 0.2 μg/ml Ab-8 (rabbit antiserum; Neomarkers); 1 μg/ml Ab-2 (monoclonal; Neomarkers); 10 μl anti-mTSP-1 (monoclonal; gift of Dr. D. Mosher), 10 μl anti-mTSP-2 (monoclonal; gift of Dr. D. Mosher) overnight at 4° C. Antibody bound beads were washed 3x with PBS and were added to 300 μl concentrated ACM for 1–3 hours at RT. Beads were spun down and the supernatant was removed and used as TSP-depleted ACM. To determine the amount of TSP depletion, a sample was saved for Western blot analysis before addition to neuron-only cultures. To determine the amount of TSP pulldown, beads were washed 3 times with PBS, 3 times with PBS plus 0.25 M NaCl, and 3 times with PBS, 50 μl loading dye was added, samples were boiled and analyzed by Western blot.
Trypsinized ACM
Trypsinized ACM was prepared by treating concentrated 1 ml of ACM with 10–100 ng of trypsin for 1 hour at 37 degrees followed by 1 hour incubation with equivalent amount of soybean trypsin inhibitor to stop the enzymatic digestion. SDS-PAGE followed by silverstain analysis showed that proteins in ACM were digested by trypsin treatment (data not shown). Neuron-only cultures were treated from 1–7 div with ACM inserts, ACM media, trypsinized ACM, ACM + trypsin inhibitor or ACM inserts+ trypsinsized ACM, then immunostained.
Western blot analyses
Samples of concentrated ACM, TSP-depleted ACM or TSP bound to beads were resolved by SDS-PAGE, transferred to nitrocellulose membranes and probed with antibodies against TSP-1 (1.5 μg/ml Ab-2; monoclonal, Neomarkers), TSP-2 (1:250; monoclonal, BDTransduction, San Jose, CA) or ApoE (1:2000; polyclonal, Biodesign, Saco, ME) followed by alkaline phosphatase-conjugated secondary antibody (1:5000; polyclonal, Applied Biosystems, Foster City, CA). Signals were visualized using chemiluminescence (WesternStar Detection System, Applied Biosystems). Films were digitally scanned and signals were quantified using ImageJ software (available at http://rsb.info.nih.gov/ij/).
Quantification and statistical analysis
For all experiments, neurons were randomly selected for analysis. GABAergic neurons were distinguished from glutamatergic neurons by anti-GAD immunoreactivity. In a majority of experiments, the number and proportion of glutamatergic and GABAergic neurons were determined by counting GAD+ and GAD− neurons in a minimum of 5 randomly chosen fields on each coverslip (750 × 750 microns). To assess neuronal morphology, GAD+ soma diameter was measured and the number and length of MAP2+ dendrites and the length of tau+ axons were determined from confocal fluorescence images using interactive software (MetaMorph, Molecular Devices, Downingtown, PA; or ImageJ). We assayed the length of tau+ axons, as tau immunostaining levels do not change in intensity among conditions. Moreover, when axon length was independently assayed after either tau or GAD immunostaining, no difference in axon length measurements was observed (data not shown). The number of immunostained pre- or postsynaptic clusters was determined from confocal images using interactive software (MetaMorph or ImageJ). To measure synapse density per axon, the longest neurite which formed synapses was identified as the axon. Because of limitations of spectral overlap among secondary antibodies and three fluorescence channels, pre- and postsynaptic specializations of GABAergic neurons were labeled with VGAT, GABAAR-β3, and GAD antibodies; tau antibody wasn’t used to specifically label axons. Confocal images of neurons were segmented automatically using an iterative thresholding technique (Bergsman et al., 2006), and the number and area of individual clusters were determined using interactive software (MetaMorph, Molecular Devices, Downingtown, PA; or custom-written ImageJ macros). Clusters with more than 20% pixel overlap of pre- and postsynaptic markers were considered colocalized and thus synaptic.
Values for axon length, branch number and cluster number were compared across conditions using the Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, unless otherwise indicated. All values are presented as mean ± s.d.
Supplementary Material
Hippocampal neurons were cultured alone, with astrocytes conditioned medium (ACM), or with fibroblast conditioned medium (FCM), and were immunostained at 4 and 7 div with antibodies against GAD (green) and tau (red) to label GABAergic axons. (A), GABAergic axon length and branching were increased in neurons cultured with ACM (middle) but not when neurons were cultured with FCM (right) compared to neurons cultured alone (left) at 4 div. Areas within white boxes are shown below at higher magnification. Scale bar = 25 (top), 10 (bottom) μm. (B), Quantification of the effect of ACM or FCM on GABAergic axon length (left) and branching (right). All values are shown as mean ± s.d. (n = 77 cells, 3 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
(A), Hippocampal neurons were cultured alone, with astrocytes, or with ACM, treated with tetrodotoxin (TTX) were immunostained with antibodies against GAD, tau, and VGAT. TTX treatment had no effect on GABAergic axon length (top), branching (middle), or presynaptic terminal density (bottom) in neurons cultured with astrocytes or ACM compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 88 cells, 3 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (B), Hippocampal neurons were cultured alone, with astrocytes, or with ACM, treated with TrkB-IgG or TrkC-IgG were immunostained with antibodies against GAD, tau, and VGAT. Compared to control cultures treated with IgG (2.0 μg/ml final concentration), TrkB-IgG or TrkC-IgG had no effect on GABAergic axon length (top), branching (middle), or presynaptic terminal density (bottom) in neurons cultured with astrocytes or ACM at 7 div compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 116 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (C), Hippocampal neurons were cultured alone, with cholesterol or vehicle were immunostained with antibodies against GAD, tau, VGAT and GABAAR. Cholesterol treatment had no effect on GABAergic axon length (top) or branching (middle) compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 125 cells, 5 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
TSP antibodies were incubated with protein A/G beads, then added to 10-fold concentrated ACM. After incubation, equivalent samples of TSP-depleted ACM was compared to ACM incubated with protein A/G beads alone, along with the TSP that was bound to the beads. (A), Immunoblotting with TSP-1 specific antibodies shows that TSP-1 is depleted from ACM, and was bound to protein A/G beads (top). Immunoblotting with TSP-2 specific antibodies shows that TSP-2 is depleted from ACM and remains bound to protein A/G beads. (middle). Immunoblotting for ApoE was used as a lane loading control (bottom). Note the lack of an ApoE specific band in the TSP bound beads lane shows the specificity of the TSP immunodepletion. (B), Quantification of the amount of TSP1 (left) and TSP2 (right) immunodepleted from ACM. All values are shown as mean ± s.d. (n = 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.05).
Acknowledgments
We thank M. Maronski and M.O. Scott for technical assistance; and Drs. B. Barres, H. Ischiropoulos and S. Scherer, and members of the Balice-Gordon lab, for helpful discussions. This work was supported by grants from the NIH (NS046490 and MH057683) to R.B.-G. and an NIH NRSA (NS056549) to E.G.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967;6:716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Aoyagi A, Nishikawa K, Saito H, Abe K. Characterization of basic fibroblast growth factor-mediated acceleration of axonal branching in cultured rat hippocampal neurons. Brain Res. 1994;661:117–126. doi: 10.1016/0006-8993(94)91188-6. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bergsman JB, Krueger SR, Fitzsimonds RM. Automated criteria-based selection and analysis of fluorescent synaptic puncta. J Neurosci Methods. 2006;152:32–39. doi: 10.1016/j.jneumeth.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFreitas MF, Yoshida CK, Frazier WA, Mendrick DL, Kypta RM, Reichardt LF. Identification of integrin alpha 3 beta 1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Dowell JA, Johnson JA, Li L. Identification of Astrocyte Secreted Proteins with a Combination of Shotgun Proteomics and Bioinformatics. J Proteome Res. 2009 doi: 10.1021/pr900248y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16:197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene SD, Greco TM, Parastatidis I, Lee SH, Hughes EG, Balice-Gordon RJ, Speicher DW, Ischiropoulos H. Mass spectrometric and computational analysis of cytokine-induced alterations in the astrocyte secretome. Proteomics. 2009;9:768–782. doi: 10.1002/pmic.200800385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R, Esquenazi S. Astrocytes mediate cerebral cortical neuronal axon and dendrite growth, in part, by release of fibroblast growth factor. Neurol Res. 2002;24:81–92. doi: 10.1179/016164102101199459. [DOI] [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Chang YH, Vaszil K, Barker JL. Astrocytes regulate amino acid receptor current densities in embryonic rat hippocampal neurons. J Neurobiol. 1997;33:848–864. [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Li YX, Dunlap V, Barker JL. Upregulation of GABAA current by astrocytes in cultured embryonic rat hippocampal neurons. J Neurosci. 1996;16:2912–2923. doi: 10.1523/JNEUROSCI.16-09-02912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens H, Weston MC, Boulland JL, Gronborg M, Grosche J, Kacza J, Hoffmann A, Matteoli M, Takamori S, Harkany T, Chaudhry FA, Rosenmund C, Erck C, Jahn R, Hartig W. Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J Neurosci. 2008;28:13125–13131. doi: 10.1523/JNEUROSCI.3887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthiessen HP, Schmalenbach C, Muller HW. Astroglia-released neurite growth-inducing activity for embryonic hippocampal neurons is associated with laminin bound in a sulfated complex and free fibronectin. Glia. 1989;2:177–188. doi: 10.1002/glia.440020307. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Miller MW. Maturation of rat visual cortex. III. Postnatal morphogenesis and synaptogenesis of local circuit neurons. Brain Res. 1986;390:271–285. doi: 10.1016/s0006-8993(86)80236-0. [DOI] [PubMed] [Google Scholar]
- Moore NH, Costa LG, Shaffer SA, Goodlett DR, Guizzetti M. Shotgun proteomics implicates extracellular matrix proteins and protease systems in neuronal development induced by astrocyte cholinergic stimulation. J Neurochem. 2009;108:891–908. doi: 10.1111/j.1471-4159.2008.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol. 2001;533:665–679. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Steinmetz CC, Buard I, Claudepierre T, Nagler K, Pfrieger FW. Regional variations in the glial influence on synapse development in the mouse CNS. J Physiol. 2006;577:249–261. doi: 10.1113/jphysiol.2006.117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol Cell Neurosci. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hippocampal neurons were cultured alone, with astrocytes conditioned medium (ACM), or with fibroblast conditioned medium (FCM), and were immunostained at 4 and 7 div with antibodies against GAD (green) and tau (red) to label GABAergic axons. (A), GABAergic axon length and branching were increased in neurons cultured with ACM (middle) but not when neurons were cultured with FCM (right) compared to neurons cultured alone (left) at 4 div. Areas within white boxes are shown below at higher magnification. Scale bar = 25 (top), 10 (bottom) μm. (B), Quantification of the effect of ACM or FCM on GABAergic axon length (left) and branching (right). All values are shown as mean ± s.d. (n = 77 cells, 3 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
(A), Hippocampal neurons were cultured alone, with astrocytes, or with ACM, treated with tetrodotoxin (TTX) were immunostained with antibodies against GAD, tau, and VGAT. TTX treatment had no effect on GABAergic axon length (top), branching (middle), or presynaptic terminal density (bottom) in neurons cultured with astrocytes or ACM compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 88 cells, 3 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (B), Hippocampal neurons were cultured alone, with astrocytes, or with ACM, treated with TrkB-IgG or TrkC-IgG were immunostained with antibodies against GAD, tau, and VGAT. Compared to control cultures treated with IgG (2.0 μg/ml final concentration), TrkB-IgG or TrkC-IgG had no effect on GABAergic axon length (top), branching (middle), or presynaptic terminal density (bottom) in neurons cultured with astrocytes or ACM at 7 div compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 116 cells, 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001). (C), Hippocampal neurons were cultured alone, with cholesterol or vehicle were immunostained with antibodies against GAD, tau, VGAT and GABAAR. Cholesterol treatment had no effect on GABAergic axon length (top) or branching (middle) compared to neurons cultured alone. All values are shown as mean ± s.d. (n = 125 cells, 5 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.001).
TSP antibodies were incubated with protein A/G beads, then added to 10-fold concentrated ACM. After incubation, equivalent samples of TSP-depleted ACM was compared to ACM incubated with protein A/G beads alone, along with the TSP that was bound to the beads. (A), Immunoblotting with TSP-1 specific antibodies shows that TSP-1 is depleted from ACM, and was bound to protein A/G beads (top). Immunoblotting with TSP-2 specific antibodies shows that TSP-2 is depleted from ACM and remains bound to protein A/G beads. (middle). Immunoblotting for ApoE was used as a lane loading control (bottom). Note the lack of an ApoE specific band in the TSP bound beads lane shows the specificity of the TSP immunodepletion. (B), Quantification of the amount of TSP1 (left) and TSP2 (right) immunodepleted from ACM. All values are shown as mean ± s.d. (n = 4 independent expts.; Kruskal-Wallis nonparametric ANOVA test followed by Dunn’s pairwise multiple comparison test, p < 0.05).