Abstract
Importance of the field
Hematopoietic stem cell transplantation (HSCT) is the treatment of choice for many hematological malignancies and genetic disorders. A majority of patients do not have a human leukocyte antigen (HLA) identical sibling donor, and alternative stem cell sources include HLA-matched or mismatched unrelated donors and haploidentical related donors. However, alternative donor HSCT are associated with three major complications (i) graft rejection, (ii) graft-versus-host disease (GvHD) and (iii) delayed immune reconstitution leading to viral infections and relapse.
Areas covered in this review
Graft rejection and the risk of GvHD can be significantly reduced by using intensive conditioning regimens, including in vivo T cell depletion as well as ex vivo T cell depletion of the graft. However, the benefits of removing alloreactive T cells from the graft are offset by the concomitant removal of T cells with anti-viral or anti-tumor activity as well as the profound delay in endogenous T cell recovery post-transplant. Thus, opportunistic infections, many of which are not amenable to conventional small-molecule therapeutics, are frequent in these patients and are associated with significant morbidity and high mortality rates. This review discusses current cell therapies to prevent or treat viral infections/reactivations post-transplant.
What the reader will gain
The reader will gain an understanding of the current state of cell therapy to prevent and treat viral infections post-HSCT, and will be introduced to preclinical studies designed to develop and validate new manufacturing procedures intended to improve therapeutic efficacy and reduce associated toxicities.
Take home message
Reconstitution of HSCT recipients with antigen-specific T cells, produced either by allodepletion or in vitro reactivation, can offer an effective strategy to provide both immediate and long-term protection without harmful alloreactivity.
Viral Infections After HSCT
Increasing numbers of viral pathogens have been implicated in infectious complications after HSCT, due to a combination of more intensive screening using improved detection methods and the extension of HSCT to higher risk patients who either receive more extensively manipulated products or who require more intensive and prolonged post-transplant immunosuppression1–6. Infections caused by endogenous herpesviruses like Epstein-Barr virus (EBV) and cytomegalovirus (CMV) are well documented, while more recently infections caused by human herpesvirus (HHV)-6, BK virus and the respiratory viruses respiratory syncytial virus (RSV), parainfluenza, metapneumovirus, adenovirus, and bocavirus are increasingly reported1–3;5;7–19 (Table 1). Pharmacologic agents are standard therapy for some infections, but most have substantial toxicities, drive the outgrowth of resistant viral variants, and are not effective against all viruses. Since the use of anti-virals does not improve virus-specific immunity, infections frequently recur after termination of treatment. In contrast, reconstitution of HSCT recipients with antigen-specific T cells can offer an effective non-toxic strategy to provide both immediate and long-term protection. Such immunotherapeutic strategies have been explored by a number of groups.
Table 1.
Emerging viral pathogens implicated in complications after HSCT and SOT.
| Virus | Incidence in bone marrow and solid organ transplant |
|---|---|
| Adenovirus | SOT and HSCT5;6;11;12;118–121 |
| Bocavirus | HSCT122 |
| Coronavirus | SOT and HSCT123–128 |
| HHV6 | SOT and HSCT18;19;129–138 |
| LCMV | SOT139–142 |
| Mumps and Measles | SOT and HSCT143–146 |
| Metapneumovirus | SOT and HSCT147–156 |
| Parainfluenza | SOT and HSCT1;128;157–162 |
| Parvovirus B19 | SOT and HSCT163–174 |
| RSV | SOT and HSCT13;128;175–178 |
| Rotavirus | SOT179–181 |
| West Nile virus | SOT and HSCT182–190 |
| BK Virus | SOT and HSCT17;191–195 |
Donor lymphocyte infusions
The first adoptive T cell transfer protocols in the allogeneic HSCT setting were based on the premise that donor peripheral blood contained T cells able to mediate antitumor and/or antiviral activity in the HSCT recipient. Accordingly, donor lymphocyte infusions (DLI) have been extensively used to provide anti-tumor immunity20–23, and to a lesser extent, antiviral immunity. DLIs should contain memory T cells specific for a broad range of viruses, however, while successful for the treatment of a proportion of infections with adenovirus and EBV24;25, the efficacy of this therapy is limited by the low frequency of T cells specific for many common “acute” viruses (such as RSV and parainfluenza) and the relatively high frequency of alloreactive T cells. The high ratio of alloreactive to virus-specific T cells is especially problematic in recipients of haploidentical transplants in whom a higher incidence of GvHD limits the tolerable DLI dose, severely limiting the dose of virus-specific T cells received 26;27.
Depletion of alloreactive T cells
To preserve the benefits and enhance the safety of DLI, strategies for the selective removal or inactivation of recipient-specific alloreactive T cells have been evaluated.
Induction of anergy
Antigen specific T-cell anergy can be induced ex vivo by T cell receptor (TCR) signaling in the absence of costimulation. T cells require at least two signals to become activated; signal 1 involves TCR engagement with peptide-loaded MHC molecules, while signal 2 is mediated by co-stimulatory molecules on T cells engaging their ligands on APCs. The interaction between the CD28 receptor on T cells and its ligands, B7-1 (CD80) and B7-2 (CD86) on APCs is one of the major positive co-stimulatory signals, and this can be blocked by fusion proteins, such as CTLA4-Ig, or monoclonal antibodies to CD80 or CD86. Guinan and colleagues investigated whether blockade of this interaction could be used clinically to render alloreactive donor T cells anergic. In their initial study they showed that co-culture of whole bone marrow with irradiated recipient cells in the presence of CTLA4-Ig for 36hrs reduced the frequency of alloreactive T cells, while reactivity to 3rd party cells was unaffected28. In 11 evaluable patients the alloanergized marrow could reliably engraft, and infusion of relatively large cell doses (median 28x106 CD3+ T cells/kg) was not associated with excessive GvHD 28.
In two follow-up phase I clinical trials the same group analyzed immune reconstitution, infection, and development of acute and chronic GvHD in a larger patient cohort who received haploidentical HSCT after ex vivo induction of alloantigen-specific anergy in donor T cells, again achieved using CTLA4-Ig. Again they found that alloanergization did not appear to impair immune reconstitution. The median absolute lymphocyte count (ALC) on day +30 was 0.42×109/L, which is similar to that of patients receiving unmanipulated BMT from HLA-matched sibling donors29;30. Further, despite relatively high numbers of anergized T cells (median CD3+ T cell dose was 29×106/kg) was not associated with an increased incidence of steroid-refractory acute or chronic GvHD. Of 11 high-risk patients (donor and/or recipient CMV seropositive), 5 reactivated CMV, but all were able to clear the infection with a short course (3 days) of antiviral therapy, and none developed either CMV disease or EBV PTLD. The authors are currently conducting a follow-up study using escalating doses of alloanergized T cells to define the optimal cell dose to improve immune reconstitution without causing severe GvHD.
Comoli and colleagues investigated a similar approach to induce alloantigen-specific T cell anergy by adding a combination of CTLA4-Ig and cyclosporine A (CsA) to in vitro primary mixed lymphocyte reactions. This induced a state of unresponsiveness to recipient alloantigens in donor PBMC, leaving anti-viral activity intact 31. However, the efficacy of this strategy remains to be tested clinically.
An alternative route to alloantigen-specific immune tolerance is to use the inhibitory/suppressive characteristics associated with regulatory T cells (Treg)32;33. To establish a role for Tregs in preventing GvHD after allogeneic SCT Rezvani and colleagues quantified the number of CD4+FOXP3+ Tregs in 32 donor grafts infused into HLA-matched siblings and found that a high frequency of Tregs in the donor was associated with a reduced risk of GvHD, while in 21 SCT recipients they found that a low CD4+FOXP3+ cell count early (day 30) after transplant was associated with an increased risk of GvHD34. The authors suggest that assessmentof Treg content can be used as a predictor of risk for acute GvHD and that ex vivo expanded Treg infusions could prevent or treat GvHD34. To this end, Hoffmann and colleagues validated the ex vivo expansion of large numbers of functional Tregs using cross-linked anti-CD3 and anti-CD28 antibodies together with high dose IL235. Recently this has been translated to the clinic and Trzonkowski et al adoptively transferred ex vivo expanded Tregs to two patients, one with chronic and one with acute GvHD. The infusions were associated with clinical benefit. In the case of the patient with chronic GvHD complete resolution of symptoms was achieved, while the patient with grade IV acute GvHD showed a transient clinical improvement36.
Selective allodepletion
A potentially more permanent approach to GVHD is to remove alloreactive T cells from the donor graft prior to infusion. Recipient-specific T cells activated by in vitro exposure to recipient cells, such as EBV-transformed lymphoblastoid cell lines (EBV-LCL) 37;38, activated lymphocytes39 or fibroblasts40, upregulate activation markers such as CD2537;41–45, CD6943;45;46, and CD13740;47, and proliferate, allowing their removal or elimination by immunomagnetic depletion47;49, apoptosis induction50;51, photodepletion39;48;52;53, or immunotoxin-conjugated antibodies37;38;42;54;55.
Three clinical trials using allodepleted T cells have been reported, all prepared using the CD25-immunotoxin42;54;55. Two were performed in pediatric recipients of haploidentical stem cell transplants and one in adults receiving an HLA-matched related donor transplant. Compared to earlier studies reporting GvHD of grade II or greater in 40% of patients after infusion of 1×105 unmanipulated donor T lymphocytes/kg56;57, Andre-Schmutz and colleagues found that infusion of doses as high as 8×105 allodepleted T cells/kg were safe and retained a virus-specific immune component since three of the infused patients with active CMV had a rapid increase in antigen-specific T cells post-infusion and subsequent resolution of their infections55. Solomon et al infused 16 elderly patients at high risk of severe GvHD with allodepleted T cells42. In eight patients who developed acute GvHD ranging from grade I/II (6 patients) to grade III/IV (2 patients), and the severity of disease correlated with the efficiency of depletion42. Finally, Amrolia and colleagues showed that the infusion of allodepleted cells to haploidentical SCT recipients was safe but a minimum dose of 1×105 cells/kg was required to produce accelerated anti-viral T cell recovery54.
Taken together these studies demonstrated the feasibility of add-back T cell therapy for clinical use but they also highlighted a number of limitations with current strategies. First, the availability of the clinical grade IT may be an issue for larger phase II/III studies. Second, in the study from Amrolia and colleagues, recipient-derived EBV-LCLs, which require 4–6 weeks to establish, were used as the T cell allo-stimulus. While EBV-LCLs provide an unlimited source of tumor-free professional antigen-presenting cells (APCs), LCL production increased the time required for T cell preparation54. This study also demonstrated the small window between the minimum cell dose for immune reconstitution and the maximum tolerate dose54. Third, achieving sufficient T cells for infusion can be challenging since the recovery of donor cells after allodepletion is approximately 10%. Thus a donor leukapheresis may be required and this is not feasible for unrelated stem cell donors. Finally, T cells specific for a most of pathogens circulate with lower frequency than those specific for persistent viruses like EBV and CMV58–60, therefore, even higher doses of allodepleted T cell may be required to provide full spectrum protection.
To allow the safe administration of larger T cell doses, suicide transgenes have been evaluated to mediate self destruction in case of adverse effects in vivo. The thymidine kinase gene from herpes simplex virus I (HSV-tk) has been used and validated clinically. TK phosphorylates the nontoxic prodrug ganciclovir, which then becomes phosphorylated by endogenous kinases to GCV-triphosphate, causing chain termination and single-strand breaks upon incorporation into DNA, which kills dividing cells. Several phase I-II studies, and a more recent Phase III study have shown that Ganciclovir administration can be used to reduce transferred TK-modified cells in vivo61–64. However, the TK gene product may be immunogenic and specific immune responses directed to this transgenic protein have been detected in vivo which may lead to the premature and unintentional elimination of infused cells65. Our group has investigated an alternative minimally-immunogenic approach in which allodepleted T cells were transduced with a retroviral vector encoding an inducible human caspase 9 (iCasp9) suicide gene66 and a selectable marker (truncated human CD19)67. Even after allodepletion, donor T cells could be efficiently transduced, expanded, and subsequently enriched by CD19 immunomagnetic selection, and that the engineered cells retained anti-viral specificity. Following, following iCasp activation with a small-molecule dimerizer over 90% of cells underwent apoptosis67. Thus, the scale-up of allodepletion doses should be feasible, and this is currently being tested at our Center in the Haploidentical transplant setting.
Infusion of ex vivo expanded CTL
An alternative strategy to prevent and treat specific viral infections after HSCT is the adoptive transfer of ex vivo-expanded T cells with antiviral activity. The specific expansion of virus-reactive T cells has the advantage of increasing the numbers of virus-specific T cells that can be infused without increasing alloreactive T cells.
Cytomegalovirus (CMV)
CMV is a persistent beta-herpesvirus that is frequently reactivated from recipient or host tissues after allogeneic SCT. Fatal pneumonitis may follow new infection or reactivation, and available therapies may fail or prove toxic10. Riddell and colleagues infused in vitro expanded cytomegalovirus (CMV) reactive CD8+ T cell clones into 14 allogeneic HSCT patients to prevent CMV reactivation, and found that the cells were safe and able to restore anti-viral immunity in vivo. The transferred cells persisted for at least 8 weeks based on T cell Receptor (TCR) clonotyping studies, but progressively declined in patients who did not develop a concomitant endogenous CMV-specific CD4+ T helper response68. Subsequently, Einsele and colleagues generated polyclonal CMV-specific CTL lines containing both CD4+ and CD8+ T cells and infused them in patients with antiviral chemotherapy-resistant CMV viremia. The clinical results were impressive, and infusion of small numbers of cells (107cells/m2) significantly reduced the viral load in 7 evaluable patients, an effect that was sustained long term in 5 subjects but was transient in the two who had the highest virus load (>105 CMV-DNA copies/mL). A second T cell infusion controlled infection completely in one patient, but the other eventually succumbed to fatal CMV encephalitis after refusing a second dose of CTL.
Similarly encouraging results using polyclonal CMV-specific CTL lines were published by Peggs and colleagues and more recently by Micklethwaite et al, although the CTL lines were generated using different antigen sources69;70. Peggs et al used DCs loaded with inactivated CMV antigen produced from human lung fibroblast cell cultures infected with human CMV (Towne strain) to stimulate PBMCs from allogeneic HSCT donors 69;71. Sixteen patients were treated with 1×105 cultured CMV-specific T cells/kg at a median of 36 days post-transplant, after the first episode of CMV viremia. The infused cells were safe, did not cause GvHD, and expanded in vivo as confirmed by tetramer analysis in donors with informative HLA types. Furthermore, the cells appeared to be effective, resulting in reconstitution of viral immunity and in eight of the ten cases additional antiviral drugs were not required69. Micklethwaite and colleagues generated donor-derived, CMV-specific T cells for prophylactic use in 12 adult HSCT patients by stimulating polyclonal CD4+ and CD8+ T cells with DCs transduced with a chimeric adenoviral vector encoding the immunodominant CMV antigen pp65. There was no infusion-related toxicity and although four patients reactivated CMV the titer was low and antiviral therapy was not needed to achieve viral control70.
Epstein Barr Virus (EBV)
T cell therapy has also been successfully used to prevent and treat viral EBV associated lymphoproliferative disorders (post transplant lymphoproliferative disease; PTLD) after HSCT or solid organ grafting. Although most patients respond to withdrawal of immunosuppression and/or the anti-B cell antibody rituximab72, the disease may progress, with a fatal outcome. Rooney and colleagues generated EBV-specific CTL using EBV-LCLs as stimulators and transferred them to immunocompromised patients at risk of developing EBV-associated PTLD. Since 1993 this group has infused over 100 SCT recipients with donor-derived polyclonal T cell lines and established that a dose of 2×107 CTL/m2 is safe and effective for both prophylaxis and treatment73–75. A similar approach has been used by other groups to achieve similar results76;77. The first 26 patients enrolled in the Rooney study received CTLs which were genetically marked with a retroviral vector containing the neomycin resistance gene (neo). Long-term follow-up showed that the marked cells could be detected for as long as 9 years post infusion.
Although effective in a majority of patients treated for active disease, CTL therapy for EBV has failed in exceptional cases. Although the EBV-LCLs used as APCs express a range of viral latent and early lytic antigens, their immunogenicity is hierarchical and HLA dependent and some CTL lines display specificity for a limited number of epitopes from 1 or 2 viral proteins78–80. Therefore, efforts to treat EBV-LPD may fail if the tumor mutates an immunodominantviral target antigen which is the major specificity contained within the CTL line. This complication was discovered in a patient with EBV-LPD whose CTL line was largely HLA-A11-restricted with specificity for 2 epitopes in EBNA3B, both of which were deleted in the tumor virus81 This highlights the importance of infusing a CTL product which is polyclonal (CD4+ and CD8+) with broad antigen and epitope specificity in order to minimize the potential for tumor immune evasion.
Multivirus CTL
More recently the safety and efficacy of CTL lines simultaneously targeting EBV, CMV, and adenovirus (Adv) has been demonstrated in HSCT recipients. APCs were produced by expressing the immunodominant CMV-pp65 antigen in activated monocytes82 and EBV-LCLs using a chimeric adenoviral vector83. These APCs consistently reactivated CTLs specific for all three viruses in a single culture, although their specificity was dominated by CMV-reactive T cells, with a smaller fraction of EBV- and adenovirus-reactive T cells84;85. Infusion of donor-derived, trivirus-specific CTLs was safe in recipients of HLA-matched related or unrelated donors and the infused cells demonstrated apparent activity against all three viruses in vivo. Strikingly, however, only the CTLs directed to EBV and CMV showed evidence of in vivo expansion and persistence. By contrast, adenovirus-specific CTLs were detected in the peripheral blood after infusion only in patients who also had positive adenoviral cultures, demonstrating the importance of antigen in vivo as a stimulus for the infused cells84. However, none of the trivirus-specific CTL recipients developed adenovirus infections, by contrast to 68% of similar patients who did not receive CTLs12, suggesting that the adenovirus-specific CTLs may survive and enter memory, likely residing in the spleen and circulating only during periods of infection. This supposition was supported by the observation that adenovirus-specific T cells could be detected if first expanded by antigenic restimulation in vitro86. Thus it appears that broad spectrum antiviral protection and treatment can be provided from a single infusion of cells and small numbers of T cells can provide long term anti-viral protection.
Limitations of current CTL generation protocols
Although the administration of ex vivo activated and expanded antigen-specific T cells with single or multivirus specificity appears to be a safe and effective means of preventing and/or treating viral infections that arise in the immunocompromised host, there are a number of limitations to the broader implementation of T cell immunotherapy. These include; (i) time taken to produce clinical grade CTL, (ii) costs associated with CTL production, (iii) complexity of production, (iv) competition between multiple viral antigens for HLA molecules on APCs and (v) the wide range of viruses that require coverage.
Time to manufacture CTL lines
In the case of EBV and trivirus CTL, the generation of the EBV-LCL used as APCs requires 4 to 6 weeks followed by an additional 4 to 6 weeks for CTL activation and expansion, followed by a 1–2 weeks to perform identity, sterility and potency testing. This precludes urgent treatment of seriously ill patients, and CTL must be prepared speculatively and in advance for patients judged to be high risk so that they are available if needed.
Cost
Besides the infrastructure cost of building and maintaining a GMP facility and maintaining regulatory components (quality assurance, quality control, data management), there are a number of production costs which must also be taken into consideration including the technician time to produce APCs and CTL for clinical use, the cost of manufacturing and testing clinical grade viral vectors that are used for genetic modification of T cells and APCs, the reagents and media for CTL production, and the release testing that must be performed on CTL lines prior to infusion to ensure identity, purity and potency. In 2009 the cost for manufacturing, testing and infusing of an EBV CTL line was $6,09587, while the generation of a trivirus line was $10,559, excluding professional time. Although each line is a patient-specific product, it should be noted that this therapeutic modality nevertheless compares favorably with others; for example, CD20 monoclonal antibody therapy for treatment of EBV-LPD is $9,000 per dose.
Complexity
The production process itself is relatively complex, necessitating the generation and genetic modification of APCs for weekly CTL stimulation, repeat feeding of open culture systems, and multiple skilled “judgment calls”, which also serves to limit scalability.
Antigenic competition
While in our study trivirus-specific CTL could be generated consistently in a single culture84 the lines were heavily dominated by CMV-reactive T cells85. This competition is likely due to a combination of factors including the lower frequency of circulating adenovirus-specific T cells relative to EBV and CMV-specific T cells in healthy donors60;80;88, and to competition from the high affinity or more stable CMV and EBV epitopes for presentation by HLA molecules in the APCs89;90. This may limit the number of organisms to which a single CTL line can be reactive.
Spectrum of viruses
The range of viruses detected post-transplant is continually increasing as more reagents become available for screening and detection (Table 1). Some of these such as EBV, CMV, Adv, BK virus and HHV6 are clearly associated with graft failure and/or morbidity and mortality post-transplant, while more recently identified viruses such as metapneumovirus and bocavirus, though detected in the post-transplant setting, have not been definitively connected with severe disease1;6. Nevertheless, these emerging viruses must be considered in the development of future CTL protocols, should prospective studies identify them as causative factors in post-transplant morbidity and mortality.
Overcoming limitations of CTL therapies
Rapid CTL production
The direct isolation of HLA-multimer-binding T cells, or the selection of IFN-γ expressing T cells following stimulation with either recombinant protein or peptide stimulation allow rapid selection of virus-reactive T cells for direct infusion into patients. Several groups have demonstrated that small numbers of ex vivo selected, antigen-specific T cells can expand substantially after infusion into HSCT recipients and protect against the targeted pathogen91–93; a median of 8.6×103 per kg of tetramer selected and 1.2–50×103/kg per kg of T cells selected by their secretion of γ–IFN in response to antigen stimulation in the Miltenyi gamma catch system proved clinically effective. However, there are also limitations to these approaches; tetramer selection is restricted to CD8+ T cells with known epitope specificities and to viruses, such as CMV, with a high frequency of circulating reactive T cells91;94. The IFN-γ-capture assay provides an HLA unrestricted means to select specific T cells with both effector and central memory characteristics95–98 that should persist in vivo and mediate long-term protection against viral challenge99. However a low frequency of circulating cells specific for certain viruses may limit T-cell recovery100. One potential approach to enhance T-cell recovery is to stimulate T cells with combinations of whole antigens from different viruses96. Increased numbers of activated and selected cells may support the survival of low frequency antigen-reactive cells, however a minimum effective dose for infusion is yet to be established.
Third party banks
To bypass the need to grow CTLs for individual patients, banks of CTL lines that are available as an “off the shelf” product for immediate use have been evaluated. Since it is unlikely that a completely HLA-matched line will be available, the most closely HLA-matched line is administered. This raises two potential concerns; (i) the risk of inducing GvHD by administering a 3rd party CTL product101–103 and (ii) limited in vivo persistence, due to recipient alloreactivity to non-shared HLA antigens. However, a number of small studies have shown the feasibility of this approach and reported clinical responses in the patients with EBV lymphoma arising after HSCT or solid organ transplant104;105. Haque and colleagues used 3rd party EBV-specific CTLs to treat PTLD after solid organ transplant or SCT and showed an encouraging response rate of 64% and 52% at 5 weeks and 6 months, respectively106. In this study patients received 4 doses of 2×106 CTL/kg at weekly intervals. Lines were selected for matching by low resolution typing and screened for high level killing of donor EBV-LCLs and low level killing of patient PHA blasts. The degree of HLA matching ranged from 2/6 to 5/6 antigens and there was a statistically significant trend towards a better outcome with closer matching at 6 months. Importantly no patient developed GVHD post CTL administration106. In another report two solid organ recipients with CNS lymphoma received closely matched EBV-specific T cells resulting in complete resolution of their brain lesions107.
Given the promising results using “allogeneic” EBV-specific CTL we are currently evaluating the safety and feasibility of using “off-the-shelf” trivirus CTL for treating HSCT recipients with CMV, adenovirus or EBV infections that persist despite standard therapy. In this multicenter phase I clinical trial, CTL lines for infusion are chosen based first on the presence of activity against the problem virus through the shared HLA allele(s), and second on the overall degree of HLA matching. For example, we would favor a CTL line matched at a single allele through which there was documented antiviral activity over a line matched at three alleles through which antiviral reactivity was not detected. Preliminary results in ten recipients, most of whom had received alternative donor transplants were encouraging. None experienced acute GvHD and complete or partial responses were achieved in 5 of 8 evaluable recipients. If this trend continues we will generate a larger CTL bank to cover as many racial groups as possible and progress to a Phase II clinical trial where we can ask more specific questions regarding the persistence and function of the CTL in vivo.
Reducing the production time and increasing the efficacy of APCs
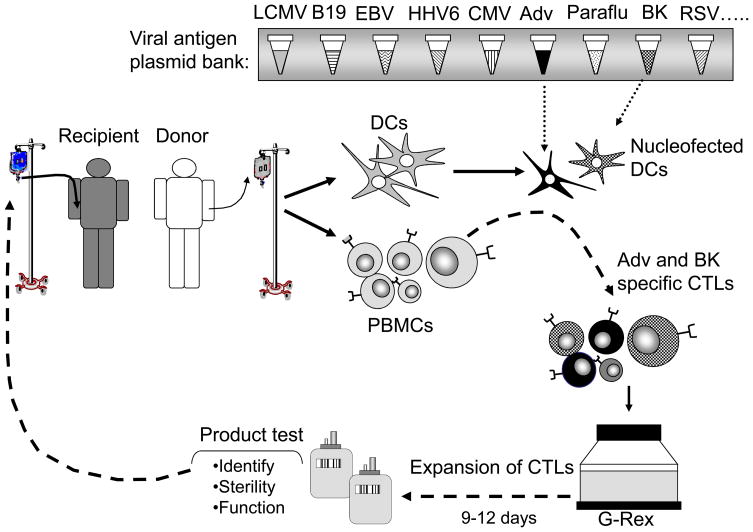
Trivirus-specific T cells are produced by reactivating peripheral blood T cells with autologous monocytes transduced with an Ad5f35 vector expressing CMV-pp65, followed on days 9, 16 and 23 by restimulation with autologous EBV-LCL transduced with the same vector84. The infectious viruses, EBV for EBV-LCL production and clinical grade adenoviral vector required for CTL stimulation are expensive to make and test. To reduce costs and avoid the use of viral vectors, we have investigated alternative sources of antigen, and have evaluated DNA plasmids that encode antigens from all three viruses and can be introduced into APCs, such as monocytes or DCs, using the clinically applicable AMAXA nucleofection system. After transfer, high level transgene expression is achieved with good APC viability during the period of T cell activation. Plasmids are non-infectious, non-replicative, and integrate poorly into the transfected cell genome, and clinical grade DNA can be rapidly and cost-effectively produced in scalable quantities with excellent long term stability. We estimate that the substitution of plasmids reduces the cost of manufacture by more than 50%, by eliminating LCL manufacture and viral vector testing testing, since the cost of plasmid testing is about one tenth that of adenovirus vector and EBV testing. Plasmids also reduce antigenic competition for HLA molecules since APCs can be nucleofected separately with each plasmid. Further, we can add to our clinical grade plasmid library as new protective antigens from other viruses are identified, which will allow us the flexibility to increase the spectrum of antigens targeted by our CTL as and when needed.
Simplifying CTL production
Most current protocols for the activation and expansion of antigen-specific CTL ex vivo are complicated and labor intensive, limiting the broad application of this therapy. Many groups expand antigen-specific CTL for clinical use in the 2cm2 wells of 24-well plates, which are not suitable for routine production of large cell numbers. In standard static culture vessels, the depth of medium is limited by oxygen diffusion to about 1mL/cm2, a volume that limits the supply of nutrients and concentrates waste products including lactic acid and CO2. As a result, the maximum cell density that can be achieved is about 2 × 106 cells/cm2/mL. Consequently, to produce large T cell numbers, skilled GMP technologists must frequently divide the cultures and replenish media and growth factors to sustain expansion.
To improve cell output with minimal cell handling, a number of closed-system bioreactors have been explored. Mechanical rocking or stirring can be used to increase the availability of O2 in the culture, while media and nutrients can be exchanged by perfusion109–113. Examples of such bioreactors include stirred tank bioreactors as well as static hollow fiber bioreactors. Stirred bioreactors allow high density cell growth and can readily be scaled up, but shear stress associated with the stirring rate reduces cell viability, and cultures require frequent medium sampling to evaluate growth-limiting factors like glucose and waste metabolites. In contrast, constant medium perfusion in the hollow fiber bioreactors results in the dilution of metabolites without shear stress, but cell sampling to assess T cell status during the culture is difficult. High cell densities can also be achieved in culture bags on rocking platforms, and the Wave Bioreactor has been used by Jensen and colleagues for therapeutic T cell production114. Although all are GMP applicable and can produce large numbers of cells, their disadvantages are the cost of purchase and the space required for specialized equipment, as well as the complexity of running and maintaining the equipment. Moreover, although genetically-engineered, mitogenically-activated T cells can be cultured in bioreactors, they have proven inefficient for antigen-specific CTL production, since CTL have strict requirements for prolonged interaction with APCs and feeder cells that are disrupted by mechanical agitation.
Vera and co-workers have described an improved manufacturing system for virus-specific CTL using optimized cell seeding densities in a novel cell gas permeable rapid expansion device (G-Rex) that supports medium to large-scale production of cells for clinical use (Vera et al, J. of Immunotherapy In press). By using cultureware that promotes optimal O2 and CO2 exchange, the initial input volume of medium can be increased, which in turn increases the available nutrients and dilutes waste products without the need for culture agitation, frequent culture feeding, or continuous medium perfusion. This allows higher antigen-specific T-cell densities per unit surface area to be achieved (8–10 × 106 per cm2 compared to 2 to 3 × 106 per cm2 in wells), and simplifies production by minimizing the number and complexity of manipulations. The G-Rex supports and promotes more rapid cell expansion than our current systems that can be further increased by the addition of enhancing cytokines. Thus cells can be made and are available for infusion sooner that would otherwise be possible. It is important to note that this rapid expansion and increased cell numbers are due to reduced cell death rather than increased cell division, thus the CTL are not functionally “exhausted” prior to adoptive transfer. Rapid expansion produces over two logs expansion of virus-specific T cells, with a concomitant but difficult to quantify loss of alloreactive T cells, that favors the ratio of virus-specific to alloreactive T cells. It should also be noted that although the G-Rex device probably cannot readily support the production of massive T cell numbers ( up to 109 CTLs per G-Rex500) a number of studies have shown that massive quantities of cells are not required for reconstitution of virus-specific immunity after HSCT74;84;91;92.
Extending CTL therapy to recipients of grafts from virus-naive donors
Despite the promising results of CTL therapy in both the HLA matched and mismatched allogeneic HSCT setting, it may be more challenging to translate this therapeutic modality to recipients of grafts from seronegative donors or to the cord blood transplant setting - an increasingly important alternative source of HLA mismatched stem cells. Generation of a virus-specific T cell product for infusion is complicated by the naive phenotype of virus-reactive T cells and their vanishingly low frequency. Hence, the generation of CTL requires the priming and extensive expansion of naive T cells rather than the more simple direct expansion of a pre-existing virus-specific memory T cell population. Modifications to traditional CTL generation schema using optimized APCs and enhancing cytokines have allowed functional CTLs to be generated even from this starting population115;116. Whether CTL derived from naive T cells will have the same in vivo persistence and antiviral activity as CTLs from peripheral blood remains to be evaluated.
Expert opinion section
Ideally, viral antigen-specific CTL preparation from allogeneic donors should rapidly and selectively produce CTL in numbers sufficient to reproducibly provide therapeutic benefit without harmful alloreactivity. This should require small amounts of donor blood, which could be obtained and cryopreserved at the time of transplant, even from unrelated donors. The CTL product should protect against a wide range of infectious agents, and not only the commonly detected CMV, Adv, EBV, BK virus, and HHV6 but also less common viruses including RSV and parainfluenza (Table 1). While these viruses are detected less frequently or may have a seasonal detection pattern, taken together they contribute significantly to patient care costs and virus-related mortality rates after allogeneic HSCT, and thus rapidly-generated broad-spectrum CTL may offer a cost-effective and safe therapy.
In the previous sections we have outlined various improvements to CTL generation protocols and we are currently combining these strategies to develop and validate new manufacturing procedures which simplify and shorten CTL production and extend the number of viruses targeted. Immunogenic antigens from a range of viruses will be expressed from plasmids after nucleofection into DCs or monocytes117. The CTL will be expanded in the G-Rex device to support optimal expansion, and after 9–12 days in culture the CTL will be tested for identify, sterility and function and then can be infused either prophylactically or therapeutically (Figure 1). Implementation of these modifications in our CTL production processes will enable the extension of T cell therapy to a broad spectrum of clinically relevant viruses using a single CTL product which will be more cost-effective (we predict a reduction in the cost of CTL manufacture from $10,559 to $3,505) and less toxic that administering multiple antiviral agents, even if these were able to deliver the same breadth of protection.
Figure 1. Rapid generation of multivirus-specific CTL.
Our CTL manufacturing process will be shortened from >10 to <2 weeks by using plasmid nucleofected DCs to activate T cells, which will then be efficiently and rapidly expanded in the G-Rex.
Article highlights box
Viral infections are frequent after hematopoietic stem cell transplant
T cell therapy can offer an effective non-toxic strategy to provide both immediate and long-term protection
Alloreactive T cells must be removed or inactivated to enhance the safety and improve the efficacy of donor leukocyte infusions
Adoptive transfer of cytotoxic T lymphocyte (CTL) lines targeting single or multiple viruses simultaneously can prevent and treat infections in immunocompromised individuals
Current preclinical work aims to overcome manufacturing limitations to allow the broad implementation of T cell therapy
Acknowledgments
A.M.L., T.T. and C.M.R. are supported by a PO1 NIH-NCI CA094237, a Specialized Centers for Cell-based Therapy Grant NIH-NHLBI 1 U54 HL081007, the Dan L. Duncan Cancer Center, the HHV6 Foundation, and an Amy Strelzer Manasevit Scholar Award (to A.M.L).
Reference List
- 1.Boeckh M, Erard V, Zerr D, Englund J. Emerging viral infections after hematopoietic cell transplantation. Pediatr Transplant. 2005;9 (Suppl 7):48–54. doi: 10.1111/j.1399-3046.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 2.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerr DM, Corey L, Kim HW, et al. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 4.Bruno B, Gooley T, Hackman RC, et al. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9:341–352. doi: 10.1016/s1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 5.Leen AM, Rooney CM. Adenovirus as an emerging pathogen in immunocompromised patients. Br J Haematol. 2005;128:135–144. doi: 10.1111/j.1365-2141.2004.05218.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–1339. doi: 10.1097/TP.0b013e31818b6548. [DOI] [PubMed] [Google Scholar]
- 7.Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 9.Leen AM, Heslop HE. Cytotoxic T lymphocytes as immune-therapy in haematological practice. Br J Haematol. 2008;143:169–179. doi: 10.1111/j.1365-2141.2008.07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 11.Flomenberg P, Babbitt J, Drobyski WR, et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 12.Myers GD, Krance RA, Weiss H, et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath) Bone Marrow Transplant. 2005 doi: 10.1038/sj.bmt.1705164. [DOI] [PubMed] [Google Scholar]
- 13.Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–638. [PubMed] [Google Scholar]
- 14.Giraud G, Priftakis P, Bogdanovic G, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplant. 2008;41:737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 15.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egli A, Binggeli S, Bodaghi S, et al. Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant. 2007;22(Suppl 8):viii72–viii82. doi: 10.1093/ndt/gfm648. [DOI] [PubMed] [Google Scholar]
- 17.Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7:2727–2735. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 18.de Pagter PJ, Schuurman R, Meijer E, et al. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2008;43:361–366. doi: 10.1016/j.jcv.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 19.de Pagter PJ, Schuurman R, Visscher H, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14:831–839. doi: 10.1016/j.bbmt.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994;330:100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 21.Porter DL, Collins RH, Jr, Shpilberg O, et al. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant. 1999;5:253–261. doi: 10.1053/bbmt.1999.v5.pm10465105. [DOI] [PubMed] [Google Scholar]
- 22.Kolb H-J, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte infusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 23.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 24.Hromas R, Cornetta K, Srour E, Blanke C, Broun ER. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood. 1994;84:1689–1690. [PubMed] [Google Scholar]
- 25.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 26.MacKinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy using donor leukocytes following bone marrow transplantation for chronic myeloid leukemia: is T cell dose important in determining biological response? Bone Marrow Transplant. 1995;15:591–594. [PubMed] [Google Scholar]
- 27.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 28.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts [see comments] N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 29.Davies JK, Gribben JG, Brennan LL, et al. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 phase 1 studies. Blood. 2008;112:2232–2241. doi: 10.1182/blood-2008-03-143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powles R, Singhal S, Treleaven J, et al. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–3486. [PubMed] [Google Scholar]
- 31.Comoli P, Locatelli F, Moretta A, et al. Human alloantigen-specific anergic cells induced by a combination of CTLA4-Ig and CsA maintain anti-leukemia and antiviral cytotoxic responses. Bone Marrow Transplant. 2001;27:1263–1273. doi: 10.1038/sj.bmt.1703063. [DOI] [PubMed] [Google Scholar]
- 32.Blazar BR, Taylor PA, Noelle RJ, Vallera DA. CD4(+) T cells tolerized ex vivo to host alloantigen by anti-CD40 ligand (CD40L:CD154) antibody lose their graft-versus-host disease lethality capacity but retain nominal antigen responses. J Clin Invest. 1998;102:473–482. doi: 10.1172/JCI3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- 34.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+C. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Amrolia PJ, Muccioli-Casadei G, Yvon E, et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102:2292–2299. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- 38.Amrolia PJ, Mucioli-Casadei G, Huls H, et al. Add-back of allodepleted donor T cells to improve immune reconstitution after haplo-identical stem cell transplantation. Cytotherapy. 2005;7:116–125. doi: 10.1080/14653240510018181. [DOI] [PubMed] [Google Scholar]
- 39.Mielke S, Nunes R, Rezvani K, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111:4392–4402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nonn M, Herr W, Khan S, et al. Selective depletion of alloreactive T lymphocytes using patient-derived nonhematopoietic stimulator cells in allograft engineering. Transplantation. 2008;86:1427–1435. doi: 10.1097/TP.0b013e31818810d6. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SR, Tran T, Carter CS, et al. Optimized clinical-scale culture conditions for ex vivo selective depletion of host-reactive donor lymphocytes: a strategy for GvHD prophylaxis in allogeneic PBSC transplantation. Cytotherapy. 2002;4:395–406. doi: 10.1080/146532402320775982. [DOI] [PubMed] [Google Scholar]
- 42.Solomon SR, Mielke S, Savani BN, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehse B, Frerk O, Goldmann M, Bulduk M, Zander AR. Efficient depletion of alloreactive donor T lymphocytes based on expression of two activation-induced antigens (CD25 and CD69) Br J Haematol. 2000;109:644–651. doi: 10.1046/j.1365-2141.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 44.Mavroudis DA, Jiang YZ, Hensel N, et al. Specific depletion of alloreactivity against haplotype mismatched related individuals by a recombinant immunotoxin: a new approach to graft-versus-host disease prophylaxis in haploidentical bone marrow transplantation. Bone Marrow Transplant. 1996;17:793–799. [PubMed] [Google Scholar]
- 45.van Dijk AM, Kessler FL, Stadhouders-Keet SA, et al. Selective depletion of major and minor histocompatibility antigen reactive T cells: towards prevention of acute graft-versus-host disease. Br J Haematol. 1999;107:169–175. doi: 10.1046/j.1365-2141.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 46.Davies JK, Koh MB, Lowdell MW. Antiviral immunity and T-regulatory cell function are retained after selective alloreactive T-cell depletion in both the HLA-identical and HLA-mismatched settings. Biol Blood Marrow Transplant. 2004;10:259–268. doi: 10.1016/j.bbmt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Wehler TC, Nonn M, Brandt B, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109:365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 48.Perruccio K, Topini F, Tosti A, et al. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2008;40:76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Hartwig UF, Nonn M, Khan S, et al. Depletion of alloreactive T cells via CD69: implications on antiviral, antileukemic and immunoregulatory T lymphocytes. Bone Marrow Transplant. 2006;37:297–305. doi: 10.1038/sj.bmt.1705238. [DOI] [PubMed] [Google Scholar]
- 50.Hartwig UF, Nonn M, Khan S, et al. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol Blood Marrow Transplant. 2008;14:99–109. doi: 10.1016/j.bbmt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Sathe A, Ortega SB, Mundy DI, Collins RH, Karandikar NJ. In vitro methotrexate as a practical approach to selective allodepletion. Biol Blood Marrow Transplant. 2007;13:644–654. doi: 10.1016/j.bbmt.2007.01.081. [DOI] [PubMed] [Google Scholar]
- 52.Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002;99:3083–3088. doi: 10.1182/blood.v99.9.3083. [DOI] [PubMed] [Google Scholar]
- 53.Guimond M, Balassy A, Barrette M, et al. P-glycoprotein targeting: a unique strategy to selectively eliminate immunoreactive T cells. Blood. 2002;100:375–382. doi: 10.1182/blood-2001-12-0353. [DOI] [PubMed] [Google Scholar]
- 54.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andre-Schmutz I, Le DF, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 56.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 57.MacKinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 58.Tan LC, Gudgeon N, Annels NE, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 59.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leen AM, Sili U, Savoldo B, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 61.Bonini C, Ciceri F, Marktel S, Magnani Z, Cazzaniga S, Zappone E, Servida P, Pescarollo A, Callegaro L, Bernardi M, Bregni M, Bordignon C. Abrogation of GvHD and Early Immune Reconstitution after Infusion of HSV-TK Engineered Donor Lymphocytes after Haplo-Identical Hematopoietic Stem cell Transplantation. Blood. 100(11):115a. 11-16-2002. [Google Scholar]
- 62.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft versus leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 63.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 64.Munshi NC, Govindarajan R, Drake R, et al. Thymidine kinase (TK) gene-transduced human lymphocytes can be highly purified, remain fully functional, and are killed efficiently with ganciclovir. Blood. 1997;89:1334–1340. [PubMed] [Google Scholar]
- 65.Traversari C, Marktel S, Magnani Z, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 66.Straathof KC, Pule MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 69.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 70.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 71.Peggs K, Verfuerth S, MacKinnon S. Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: a novel culture system free of live CMV virions. Blood. 2001;97:994–1000. doi: 10.1182/blood.v97.4.994. [DOI] [PubMed] [Google Scholar]
- 72.Kuehnle I, Huls MH, Liu Z, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95:1502–1505. [PubMed] [Google Scholar]
- 73.Heslop HE, Ng CYC, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 74.Rooney CM, Smith CA, Ng C, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 75.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 76.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 77.Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7:1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 78.Gavioli R, Kurilla MG, de Campos-Lima PO, et al. Multiple HLA-A11-restricted cytotoxic T lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol. 1993;67:1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gavioli R, de Campos-Lima PO, Kurilla M, et al. Recognition of the Epstein-Barr virus-encoded nuclear antigens, EBNA4 and EBNA6 by HLA-A11-restricted cytotoxic T lymphocytes: implications for down-regulation of HLA-A11 in Burkitt lymphoma. Proc Natl Acad Sci USA. 1992;89:5862–5866. doi: 10.1073/pnas.89.13.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 81.Gottschalk S, Ng CYC, Smith CA, et al. An Epstein-Barr virus deletion mutant that causes fatal lymphoproliferative disease unresponsive to virus-specific T cell therapy. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 82.Leen A, Ratnayake M, Foster A, et al. Contact-activated monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J Immunother (1997) 2007;30:96–107. doi: 10.1097/01.cji.0000211325.30525.84. [DOI] [PubMed] [Google Scholar]
- 83.Sili U, Huls MH, Davis AR, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocytes using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–256. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 85.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplant. Blood. 2009 doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heslop HE, Slobod KS, Pule MA, et al. Long term outcome of EBV specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2009 doi: 10.1182/blood-2009-08-239186. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kern F, Faulhaber N, Frommel C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein- spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 89.Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 91.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 93.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40:63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97:1232–1240. doi: 10.1182/blood.v97.5.1232. [DOI] [PubMed] [Google Scholar]
- 95.Feuchtinger T, Lang P, Hamprecht K, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004;32:282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Fujita Y, Leen AM, Sun J, et al. Exploiting Cytokine Secretion to Rapidly Produce Multivirus-specific T Cells for Adoptive Immunotherapy. J Immunother. 2008 doi: 10.1097/CJI.0b013e318181b4bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to Generate Multivirus-specific T Cells for Prevention or Treatment of Viral Infections in the Immunocompromised Host. Mol Ther. 2009 doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rauser G, Einsele H, Sinzger C, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004;103:3565–3572. doi: 10.1182/blood-2003-09-3056. [DOI] [PubMed] [Google Scholar]
- 99.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to Generate Multivirus-specific T Cells for Prevention or Treatment of Viral Infections in the Immunocompromised Host. Mol Ther. 2009 doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landais E, Morice A, Long HM, et al. EBV-specific CD4+ T cell clones exhibit vigorous allogeneic responses. J Immunol. 2006;177:1427–1433. doi: 10.4049/jimmunol.177.3.1427. [DOI] [PubMed] [Google Scholar]
- 102.Urbani S, Amadei B, Fisicaro P, et al. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–680. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elkington R, Khanna R. Cross-recognition of human alloantigen by cytomegalovirus glycoprotein-specific CD4+ cytotoxic T lymphocytes: implications for graft-versus-host disease. Blood. 2005;105:1362–1364. doi: 10.1182/blood-2004-07-2602. [DOI] [PubMed] [Google Scholar]
- 104.Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 105.Sun Q, Burton R, Reddy V, Lucas KG. Safety of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for patients with refractory EBV-related lymphoma. Br J Haematol. 2002;118:799–808. doi: 10.1046/j.1365-2141.2002.03683.x. [DOI] [PubMed] [Google Scholar]
- 106.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 107.Gandhi MK, Wilkie GM, Dua U, et al. Immunity, homing and efficacy of allogeneic adoptive immunotherapy for posttransplant lymphoproliferative disorders. Am J Transplant. 2007;7:1293–1299. doi: 10.1111/j.1600-6143.2007.01796.x. [DOI] [PubMed] [Google Scholar]
- 108.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to Generate Multivirus-specific T Cells for Prevention or Treatment of Viral Infections in the Immunocompromised Host. Mol Ther. 2009 doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carswell KS, Papoutsakis ET. Culture of human T cells in stirred bioreactors for cellular immunotherapy applications: shear, proliferation, and the IL-2 receptor. Biotechnol Bioeng. 2000;68:328–338. doi: 10.1002/(sici)1097-0290(20000505)68:3<328::aid-bit11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 110.Foster AE, Forrester K, Gottlieb DJ, et al. Large-scale expansion of cytomegalovirus-specific cytotoxic T cells in suspension culture. Biotechnol Bioeng. 2004;85:138–146. doi: 10.1002/bit.10801. [DOI] [PubMed] [Google Scholar]
- 111.Knazek RA, Wu YW, Aebersold PM, Rosenberg SA. Culture of human tumor infiltrating lymphocytes in hollow fiber bioreactors. J Immunol Methods. 1990;127:29–37. doi: 10.1016/0022-1759(90)90337-u. [DOI] [PubMed] [Google Scholar]
- 112.Malone CC, Schiltz PM, Mackintosh AD, et al. Characterization of human tumor-infiltrating lymphocytes expanded in hollow-fiber bioreactors for immunotherapy of cancer. Cancer Biother Radiopharm. 2001;16:381–390. doi: 10.1089/108497801753354285. [DOI] [PubMed] [Google Scholar]
- 113.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tran CA, Burton L, Russom D, et al. Manufacturing of large numbers of patient-specific T cells for adoptive immunotherapy: an approach to improving product safety, composition, and production capacity. J Immunother. 2007;30:644–654. doi: 10.1097/CJI.0b013e318052e1f4. [DOI] [PubMed] [Google Scholar]
- 115.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108:1770–1773. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T-cells that target CMV, adenovirus and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to Generate Multivirus-specific T Cells for Prevention or Treatment of Viral Infections in the Immunocompromised Host. Mol Ther. 2009 doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howard DS, Phillips GL, II, Reece DE, et al. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 1999;29:1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- 119.Runde V, Ross S, Trenschel R, et al. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant. 2001;28:51–57. doi: 10.1038/sj.bmt.1703083. [DOI] [PubMed] [Google Scholar]
- 120.Hale GA, Heslop HE, Krance RA, et al. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999;23:277–282. doi: 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- 121.Funk GA, Gosert R, Hirsch HH. Viral dynamics in transplant patients: implications for disease. Lancet Infect Dis. 2007;7:460–472. doi: 10.1016/S1473-3099(07)70159-7. [DOI] [PubMed] [Google Scholar]
- 122.Schenk T, Strahm B, Kontny U, et al. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis. 2007;13:1425–1427. doi: 10.3201/eid1309.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3:977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farcas GA, Poutanen SM, Mazzulli T, et al. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garbino J, Crespo S, Aubert JD, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (Non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43:1009–1015. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pene F, Merlat A, Vabret A, et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37:929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 129.Harma M, Hockerstedt K, Krogerus L, Lautenschlager I. Pretransplant human herpesvirus 6 infection of patients with acute liver failure is a risk factor for posttransplant human herpesvirus 6 infection of the liver. Transplantation. 2006;81:367–372. doi: 10.1097/01.tp.0000195771.83614.0b. [DOI] [PubMed] [Google Scholar]
- 130.Benito N, Ricart MJ, Pumarola T, et al. Infection with human herpesvirus 6 after kidney-pancreas transplant. Am J Transplant. 2004;4:1197–1199. doi: 10.1111/j.1600-6143.2004.00449.x. [DOI] [PubMed] [Google Scholar]
- 131.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193:68–79. doi: 10.1086/498531. [DOI] [PubMed] [Google Scholar]
- 132.Yoshikawa T, Suga S, Asano Y, et al. A prospective study of human herpesvirus-6 infection in renal transplantation. Transplantation. 1992;54:879–883. doi: 10.1097/00007890-199211000-00022. [DOI] [PubMed] [Google Scholar]
- 133.Cole PD, Stiles J, Boulad F, et al. Successful treatment of human herpesvirus 6 encephalitis in a bone marrow transplant recipient. Clin Infect Dis. 1998;27:653–654. doi: 10.1086/517145. [DOI] [PubMed] [Google Scholar]
- 134.Rosenfeld CS, Rybka WB, Weinbaum D, et al. Late graft failure due to dual bone marrow infection with variants A and B of human herpesvirus-6. Exp Hematol. 1995;23:626–629. [PubMed] [Google Scholar]
- 135.Singh N, Carrigan DR, Gayowski T, Marino IR. Human herpesvirus-6 infection in liver transplant recipients: documentation of pathogenicity. Transplantation. 1997;64:674–678. doi: 10.1097/00007890-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 136.Isegawa Y, Hara J, Amo K, et al. Human herpesvirus 6 ganciclovir-resistant strain with amino acid substitutions associated with the death of an allogeneic stem cell transplant recipient. J Clin Virol. 2009;44:15–19. doi: 10.1016/j.jcv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Wang LR, Dong LJ, Zhang MJ, Lu DP. Correlations of human herpesvirus 6B and CMV infection with acute GVHD in recipients of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;42:673–677. doi: 10.1038/bmt.2008.238. [DOI] [PubMed] [Google Scholar]
- 138.Dzieciatkowski T, Przybylski M, Torosian T, Tomaszewska A, Luczak M. Prevalence of human herpesvirus 6 antibodies and DNA in allogeneic stem cell transplant patients: two-year single centre experience. Arch Immunol Ther Exp (Warsz) 2008;56:201–206. doi: 10.1007/s00005-008-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 140.Lymphocytic choriomeningitis virus infection in organ transplant recipients--Massachusetts, Rhode Island, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:537–539. [PubMed] [Google Scholar]
- 141.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358:991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 142.Brief report: Lymphocytic choriomeningitis virus transmitted through solid organ transplantation--Massachusetts, 2008. MMWR Morb Mortal Wkly Rep. 2008;57:799–801. [PubMed] [Google Scholar]
- 143.Wong RD, Goetz MB. Clinical and laboratory features of measles in hospitalized adults. Am J Med. 1993;95:377–383. doi: 10.1016/0002-9343(93)90306-a. [DOI] [PubMed] [Google Scholar]
- 144.Baas MC, van Donselaar KA, Florquin S, et al. Mumps: Not an Innocent Bystander in Solid Organ Transplantation. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- 145.Park SB, Jin KB, Hwang EA, et al. Case of adult mumps infection after renal transplantation. Transplant Proc. 2008;40:2442–2443. doi: 10.1016/j.transproceed.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 146.Turner A, Jeyaratnam D, Haworth F, et al. Measles-associated encephalopathy in children with renal transplants. Am J Transplant. 2006;6:1459–1465. doi: 10.1111/j.1600-6143.2006.01330.x. [DOI] [PubMed] [Google Scholar]
- 147.Sumino KC, Agapov E, Pierce RA, et al. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis. 2005;192:1052–1060. doi: 10.1086/432728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Larcher C, Geltner C, Fischer H, et al. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24:1891–1901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 149.Richards A, Chuen JN, Taylor C, et al. Acute respiratory infection in a renal transplant recipient. Nephrol Dial Transplant. 2005;20:2848–2850. doi: 10.1093/ndt/gfi167. [DOI] [PubMed] [Google Scholar]
- 150.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 151.Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78:408–416. doi: 10.1002/jmv.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Debiaggi M, Canducci F, Sampaolo M, et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 153.Raza K, Ismailjee SB, Crespo M, et al. Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J Heart Lung Transplant. 2007;26:862–864. doi: 10.1016/j.healun.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–881. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 155.Dare R, Sanghavi S, Bullotta A, et al. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol. 2007;45:548–552. doi: 10.1128/JCM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kamble RT, Bollard C, Demmler G, LaSala PR, Carrum G. Human metapneumovirus infection in a hematopoietic transplant recipient. Bone Marrow Transplant. 2007;40:699–700. doi: 10.1038/sj.bmt.1705781. [DOI] [PubMed] [Google Scholar]
- 157.Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clin Infect Dis. 2001;32:413–418. doi: 10.1086/318498. [DOI] [PubMed] [Google Scholar]
- 158.Vilchez RA, McCurry K, Dauber J, et al. The epidemiology of parainfluenza virus infection in lung transplant recipients. Clin Infect Dis. 2001;33:2004–2008. doi: 10.1086/324348. [DOI] [PubMed] [Google Scholar]
- 159.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 160.Zambon M, Bull T, Sadler CJ, Goldman JM, Ward KN. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol. 1998;36:2289–2293. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hohenthal U, Nikoskelainen J, Vainionpaa R, et al. Parainfluenza virus type 3 infections in a hematology unit. Bone Marrow Transplant. 2001;27:295–300. doi: 10.1038/sj.bmt.1702776. [DOI] [PubMed] [Google Scholar]
- 162.Cortez KJ, Erdman DD, Peret TC, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 163.Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43:40–48. doi: 10.1086/504812. [DOI] [PubMed] [Google Scholar]
- 164.Klumpen HJ, Petersen EJ, Verdonck LF. Severe multiorgan failure after parvovirus B19 infection in an allogeneic stem cell transplant recipient. Bone Marrow Transplant. 2004;34:469–470. doi: 10.1038/sj.bmt.1704612. [DOI] [PubMed] [Google Scholar]
- 165.Laurenz M, Winkelmann B, Roigas J, et al. Severe parvovirus B19 encephalitis after renal transplantation. Pediatr Transplant. 2006;10:978–981. doi: 10.1111/j.1399-3046.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 166.Yango A, Jr, Morrissey P, Gohh R, Wahbeh A. Donor-transmitted parvovirus infection in a kidney transplant recipient presenting as pancytopenia and allograft dysfunction. Transpl Infect Dis. 2002;4:163–166. doi: 10.1034/j.1399-3062.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 167.Wasak-Szulkowska E, Grabarczyk P, Rzepecki P. Pure red cell aplasia due to parvovirus B19 infection transmitted probably through hematopoietic stem cell transplantation. Transpl Infect Dis. 2008;10:201–205. doi: 10.1111/j.1399-3062.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 168.Muetherig A, Christopeit M, Muller LP, et al. Human parvovirus B19 infection with GvHD-like erythema in two allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2007;39:315–316. doi: 10.1038/sj.bmt.1705589. [DOI] [PubMed] [Google Scholar]
- 169.Plentz A, Hahn J, Holler E, Jilg W, Modrow S. Long-term parvovirus B19 viraemia associated with pure red cell aplasia after allogeneic bone marrow transplantation. J Clin Virol. 2004;31:16–19. doi: 10.1016/j.jcv.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 170.Barzon L, Murer L, Pacenti M, et al. Investigation of intrarenal viral infections in kidney transplant recipients unveils an association between parvovirus B19 and chronic allograft injury. J Infect Dis. 2009;199:372–380. doi: 10.1086/596053. [DOI] [PubMed] [Google Scholar]
- 171.Pinto V, Grandy J, Zambrano P, et al. Severe anemia from parvovirus b19 infection in pediatric renal transplant recipients: two case reports. Transplant Proc. 2008;40:3261–3264. doi: 10.1016/j.transproceed.2008.03.127. [DOI] [PubMed] [Google Scholar]
- 172.Biagini P, Dussol B, Touinssi M, et al. Human parvovirus 4 in kidney transplant patients, France. Emerg Infect Dis. 2008;14:1811–1812. doi: 10.3201/eid1411.080862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ardalan MR, Shoja MM, Tubbs RS, Esmaili H, Keyvani H. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant. 2008;8:1340–1344. doi: 10.1111/j.1600-6143.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 174.Waldman M, Kopp JB. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol. 2007;3:540–550. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 175.Whimbey E, Ghosh S. Respiratory syncytial virus infections in immunocompromised adults. Curr Clin Top Infect Dis. 2000;20:232–255. [PubMed] [Google Scholar]
- 176.Ko JP, Shepard JA, Sproule MW, et al. CT manifestations of respiratory syncytial virus infection in lung transplant recipients. J Comput Assist Tomogr. 2000;24:235–241. doi: 10.1097/00004728-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 177.Harrington RD, Hooton TM, Hackman RC, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 178.Lopez-Medrano F, Aguado JM, Lizasoain M, et al. Clinical implications of respiratory virus infections in solid organ transplant recipients: a prospective study. Transplantation. 2007;84:851–856. doi: 10.1097/01.tp.0000282788.70383.8b. [DOI] [PubMed] [Google Scholar]
- 179.Stelzmueller I, Wiesmayr S, Swenson BR, et al. Rotavirus enteritis in solid organ transplant recipients: an underestimated problem? Transpl. Infect Dis. 2007;9:281–285. doi: 10.1111/j.1399-3062.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 180.Tumgor G, Arikan C, Aydin U, Kilic M, Aydogdu S. Effect of rotavirus on serum tacrolimus level in pediatric liver transplant recipients. Pediatr Int. 2008;50:592–593. doi: 10.1111/j.1442-200X.2008.02697.x. [DOI] [PubMed] [Google Scholar]
- 181.Eisengart LJ, Chou PM, Iyer K, Cohran V, Rajaram V. Rotavirus infection in small bowel transplant: a histologic comparison with acute cellular rejection. Pediatr Dev Pathol. 2009;12:85–88. doi: 10.2350/08-05-0473.1. [DOI] [PubMed] [Google Scholar]
- 182.Barshes NR, Agee EE, Zgabay T, et al. West Nile Virus encephalopathy following pancreatic islet transplantation. Am J Transplant. 2006;6:3037. doi: 10.1111/j.1600-6143.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 183.Armali Z, Ramadan R, Chlebowski A, Azzam ZS. West Nile meningo-encephalitis infection in a kidney transplant recipient. Transplant Proc. 2003;35:2935–2936. doi: 10.1016/j.transproceed.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 184.Shepherd JC, Subramanian A, Montgomery RA, et al. West Nile virus encephalitis in a kidney transplant recipient. Am J Transplant. 2004;4:830–833. doi: 10.1111/j.1600-6143.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 185.DeSalvo D, Roy-Chaudhury P, Peddi R, et al. West Nile virus encephalitis in organ transplant recipients: another high-risk group for meningoencephalitis and death. Transplantation. 2004;77:466–469. doi: 10.1097/01.TP.0000101434.98873.CB. [DOI] [PubMed] [Google Scholar]
- 186.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation. 2004;77:399–402. doi: 10.1097/01.TP.0000101435.91619.31. [DOI] [PubMed] [Google Scholar]
- 187.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, et al. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 188.Penn RG, Guarner J, Sejvar JJ, et al. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42:680–683. doi: 10.1086/500216. [DOI] [PubMed] [Google Scholar]
- 189.Hong DS, Jacobson KL, Raad II, et al. West Nile encephalitis in 2 hematopoietic stem cell transplant recipients: case series and literature review. Clin Infect Dis. 2003;37:1044–1049. doi: 10.1086/378278. [DOI] [PubMed] [Google Scholar]
- 190.Hiatt B, DesJardin L, Carter T, et al. A fatal case of West Nile virus infection in a bone marrow transplant recipient. Clin Infect Dis. 2003;37:e129–e131. doi: 10.1086/378891. [DOI] [PubMed] [Google Scholar]
- 191.Ziedina I, Folkmane I, Chapenko S, et al. Reactivation of BK Virus in the Early Period After Kidney Transplantation. Transplant Proc. 2009;41:766–768. doi: 10.1016/j.transproceed.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 192.Munoz GP. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009;41:1033–1037. doi: 10.1016/j.transproceed.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 193.Boldorini R, Allegrini S, Miglio U, Paganotti A, Veggiani C. Detection, distribution, and pathologic significance of BK virus strains isolated from patients with kidney transplants, with and without polyomavirus-associated nephropathy. Arch Pathol Lab Med. 2009;133:766–774. doi: 10.5858/133.5.766. [DOI] [PubMed] [Google Scholar]
- 194.Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant. 2008;13:569–574. doi: 10.1097/MOT.0b013e3283186b93. [DOI] [PubMed] [Google Scholar]
- 195.Comoli P, Binggeli S, Ginevri F, Hirsch HH. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8:86–94. doi: 10.1111/j.1399-3062.2006.00167.x. [DOI] [PubMed] [Google Scholar]