Abstract
Fibroblast Growth Factor Receptor (FGFR) is overexpressed in a wide variety of tumors, and therefore is an attractive target for drug delivery. Recombinant FGF-1 was purified and attached to a fifth-generation (G5) polyamidoamine dendrimer. The specific binding and internalization of this conjugate labeled with FITC was demonstrated by flow cytometry as well as by confocal microscopic analysis in cell lines expressing FGFR. The binding and uptake of FGF-conjugated dendrimers was completely blocked by excess non-conjugated FGF-1. Confocal microscopic analysis showed cytosolic as well as nuclear localization. Multivalent G5-FGF nanoparticles may serve as a platform for cytosolic as well as nuclear drug delivery in tumor cells, and as an FGF delivery agent for angiogenesis and wound healing. Our study shows for the first time the applicability of a dendrimer nanodevice for tumor cell targeting through FGFR.
The fibroblast growth factors (FGFs) are a family of 23 identified proteins which modulate diverse physiological functions such as cell proliferation, cell migration, wound healing, angiogenesis, and tumorigenesis.1–6 Their receptors (FGFR1–4), a family of four high affinity receptors, form a ternary membrane complex with an FGF and heparin sulphate. FGF-1 (acidic FGF; FGFα) is a 154-amino acid protein which preferentially binds to FGFR1 and FAFR4 and following internalization with the receptor, it is taken up also into the nuclear compartment.7, 8 FGFR is known to be overexpressed in cancers such as those of the bladder, breast, prostate, and squamous cells, and the overexpression can lead to tumor cell growth, tumor invasion, and metastasis.1–6 Although FGFR is also expressed in normal cells,9 the overexpression of this protein in tumors can be exploited as a therapeutic strategy for tumor-targeted drug delivery.10 Moreover, as the FGF signal pathways play a crucial role in neovascularization, the FGF-based chemotherapeutic delivery agent will compete with circulating FGF for binding to endothelial cells, leading to vascular growth inhibition and tumor cell apoptosis.11 Protein toxins directly conjugated to FGF have been demonstrated to preferentially kill tumor cells in vitro and in vivo.12–15 However, these toxin conjugates have the disadvantages of developing immunogenicity and toxicity. In this study we report the synthesis and FGFR-dependent tumor cell delivery of a dendrimer-FGF nanodevice. Dendrimer-based targeted delivery of a large payload of drugs through the FGFR will overcome the limitations of a simple `FGF-Toxin' or `FGF-Drug' conjugates.
Recombinant FGF1 was initially prepared from bacterial culture “BL21 (DE3) pLysS” carrying FGF-121–154 (kindly provided by Dr. Prudovsky, Maine Medical Center, Maine). The presence of FGF-121–154 in the culture was initially confirmed by PCR amplification of the DNA plasmid template using primers spanning the FGF-121–154 insert and additional nucleotides on the 5' and 3' region of the insert (data not shown). The purification of the FGF-1 was performed as described before.16 The purity of the protein was checked by SDS PAGE electrophoresis and staining with silver stain (Figure 1).
Figure 1.
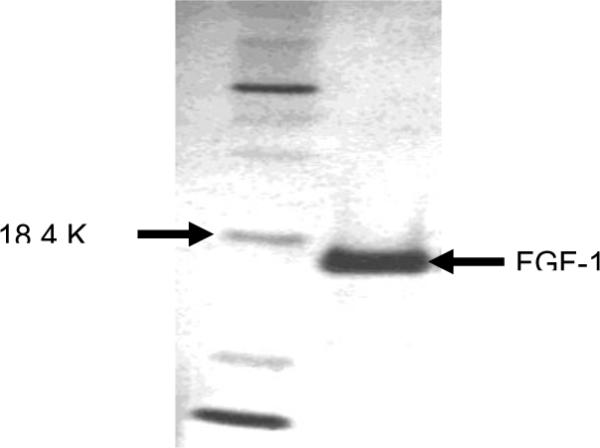
SDS-PAGE of purified recombinant FGF-1. 0.7 μg purified protein was run on a NuPAGETM 10% Bis-Tris gel (Invitrogen) for 35 minutes along with protein molecular weight standards (left lane), and the protein was stained using a Bio-Rad Silver Stain kit. The purified FGF-1 showed a single band that coincided with the theoretical molecular weight of 15.4 kDa.
The purified FGF-1 was iodinated by the Chloramine-T method at the Reproductive Biology Core facility of the University of Michigan. The specific activity of the iodinated protein was ~100,000 cpm/ng. The biological activity of the purified FGF-1 was initially confirmed by testing the 125I-FGF for its efficacy to bind onto the FGFR-expressing cell line SUM52. As shown in Figure 2, the purified protein bound to the SUM52 cell line in a dose-dependent fashion.
Figure 2.
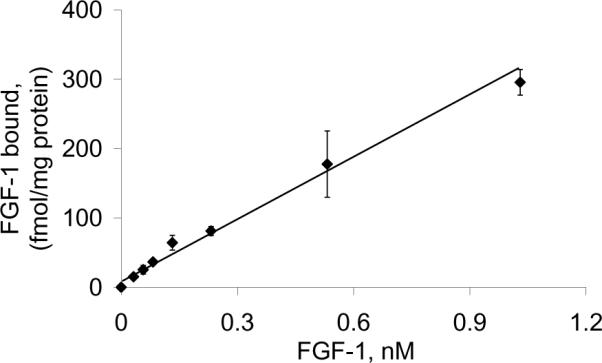
FGFR-expressing Sum52 cells cultured in 24-well plates was incubated with different concentrations of purified FGF-1 containing 32 pM 125I-FGF. Following incubation for 1 hour at 4°C, the cells were washed 4 times with phosphate-buffered saline and extracted with 0.5% Triton X-100, and the radioactivity was measured in a γ-counter.
The FGF-1 was then chemically conjugated the generation 5 (G5) PAMAM dendrimer (Scheme 1). The G5-dendrimer was prepared at the Michigan Nanotechnology Institute for Medicine and Biological Sciences, University of Michigan, and was characterized as described previously.17
Scheme 1.
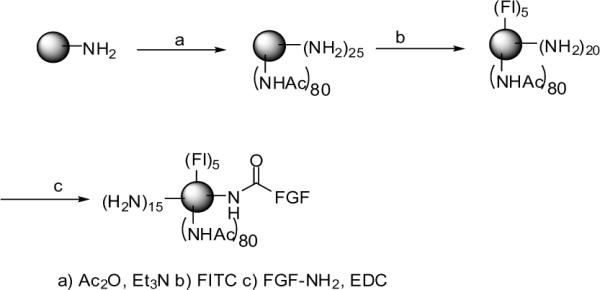
Synthesis of FGF-dendrimer conjugate
The molecular weight of the synthesized dendrimer was measured to be 26,530 g/mol by GPC, and the average number of primary amino groups was estimated to be 108 by potentiometric titration. The amine terminated dendrimer was partially acetylated to reduce the number of primary surface amines in order to minimize the charge interactions with the biologic molecules. G5 dendrimer (0.265 g, 0.0099 mmol) and triethyl amine (Et3N; 0.088 g, 0.8635 mmol) were dissolved in 30 mL anhydrous MeOH and allowed to stir for 30 minutes. A solution of acetic anhydride (Ac2O; 0.076 g, 0.744 mmol) in anhydrous MeOH (15 ml) was added dropwise while stirring. The reaction mixture was allowed to stir overnight at room temperature. After evaporation of the solvent, the residue was dissolved in H2O and dialyzed in 10,000 MWCO regenerated cellulose dialysis bags initially against PBS buffer, followed by water. The partially acetylated dendrimer was lyophilized to give a colorless powder (0.270 g, 91.2%). The purity of the partially acetylated compound and the extent of acetylation were evaluated by 1H NMR, which showed a distinct signal for the terminal NHCOCH3 protons at δ 1.85 ppm. The degree of acetylation was determined by comparing the ratio of the NHCOCH3 protons with the sum of all methylene protons in the dendrimer to a calibration curve as described previously.17
The fluorescent dye FITC was initially conjugated onto the dendrimer. For this, a DMSO solution of FITC (Fl, 0.0058 g, 15.0 μmol, 2 ml) was added dropwise to the G5-Ac (0.102 g, 3.4 μmol) in DMSO (10 ml). The reaction mixture was allowed to stir overnight. The reaction mixture was diluted 1:1 in PBS, and the free dye was separated from the conjugate (G5-FI) by gel filtration on a Sephadex G-25 column. The eluted conjugate was concentrated using a Centricon device (10,000 MWCO) and was dialyzed against PBS and H2O before lyophilization. The 1H NMR of the conjugate showed broad signals in the aromatic region corresponding to the FITC protons. The number of dye molecules attached to the dendrimer was calculated to be 3–4, based on UV/Vis spectroscopy and 1H NMR.
For conjugation of the FGF-1, to a PBS solution of FGF-1 solution (620 μg in 1.5 ml) was added N-(3-dimethylamineopropyl)-N'-ethylcarbodiimide hydrochloride (0.002g) followed by purified G5-FI (1.7 mg, 0.05362 μmol, in PBS buffer). The reaction mixture was stirred for ~48 hours and the product (G5-FI-FGF) was purified by extensive ultrafiltration against PBS and then H2O, using 10000 MWCO and 30000 MWCO Centricon devices, respectively, prior to lyophilization. The final conjugate was characterized by 1H-NMR spectroscopy that showed peaks in the aliphatic region due to the presence of the FGF peptide. UV-visible spectral analysis showed absorbance at λmax 500 nm specific for FI. As the FGF and FI absorb at 280 nm, the molar quantity of FGF present in the conjugate was difficult to ascertain by UV analysis.
The uptake of the conjugate was initially tested in the FGFR-expressing MCR5 endothelial cell line. The conjugate bound to the MCR5 cells, and the binding was reversed by excess free FGF, demonstrating receptor-specific binding (Figure 3).
Figure 3.
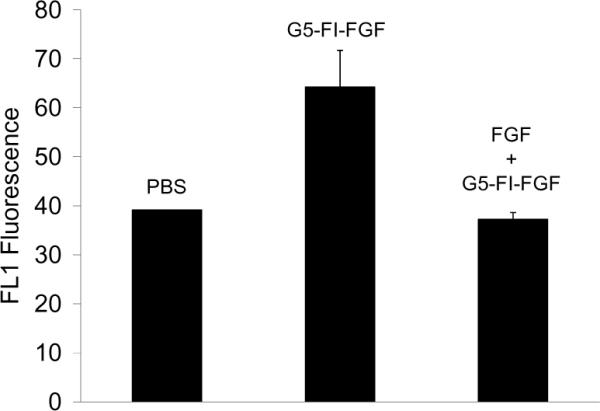
FGFR-dependent uptake of G5-FI-FGF in MCR fibroblasts. The cells were incubated with 300 nM of the conjugate for 1 hour at 37°C in the presence or absence of 15 μM free FGF and then rinsed, and the FL1 fluorescence was determined in a flow cytometer.
The binding of the conjugate was further tested in two additional cell types (Figure 4). The breast cancer cell line MCF7 showed only a modest increase in the binding of the conjugate, whereas the MCR endothelial cell line and the PC3 prostate cancer cell line showed significant specific binding. This is consistent with previous studies showing largely negative expression of the FGFR in un-stimulated MCF7 cells18 and positive expression of the FGFR4 in the PC3 prostate cancer cell line.19 The observations that the G5-FI-FGF conjugate bound to two different FGFR-expressing cell lines, that the control dendrimer G5-FI failed to bind to the FGFR-expressing cells, and that the G5-FI-FGF failed to associate with the FGFR-negative MCF7 cells all indicate receptor-specific binding of the conjugate. The competition between the binding of the conjugates and the free FGF further confirmed the specificity of binding.
Figure 4.
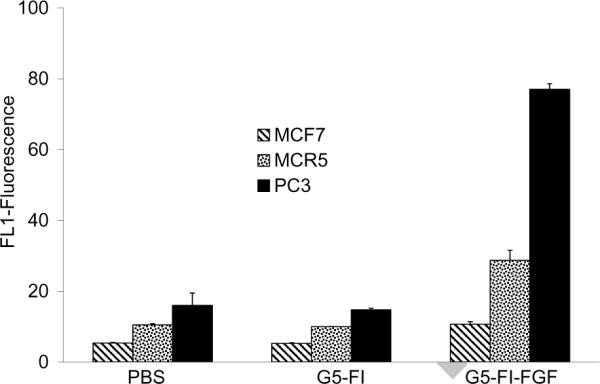
Comparison of the uptake of G5-FI-FGF in different cell lines, as determined by flow cytometry. The cells were incubated with 300 nM of the conjugate for 1 hour at 37°C and then rinsed, and FL1 fluorescence was measured.
Internalization of the conjugate was demonstrated in the PC3 cell line by confocal microscopic analysis (Figure 5). Internalization was observed as early as 1 hour, and significantly larger uptake was observed in 24 hours. The conjugate was primarily localized in the cytosolic compartment, similar to conjugates of the dendrimer with other molecules such as folic acid,20 antibodies21, 22 and EGF.23 A confocal microscopic z-series analysis of the fluorescence of single cells confirmed intracellular localization of the conjugate (data not shown). Perinuclear and nuclear localization are also observed, as has been shown before for free FGF.8, 18, 24, and for G5-EGF conjugate.23 Therefore the FGF-Dendrimer nanodevice may serve as a suitable platform for the targeted delivery of chemotherapeutic drugs with their cellular actions localized in either the cytosol (e.g. methetrexate) or in the nucleus (e.g. doxorubicin).
Figure 5.
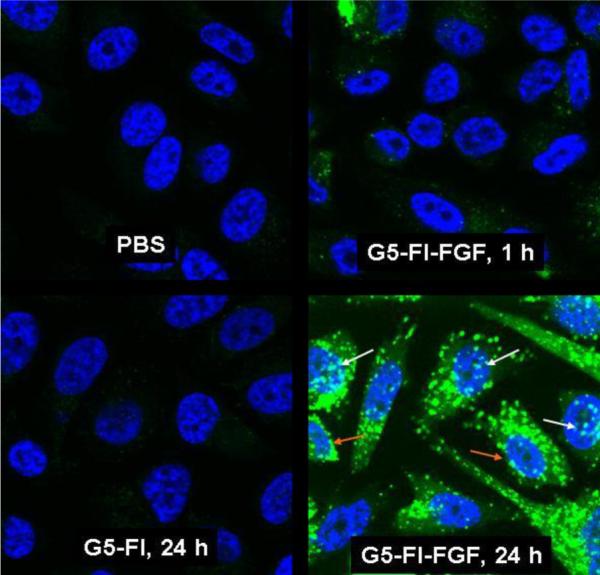
Internalization of G5-FI-FGF in PC3 cells. Cells grown on cover slips were incubated with 300 nM each of G5-FI-FGF or the control conjugate G5-FI at 37°C for 1 or 24 hours. Cells were rinsed and fixed with paraformaldehyde, mounted using solution containing the nuclei stain DAPI, and the fluorescence was measured in an Olympus confocal microscope. The green stain shows the conjugate fluorescence, and the blue stain shows the cell nuclei stained with DAPI. The white and the orange arrows show nuclear and perinuclear localization, respectively.
FGF-mediated signal transduction events play a crucial role in wound healing.24 Matrix-based systems using poly (D, L-lactic-co-glycolic acid) microspheres,25 lipid micelles,26 gelatin hydrogels,27 and collagen sponges28 have been reported for the sustained delivery of FGF. Our recent studies have shown that a polyvalent conjugate of PAMAM dendrimer with epidermal growth factor (EGF) acts as a super-agonist to stimulate cell growth.23 A polyvalent dendrimer-FGF conjugate may also serve as a sustained FGF delivery agent in therapies such as angiogenesis, wound healing and neuroprotection.25, 29–31
In conclusion, our studies show for the first time the suitability of PAMAM dendrimer as a platform for molecular delivery into cells through covalently conjugated FGF-1 on the dendrimer surface. Studies are in progress to test the suitability of this platform for in vivo tumor drug delivery.
ACKNOWLEDGMENT
This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under awards 1 R33 CA112141 and 1 R21 RR021893, and the National Institute of Biomedical Imaging and Bio-Engineering, National Institutes of Health, under award RO1 EB005028. This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases. We thank Dr. Stephen I. Lentz for his skillful technical help in the confocal microscopic analysis. Dr. James R. Baker, Jr., holds an ownership position in Avidimer Therapeutics, Inc., and is the inventor of technologies that the University has licensed to Avidimer Therapeutics, Inc, and some of these technologies are involved in this research. Avidimer Therapeutics, Inc. had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mason I. Current Biology. 2003;13:R346. doi: 10.1016/s0960-9822(03)00270-7. [DOI] [PubMed] [Google Scholar]
- 2.Ornitz DM, Itoh N. Genome Biology. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson C, Spencer-Dene B, Dillon C, Fantl V. Breast Cancer Research. 2000;2:191. doi: 10.1186/bcr53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Cytokine & Growth Factor Reviews. 2005;16:233. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Cytokine & Growth Factor Reviews. 2005;16:159. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Jeffers M, LaRochelle WJ, Lichenstein HS. Expert Opinion on Therapeutic Targets. 2002;6:469. doi: 10.1517/14728222.6.4.469. [DOI] [PubMed] [Google Scholar]
- 7.Bosman AW, Janssen HM, Meijer EW. Chem. Rev. 1999;99:1665. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 8.Bryant DM, Stow JL. Traffic. 2005;6:947. doi: 10.1111/j.1600-0854.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes SE. Journal of Histochemistry & Cytochemistry. 1997;45:1005. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 10.Marcinkowska E, Superat K, Wiedlocha A. Oncology Research. 2006;16:27. doi: 10.3727/000000006783981242. [DOI] [PubMed] [Google Scholar]
- 11.Rusnati M, Presta M. Current Pharmaceutical Design. 2007;13:2025. doi: 10.2174/138161207781039689. [DOI] [PubMed] [Google Scholar]
- 12.Coll-Fresno PM, Batoz M, Tarquin S, Birnbaum D, Coulier F. Oncogene. 1997;14:243. doi: 10.1038/sj.onc.1200826. [DOI] [PubMed] [Google Scholar]
- 13.Siegall CB, Gawlak SL, Chace DF, Merwin JR, Pastan I. Bioconjugate Chemistry. 1994;5:77. doi: 10.1021/bc00025a010. [DOI] [PubMed] [Google Scholar]
- 14.Davol PA, Frackelton AR., Jr. Prostate. 1999;40:178. doi: 10.1002/(sici)1097-0045(19990801)40:3<178::aid-pros6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Futami J, Seno M, Ueda M, Tada H, Yamada H. Protein Engineering. 1999;12:1013. doi: 10.1093/protein/12.11.1013. [DOI] [PubMed] [Google Scholar]
- 16.Engleka KA, Maciag T. Journal of Biological Chemistry. 1992;267:11307. [PubMed] [Google Scholar]; Briefly, the bacterial culture was induced to express the FGF-1 (under the control of the T7 promoter) by incubating with 4 mM IPTG for 3 hours at 37°C. The cells were harvested by centrifugation, lysed by sonication in lysis buffer (50 mM Tris, pH 8.0, 10 mM EDTA, 10 mM glucose, 150 mM NaCl, and 10 μg/ml egg lysozyme) and precipitated using solid ammonium sulfate at 50% saturation. The supernatant obtained was further precipitated with 90% ammonium sulfate saturation and the pellet was re-suspended in binding buffer (50 mM Tris, pH 7.4, 10 mM EDTA) and mixed with an equal volume of heparin sepharose CL6B pre-equilibrated with 0.6 M NaCl, 50 mM Tris, 10 mM EDTA, pH 7.4. The slurry was incubated at room temperature for 30 minutes and loaded into a 1.5 cm × 12 cm column and washed with 80 ml of binding buffer. The bound FGF-1 was collected by elution with 1.5 M NaCl in 10 mM sodium phosphate buffer, pH 7.2.
- 17.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr. Journal of Medicinal Chemistry. 2005;48:5892. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 18.Bryant DM, Wylie FG, Stow JL. Molecular Biology of the Cell. 2005;16:14. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropiquet F, Giri D, Kwabi-Addo B, Mansukhani A, Ittmann M. Cancer Research. 2000;60:4245. [PubMed] [Google Scholar]
- 20.Thomas TP, Shukla R, Majoros IJ, Myc A, Baker JR., Jr . In: Nanobiotechnology: Concepts, Methods and Perspectives. Mirkin, editor. Wiley-VCH; 2007. [Google Scholar]
- 21.Shukla R, Thomas TP, Peters JL, Desai AM, Kukowska-Latallo J, Patri AK, Kotlyar A, Baker JR., Jr. Bioconjugate Chemistry. 2006;17:1109. doi: 10.1021/bc050348p. [DOI] [PubMed] [Google Scholar]
- 22.Thomas TP, Patri AK, Myc A, Myaing MT, Ye JY, Norris TB, Baker JR., Jr. Biomacromolecules. 2004;5:2269. doi: 10.1021/bm049704h. [DOI] [PubMed] [Google Scholar]
- 23.Thomas TP, Shukla R, Kotlyar A, Liang B, Ye JY, Norris TB, Baker JR., Jr. Biomacromolecules. 2008;9:603. doi: 10.1021/bm701185p. [DOI] [PubMed] [Google Scholar]
- 24.Prudovsky I, Savion N, Zhan X, Friesel R, Xu J, Hou J, McKeehan WL, Maciag T. Journal of Biological Chemistry. 1994;269:31720. [PubMed] [Google Scholar]
- 25.Royce SM, Askari M, Marra KG. Journal of Biomaterials Science, Polymer Edition. 2004;15:1327. doi: 10.1163/1568562041960016. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Go DH, Bae JW, Lee SJ, Park KD. Journal of Controlled Release. 2007;117:204. doi: 10.1016/j.jconrel.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, Saji Y, Inui K.-i., Hashida T, Yokoyama S, Onodera R, Ikeda T, Fukushima M, Komeda M. Circulation Journal. 2007;71:1181. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- 28.Yao C-C, Yao P, Wu H, Zha Z-G. Journal of Materials Science-Materials in Medicine. 2007;18:1969. doi: 10.1007/s10856-007-3093-7. [DOI] [PubMed] [Google Scholar]
- 29.Cetin M, Aktas Y, Vural I, Capan Y, Dogan LA, Duman M, Dalkara T. Drug Delivery. 2007;14:525. doi: 10.1080/10717540701606483. [DOI] [PubMed] [Google Scholar]
- 30.Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, Pierce GF. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2002;5:517. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- 31.Lobb RR. European Journal of Clinical Investigation. 1988;18:321. doi: 10.1111/j.1365-2362.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]


