Abstract
Purpose
Bone marrow (BM) cells contribute to tumor vessel formation that supports the growth of Ewing’s sarcoma. These BM cells migrate into the tumor and differentiate into endothelial cells and pericytes. We investigated whether Delta like ligand 4 (DLL4) played a role in the formation of BM-derived pericytes/vascular smooth muscle cells (vSMCs) during tumor vessel formation.
Experimental Design
Using immunohistochemistry, we examined the expression pattern of DLL4 in 14 patient samples and 2 xenograft mouse models of Ewing’s sarcoma. We then used intratumor injections of shRNA to inhibit DLL4 expression in Ewing’s sarcoma tumors in mice, and evaluated the effect on BM-derived pericytes/vSMCs.
Results
DLL4 was expressed by perivascular cells in 12 of 14 human samples and in BM-derived pericytes/vSMCs in both A4573 and TC71 xenograft tumors. Inhibition of DLL4 expression by shRNA correlated with decreased numbers of BM-derived cells in tumor vessels and decreased numbers of α-SMA+, desmin+, and NG2+ pericytes/vSMCs, as well as increased tumor hypoxia.
Conclusions
DLL4 is important for the formation of BM-derived pericytes/vSMCs during vasculogenesis in Ewing’s sarcoma. DLL4 may be a therapeutic target for treatment of Ewing’s sarcoma by inhibition of blood vessel formation.
Keywords: Delta-like ligand 4, Notch, vasculogenesis, Ewing’s sarcoma, bone marrow
Introduction
Ewing’s sarcoma tumors depend on a functional vascular network to support the high metabolic needs of rapidly growing cancer cells. Understanding how Ewing’s sarcomas form blood vessels may identify new therapeutic targets. Two processes that contribute to tumor vessel expansion are angiogenesis, the sprouting of new vessels from preexisting ones, and vasculogenesis, the process by which bone marrow (BM) cells are recruited to the tumor and organized to form a vascular network de novo. We have previously shown that vasculogenesis plays a critical role in the growth of Ewing’s sarcoma tumors in vivo (1–4). We found that tumor vessels were comprised of both BM-derived and locally derived endothelial cells and pericytes/vascular smooth muscle cells (vSMCs). However, the molecular mechanisms controlling the differentiation of immature BM cells into mature pericytes/vSMCs are unclear.
A mature blood vessel consists of an inner tube of endothelial cells surrounded by several layers of pericytes and/or vSMCs. Pericytes/vSMCs support and shape blood vessels as well as communicate growth and quiescence signals to endothelial cells (5–8). Without pericytes/vSMCs, blood vessels become weak, leaky, and less functional(8). In adults, pericytes were originally assumed to be formed only by proliferation of local pericytes during vessel formation. Recent studies, however, have highlighted the prevalence of the differentiation of BM cells into pericytes de novo during wound healing and tumor vasculogenesis (9, 10). In addition, rat BM stem cells can differentiate into both endothelial cells and pericytes and form vessel-like structures in vitro (11).
We showed that BM progenitor cells migrate to TC71 Ewing’s sarcoma tumors and differentiate into both endothelial cells and pericytes/vSMCs (3). The majority of these BM-derived cells differentiated into pericytes/vSMCs during tumor vascular formation. BM cell participation in vasculogenesis is a complex process: cells must leave the BM, migrate to the tumor, adhere to regions of developing vessels, and differentiate into endothelial cells or pericytes/vSMCs. We demonstrated that vascular endothelial growth factor 165 (VEGF165) is necessary for BM cell migration to Ewing’s sarcoma tumors (4, 12). It is unclear, however, what molecular signals are needed for BM cell attachment to the developing vessels and differentiation into pericytes/vSMCs.
One signaling pathway that may contribute to this process is the Notch signaling pathway. The mammalian Notch family consists of four receptors, Notch 1 4, and five ligands, Jagged 1 and 2 and Delta like ligand 1, 3, and 4 (DLL1,3,4). Notch ligands and receptors are membrane bound and signal through a paracrine cell pathway (13). When stimulated by ligand binding, Notch receptors undergo two cleavage events, releasing the Notch intracellular domain (NICD). In the canonical pathway, NICD then travels to the nucleus, where it forms a complex with C-promoter binding factor 1 (CBF-1) and other transcriptional regulators and induces the expression of downstream targets such as the Hes and Hey family members (13).
DLL4 is an essential regulator of vessel development in both embryogenesis and tumor vessel formation (14–17). DLL4 is highly expressed on the endothelial cell tips of growing vessels (18), on the endothelial cells of several tumor types (19), and it is moderately expressed in adult bone marrow (20). DLL4 is selectively expressed on new arterial vessels as opposed to established arteries or veins. When DLL4 is inhibited, tumor vessels become highly branched yet less functional, with reduced pericyte coverage (21). The vascular phenotype in tumors after DLL4 inhibition suggests that DLL4-Notch signaling plays a role in the formation of pericytes/vSMCs.
Because DLL4 is expressed on BM cells and is required for proper blood vessel formation, we wished to determine whether DLL4 plays a role in the differentiation of BM cells into pericytes/vSMCs during vasculogenesis in Ewing’s sarcoma. To address this question, we used our green fluorescent protein (GFP)+ BM transplant mouse model to identify BM-derived cells and used short hairpin RNA (shRNA) to inhibit DLL4 expression. We show for the first time that DLL4 is expressed on the perivascular stromal cells, pericytes and vSMCs, in Ewing’s sarcoma tumors in vivo and in patient tumor samples. We further demonstrate that inhibition of DLL4 expression correlates with a reduction of BM-derived pericytes/vSMCs around tumor vessels, and an increase in tumor hypoxia.
Materials and Methods
Mice and murine BM cells
Experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center. Animals were housed in a pathogen-free facility. GFP transgenic mice (Jackson Laboratory strain 003115) were purchased from the M. D. Anderson Cancer Center Genetic Mouse Engineering Facility. These mice express enhanced GFP under the control of the CMV-enhanced chicken β-actin promoter; GFP expression varies by tissue. Six-week-old nu/nu athymic (T-cell deficient) nude mice were purchased from the National Cancer Institute (strain 01B70).
GFP BM transplant
To track BM cells, we used GFP transgenic mice as donors and nude mice as recipients. BM cells were flushed from the femurs of GFP+ transgenic mice with phosphate buffered saline (PBS). Nude mice were irradiated using an external cesium source (137Cs Mark 1 Irradiator; J. L. Shepherd & Associates, Glendale, CA). Mice received one dose of 9 Gy whole body irradiation. After irradiation, mice were intravenously injected with 1 × 106 GFP+ BM cells. Five weeks later, engraftment was confirmed before injection of tumor cells.
TC71 and A4573 Ewing’s sarcoma cells and mouse models
TC71 and A4573 cells contain the t(11;22) translocation. Cells were cultured as previously described (3). TC71 cells were a gift from Dr. T. Triche (University of Southern California, Los Angeles, LA) and A4573 cells were a gift from Dr. V. Soldatenkov (Georgetown University Medical Center, Washington, DC). Both cell lines have the EWS-Fli1 fusion, confirmed by PCR. For the in vivo TC71 experiments, mice transplanted with GFP+ BM cells were injected with 2 × 106 TC71 cells subcutaneously. Two weeks later, the mice were euthanized and the tumors resected and frozen.
For in vivo experiments with A4573 cells, mice transplanted with GFP+ BM cells were injected with 3.5 × 106 A4573 cells subcutaneously. Twenty-four days later, the mice were euthanized and the tumors resected and frozen.
shRNA vector and polyethylenimine (PEI)
shDLL4 was made by ligation of the DNA oligomer targeting the sequence 5 ′-GATCCAGTCACTTGGGTGCAGTGTTTCAAGAGAACACTGCACCCAAGTGACTTTTTT TGGAAA-3′ into pSilencer 2.1-U6 hygro shRNA vector (Ambion, Austin, TX). pSilencer 2.1-U6 hygro containing a sequence with minimal homology to the mouse genome was used for the sh− control. PEI (Mr 25,000, branched form; Aldrich Chemical, Milwaukee, WI) was used as the gene delivery vector. A stock solution of PEI (pH 7.0 7.5) was prepared at a concentration of 4.3 mg/ml (0.1 M nitrogen) in PBS. PEI:shRNA complexes were prepared at a PEI nitrogen:DNA phosphate ratio of 10:1 and a PEI:DNA ratio of 1.29:1 (w/w). DNA and PEI were diluted in H2O so that the final volumes were the same for each solution. DNA was then added dropwise to PEI while vortexing, for a final concentration of 0.4 μg DNA per 1 μl PEI/DNA mixture.
Transplant experiment with shRNA
GFP BM transplants were performed on nude mice as described above. Four weeks after transplantation mice were injected subcutaneously with 2 × 106 TC71 cells in both their right and left flanks. Beginning on day 5, the left-side tumors were injected with PEI/sh− control while the right-side tumors were injected with PEI/shDLL4 every other day for 24 days. Each tumor received a total of 50 μl PEI/DNA mixture (20 μg DNA) by reinsertion of the needle into multiple sites per tumor. Twenty-eight days after tumor inoculation, the mice were sacrificed and tumors were harvested and frozen for immunohistochemical analysis.
Hypoxyprobe-1 tail vein injection
Hypoxyprobe-1 (HPI, inc., Burlington, MA) was reconstituted in PBS per manufacturer’s instructions. 2.5 hours prior to sacrifice, mice were injected i.v. with 60 mg Hypoxyprobe-1 per kg body weight in 200 μl PBS. Mice were then sacrificed by cervical dislocation.
Immunohistochemical analysis and microscopy
Frozen tumor sections were fixed in acetone and blocked with 4% fish gelatin in PBS as previously described (3). Primary antibodies used were: rabbit anti-GFP, rabbit anti-mouse NG2 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human DLL4, rabbit anti-desmin, mouse anti-mouse α-SMA (Abcam, Cambridge, MA), rabbit anti-human DLL4, rabbit anti-NICD1 (Cell Signaling Technology, Danvers, MA), rat anti-mouse CD31 (BD Biosciences, Franklin Lakes, NJ), anti-a-SMA-Cy3 (Sigma-Aldrich, St. Louis, MO), and mouse anti-hypoxyprobe (HPI, inc.) Slides that were stained with mouse anti-mouse antibodies were first preblocked with mouse Fab fragment diluted in fish gel overnight. After incubation with primary antibodies, slides were incubated with the appropriate fluorochrome-labeled secondary antibodies. Alexa Flour 488 and Alexa Flour 594 labeled secondary antibodies were used for flourescence microscopy (all from Molecular Probes, Eugene, OR), and Cy3 or Cy5 labeled secondary antibodies were used for confocal microscopy (all from Jackson Immunoresearch, West Grove, PA). Nuclei were stained with Hoescht 33342 dye (Molecular Probes) or Sytox Green (Jackson Immunoresearch). Images were captured using using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 100-W Hg lamp and narrow bandpass excitation filters. Images were obtained using a cooled charge-coupled device Hamamatsu C5810 camera (Hamamatsu Photonics, Bridgewater, NJ) and Optimas imaging software (Media Cybernetics, Bethesda, MD). For confocal microscopy, images were collected using a Zeiss Laser Confocal Microscope (Carl Zeiss MicroImaging, Inc.).
Quantification of immunohistochemistry
Areas of positive staining were quantified using Simple PCI software (Hamamatsu, Sewickley, PA). Five random 10x-magnification fields were captured per slide. A minimum and maximum fluorescent intensity was set for positive staining for each antibody. For each of the five fields per slide, mean positive pixel area was measured. For quantification of hypoxic regions, total hypoxyprobe+ pixels were normalized against number of nuclei present. Measurements from each field and from each group (treatment or control) were then pooled, and data were analyzed for statistical significance using the Mann-Whitney U test.
Patient tumor samples and immunohistochemical analysis
We obtained 14 paraffin-embedded human Ewing’s sarcoma samples from the tumor bank at M. D. Anderson Cancer Center. These samples represented both primary and metastatic lesions from 10 different patients. The Institutional Review Board at M. D. Anderson approved the use of human specimens for this study.
Immunohistochemical analysis was performed to examine DLL4 expression. Tumor sections were deparaffinized by sequential bathing in xylene, ethanol, and PBS. Slides were incubated with pepsin at 37°C, then endogenous peroxidase was blocked using 3% H2O2. Slides were then incubated with rabbit anti-DLL4 (Abcam) diluted in normal horse serum and normal goat serum in PBS, followed by peroxidase-labeled biotinylated goat anti-rabbit secondary antibody (Jackson Immunoresearch). DLL4+ cells were detected with 3,3′-diaminobenzidine (DAB; Open Biosystems, Huntsville, AL) and counterstained with Gill’s hematoxylin (Sigma-Aldrich). Tumors were scored in a qualitative manner as either positive or negative for DLL4 expression.
Results
GFP+ BM cells differentiate into pericytes/vSMCs
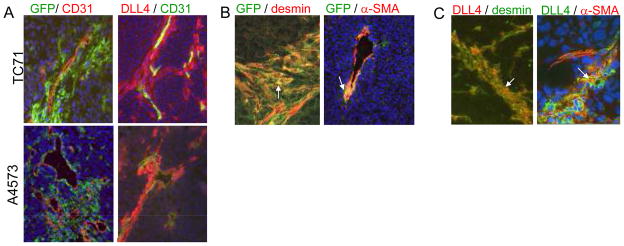
To track BM-derived cells within tumors, GFP+ BM cells were transplanted into nude mice. One month after transplant, mice were injected subcutaneously with TC71 cells. Two weeks later, the mice were sacrificed, their tumors removed, and immunohistochemical analysis was performed. GFP+ BM-derived cells surrounded CD31+ endothelial cells (Fig. 1A). These perivascular GFP+ BM-derived cells expressed the pericyte/vSMC markers desmin and α-SMA (Fig. 1B), indicating that these BM cells had differentiated into pericytes/vSMCs.
Figure 1.
DLL4+ pericytes are derived from BM cells. A, TC71 or A4573 cells were implanted subcutaneously in mice previously transplanted with GFP+ BM. Tumors were harvested and analyzed by immunohistochemistry. BM-derived cells were visualized using anti-GFP (green) and costained with anti-CD31 (red). DLL4+ cells were visualized using anti-DLL4 (red) and costained with anti-CD31 (green). B, TC71 tumors were stained using anti-GFP (green) in combination with either anti-desmin (red) or anti-α-SMA (red). BM-derived cells expressing pericyte markers are orange (white arrows). C, TC71 tumors were stained using anti-DLL4 (red) in combination with either anti-desmin (green) or anti-α-SMA (green). Colocalization of DLL4 and pericyte markers is orange (white arrows). All photos were taken at 20x magnification, except panels co-stained using anti-GFP and anti-desmin, and anti-DLL4 and anti-α-SMA, which were taken at 40x magnification. Hoescht 33342 (blue) was used to visualize nuclei.
We used a second xenograft model (A4573) to confirm that BM-derived cells participate in vasculogenesis and contribute to the pericyte pool, and that this process is not unique to the TC71 cells. A4573 cells were injected subcutaneously in nude mice that had previously received a GFP+ BMT. Mice were sacrificed twenty-four days later, and tumors were harvested and examined by immunohistochemistry. Once again, GFP+ BM-derived cells were seen in thick perivascular layers surrounding CD31+ endothelial cells in A4573 tumors, similar to what was seen in TC71 tumors (Fig. 1A).
Expression of DLL4 in TC71, A4573, and patient tumors
TC71 and A4573 tumors were analyzed by immunohistochemistry for expression of DLL4. DLL4+ cells were observed surrounding CD31+ endothelial cells in both the TC71 and A4573 tumors (Fig. 1A). The thick layers of DLL4+ perivascular cells were confirmed using a second anti-DLL4 antibody (data not shown), which has been used by other investigators to identify DLL4+ cells (22). The location and morphology of the DLL4+ cells suggested that these cells were pericytes or vSMCs. To determine whether the DLL4+ cells were pericytes/vSMCs, tumor sections were costained using anti-DLL4 and either anti-desmin or anti-α-SMA antibodies. The majority of DLL4+ perivascular cells were also desmin+ (Fig. 1C). Several desmin+DLL4− cells were also observed, indicating that DLL4 is not expressed by all desmin+ pericytes. DLL4 and α-SMA colocalized to a lesser extent than DLL4 and desmin (Fig. 1C). The α-SMA+ cells were most often observed adjacent to DLL4+ cells. There were also a few cells that were positive for both DLL4 and CD31, but thesewere seen in only a small percentage of vessels (Fig. 1A).
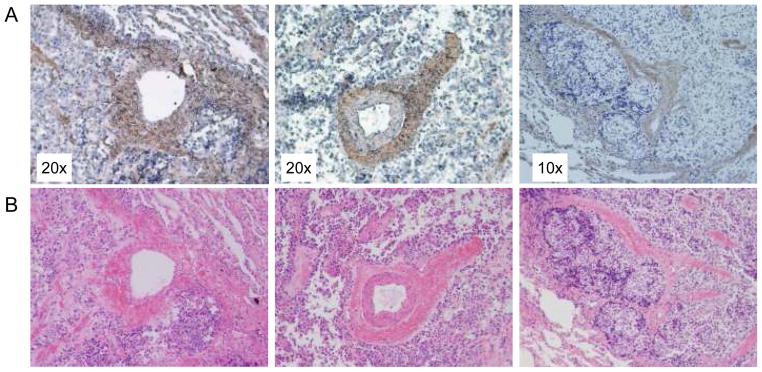
To confirm the clinical relevance of our findings, we analyzed 14 human Ewing’s sarcoma samples for DLL4 expression. These tumors were from primary and metastatic lesions of 10 different patients. Twelve of the 14 tumors were DLL4+ (Fig. 2A, B and C). DLL4 was expressed in perivascular regions in patterns similar to those seen in TC71 and A4573 tumors. This perivascular location is consistent with these cells being pericytes or vSMCs.
Figure 2.
Perivascular DLL4 expression in Ewing’s sarcoma patient samples. A, Fourteen Ewing’s sarcoma patient samples, both primary and metastatic, from 10 patients were stained using anti-DLL4. DLL4+ cells are brown. B, Sections adjacent to those stained for DLL4 were stained with hematoxylin and eosin. Magnification is indicated.
DLL4+ cells are bone marrow derived
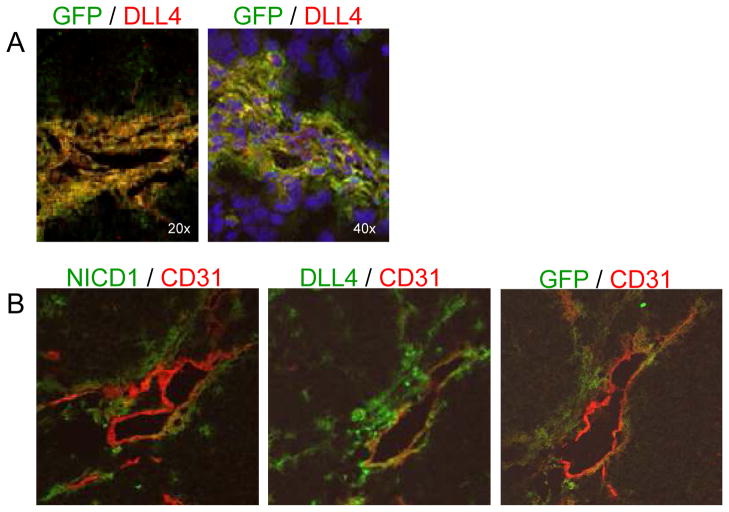
The similar perivascular locations of the GFP+ BM-derived cells and the DLL4+ cells (Fig. 1A) suggested that the DLL4+ cells were derived from the BM cells that had migrated into the tumor. To address this question, GFP+ BM-transplanted mice were injected subcutaneously with TC71 cells. Tumors were removed 2 weeks later and examined by immunohistochemistry. The majority of GFP+ perivascular cells were DLL4+. However, GFP and DLL4 did not always colocalize. Several DLL4+ cells were GFP-, indicating that these DLL4+ cells were derived from local host cells. There were also GFP+ DLL4- cells present, indicating that not all BM-derived cells were DLL4+ (Fig. 3A).
Figure 3.
GFP+ BM-derived cells express DLL4 in the same perivascular pattern as cleaved Notch1. TC71 cells were subcutaneously implanted in mice that had previously been transplanted with GFP+ BM cells. A, Immunohistochemical analysis was performed using anti-GFP (green) and anti-DLL4 (red) antibodies. Double-positive cells are orange. Nuclei were stained with Hoescht 33342 (blue). B, Serial sections were stained using anti-CD31 (red) in combination with anti-NICD1 (green), anti-DLL4 (green), or anti-GFP (green). The same vessel was located on each section and photgraphed.
Notch 1 is activated in perivascular BM-derived cells
We next determined whether Notch signaling was activated in these perivascular cells. Cleaved Notch1 implies active Notch signaling. Therefore, we performed fluorescence immunohistochemical analysis using an antibody specific for cleaved Notch1 (NICD1). Slides were dual-stained with anti-NICD1 and anti-CD31 and then analyzed by confocal microscopy. NICD1+ cells surrounded CD31+ endothelial cells in the same perivascular pattern as DLL4+ and GFP+ BM-derived cells (Fig. 3B). Taken together, these data suggest that Notch signaling is active in the BM-derived pericytes/vSMCs.
We were unable to perform triple staining. Therefore, to determine whether Notch1 was activated in the BM-derived DLL4+ perivascular cells, we performed immunohistochemical analysis on serial TC71 tumor sections from a GFP+ BM-transplanted mouse. The first section was stained for CD31 and NICD1 to identify endothelial cells and cells with active Notch signaling (Fig. 3B). The next consecutive section was stained for CD31 and GFP, to identify endothelial cells and BM-derived cells (Fig. 3B). The third consecutive tumor section was stained for CD31 and DLL4, to identify endothelial and DLL4+ cells (Fig. 3B). The same vessel was located on each consecutive tumor section for comparison of NICD1+, GFP+, and DLL4+ cell locations. Cells positive for NICD1, GFP, and DLL4 had similar perivascular locations, suggesting that Notch 1 is active in the DLL4+ GFP+ BM-derived cells (Fig. 3B).
Downregulation of DLL4 inhibits participation of BM-derived cells in the perivascular layer surrounding tumor vessels
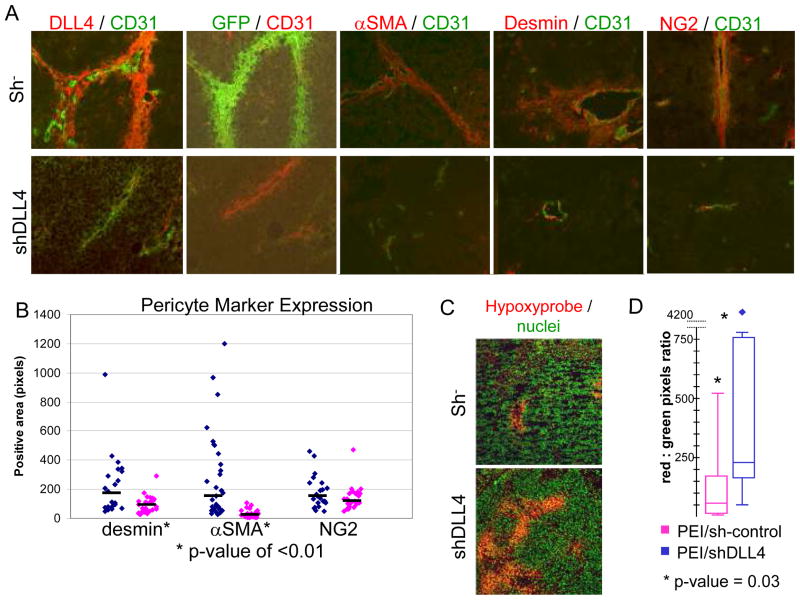
We next determined whether downregulating DLL4 expression affects the number and location of BM-derived pericytes/vSMCs. GFP+ BM-transplanted mice were injected with TC71 cells on both their right and left flanks. When tumors became palpable, we began intratumor injections three times weekly with PEI containing either shRNA control (PEI/sh−, left side) or shDLL4 (PEI/shDLL4, right side). This model allows for paired tumors (one on the right and one on the left) to share the same BM cell pool. After 24 days of treatment, the mice were sacrificed and their tumors harvested and examined by immunohistochemistry for the presence of CD31+ cells, DLL4+ cells, and GFP+ BM-derived cells. DLL4 was significantly inhibited in tumors treated with PEI/shDLL4 (Fig. 4A). Few or no GFP+ BM-derived cells were observed in the PEI/shDLL4-treated tumors, while the PEI/sh− tumors showed extensive vessel coverage with GFP+ BM-derived perivascular cells (Fig. 4A).
Figure 4.
Inhibition of DLL4 expression results in decreased tumor vessel-associated GFP+ BM-derived cells and pericytes, and increased hypoxia. A, B, TC71 cells were implanted subcutaneously on both flanks of nude mice that had previously been transplanted with GFP+ BM cells. Tumors were injected with PEI/sh− control (left side tumors) or PEI/shDLL4 (right side). A, Slides were stained with anti-CD31 in combination with anti-DLL4, anti-GFP, anti-α-SMA, anti-desmin, or anti-NG2. Colors are indicated above each panel. B, Five 10x fields from each tumor were analyzed using SimplePCI software. Diamonds represent the average positive area per slide for desmin, α-SMA, and NG2. Black bars show median values. Desmin, P < 0.01; α-SMA, P < 0.001; NG2, P not significant. C,D, Mice bearing bilateral TC71 tumors and treated with intratumor injections of PEI/sh− or PEI/shDLL4, described above, were injected with Hypoxyprobe-1 prior to sacrifice. Tumor sections were stained with anti-hypoxyprobe (red) to identify hypoxic regions and Sytox green (green) to identify nuclei. D, Five 10x fields from each tumor were analyzed. Box and whisker plots show the amount of hypoxia in PEI/sh− (pink) and PEI/shDLL4 (blue) treated tumors. P = 0.03.
Inhibition of DLL4 expression correlates with reduction of α-SMA+, desmin+, and NG2+ pericytes/vSMCs, increased hypoxia, and decreased tumor growth
Having shown that PEI/shDLL4 inhibits DLL4 expression, we next determined whether loss of DLL4 correlates with an overall loss of pericytes in the tumor. We performed immunohistochemical analysis for CD31 in combination with α-SMA, desmin, or NG2. Blood vessels in the PEI/shDLL4-treated tumors had significantly fewer αSMA+ and desmin+ cells surrounding CD31+ vessels than those in control tumors (91% and 57% reduction, respectively) (Fig. 4A and B). There was also a trend towards reduced numbers of NG2 + cells in PEI/shDLL4-treated tumors, although the reduction was not statistically significant (43% reduction). These data suggest that DLL4 is necessary for pericyte formation in Ewing’s sarcoma.
The drastic loss of pericytes/vSMC in tumors after treatment with PEI/shDLL4 should reduce vessel functionality and increase tumor hypoxia. To determine whether hypoxia was increased in PEI/shDLL4 treated tumors, we again implanted TC71 cells bilaterally in nude mice. Tumors were treated as described above with PEI/sh− (left side) or PEI/shDLL4 (right side). Prior to sacrifice, mice were injected i.v. with Hypoxyprobe-1, which forms pimonidazole adducts in hypoxic tissue and can be recognized using immunohistochemistry. PEI/shDLL4 treated tumors had significantly larger regions of hypoxia than PEI/sh− treated tumors (Fig 4C,D). This suggests reduced vascular function in PEI/shDLL4 treated tumors, leading to greater regions of hypoxia. Tumor growth was also followed. The PEI/shDLL4 tumors were significantly smaller (p-value = 0.03).
Discussion
We have previously shown that BM-derived cells contribute to the pericyte population in Ewing’s sarcoma during vasculogenesis (3), and that vasculogenesis plays a critical role in the expansion of tumor vessels and the growth of Ewing’s sarcoma in vivo (1, 2). Interfering with this process severely compromised tumor growth (1, 4, 12). Here, we demonstrate for the first time that the pericytes/vSMCs covering Ewing’s tumor vasculature are DLL4+, that these cells are derived from BM cells, and that downregulation of DLL4 inhibits the participation of BM cells in the formation of the perivascular layer surrounding the tumor vessels and increases tumor hypoxia. This was done using a BM transplant model with GFP+ BM-derived cells to track the migration and differentiation of these cells. We found extensive colocalization of GFP (BM-derived cells) and the pericyte markers α-SMA and desmin, indicating that the tumor vessel pericytes/vSMCs were derived from BM cells. Furthermore, these perivascular BM-derived cells were DLL4+. Not all pericytes were GFP+; thus, local cells also contribute to the pericyte population. We also found thick perivascular layers of DLL4+ cells in 12 of 14 human Ewing’s sarcoma samples, confirming the relevance of our findings.
DLL4 is expressed on newly forming vessels. While expression of DLL4 by endothelial cells in tumor vessels has been reported (16, 17, 21, 22), we are the first to report DLL4 expression by pericytes/vSMCs. We examined DLL4 expression in two different Ewing’s sarcoma xenograft tumors. We demonstrated the colocalization of DLL4 with α-SMA or desmin. Thick layers of DLL4+ cells surrounded the endothelial cells in both the TC71 and A4573 models. These findings were confirmed using two different anti-DLL4 antibodies. The extensive number of cells that were double-positive for DLL4 and GFP or DLL4 and either α-SMA or desmin indicates that the majority of the DLL4+ pericytes were BM-derived. In addition, 12 of the 14 Ewing’s sarcoma patient samples were positive for DLL4. DLL4 was expressed in both primary and metastatic lesions, and the majority of the DLL4 expression was in the same perivascular location as seen in our xenograft models.
We were unable to perform double staining using anti-DLL4 and pericyte markers with our patient tumor samples, as immunofluorescent staining cannot be done on paraffin-embedded tissue. However, comparison of sequential sections stained for either hematoxylin and eosin or DLL4 confirms that DLL4 expression is in perivascular cells and that these DLL4+ cells are likely to be pericytes or vSMCs. The expression of DLL4 was not always perivascular. Some tumor cells were also positive for DLL4. Taken together, these data support the relevance of our findings in the xenograft mouse model for the human disease.
Our finding of DLL4 expression by BM-derived perivascular stroma is supported by other studies. Indraccolo et al. showed that DLL4+CD31− cells were associated with tumor vessels in T-ALL in mice (23). In addition, DLL4+ lin−Sca1+ BM cells have been shown to participate in new vessel formation during wound healing (24). DLL4 has also been detected in the stromal cells of human endometrium (25). Thus, our demonstration of the expression of DLL4 by nonendothelial BM-derived cells is consistent with previous reports.
If DLL4-Notch signaling is important for BM cell differentiation into pericytes, we would expect to see active Notch signaling in BM-derived cells. We used an anti-NICD1 antibody to identify cleaved, active, Notch1. NICD1 was expressed in the same perivascular location as DLL4+ BM-derived cells on sequential sections from the same tumor, supporting our hypothesis that Notch signaling is important for BM cell differentiation into pericytes.
We are also the first to show that a reduction in DLL4 expression correlates with a reduction in BM-derived cells around tumor vessels. This was done using bilateral tumors where DLL4 was downregulated in only one tumor using PEI/shDLL4. We elected to use PEI as the delivery vector because of our extensive experience with this gene delivery system (26, 27). Intratumor injections of PEI/shDLL4 resulted in decreased DLL4 expression and a decrease in BM-derived cells around the tumor vessels.
In PEI/shDLL4 treated tumors, α-SMA+, desmin+, and NG2+ pericytes were decreased by 91%, 57%, and 43%, respectively, compared to control (Fig 4B). The decrease in the number of α-SMA+ and desmin+ cells was statistically significant (Mann-Whitney p-value<0.01). While the reduction in the number of NG2+ cells was visually apparent, it was not statistically significant. NG2+ cells are less common than α-SMA+ and desmin+ cells, even in control tumors. Therefore, a reduction in this population may be more difficult to detect. Alternatively, those pericytes that are NG2+ may be less dependent on Notch signaling for development or maintenance. The difference in expression levels of pericyte markers is most likely a reflection of the differing phases of maturity of pericytes in the tumor. Mature pericytes, for example, are less likely to express NG2 (7). Taken together, the reduction in α-SMA+, desmin+, and NG2+ pericytes/vSMCs around vessels in PEI/shDLL4-treated tumors indicates the critical role that DLL4-Notch signaling plays in pericyte formation in tumor vessels.
It is unlikely that the reduction in BM-derived cells in the PEI/shDLL4-treated tumor was the result of a systemic effect on circulating BM pericyte progenitors because the blood vessels in the control tumor on the opposite side of the same mouse had extensive BM-derived pericytes/vSMCs. If circulating BM cells had been affected by leakage of PEI/shDLL4 into the circulation, we would expect to see a reduction of BM-derived pericytes/vSMCs in both the PEI/sh− control and PEI/shDLL4-treated tumors.
We also demonstrated a reduction in tumor growth and that tumor vessel function was compromised following inhibition of DLL4, resulting in increased tumor hypoxia. Using Hypoxprobe-1 and immunohistochemical analysis, we demonstrated increased hypoxia in PEI/shDLL4-treated tumors, presumably resulting from decreased delivery of oxygenated blood to the tumor. This finding is consistent with the inhibition of pericytes/vSMCs (8).
Once a BM cell has migrated to the site of neovessel formation, it must attach to the vessel wall, extravasate out of the blood stream, and differentiate into a mature pericyte. It is unclear which of these processes are affected by loss of DLL4. DLL4-Notch signaling is necessary for expression of specific adhesion molecules such as α3β1 integrin (24). Inhibition of DLL4-Notch signaling resulted in reduced adhesion in vitro and decreased wound healing in vivo (25). This suggests that DLL4-Notch signaling is important for adhesion of BM cells to sites of developing vasculature. Notch signaling is involved in pericyte differentiation and maturation. Notch3 is necessary for mural cell differentiation, and active Notch3 induces smooth muscle gene expression (28). Inhibiting Notch activation with a gamma secretase inhibitor prevents expression of α-SMA (29). Thus, it is likely that DLL4-Notch signaling contributes to both adhesion and differentiation of BM cells into pericytes.
In conclusion, we are the first to report DLL4 expression by pericytes/vSMCs in a tumor model. DLL4+ pericytes were seen in two different xenograft models of Ewing’s sarcoma, as well as in patient tumors. Inhibition of DLL4 expression correlated with a decrease in the number of BM-derived perivascular cells, a reduction in the overall pericyte/vSMC coverage of vessels, and increased tumor hypoxia. Furthermore, we showed that Notch1 is activated in the BM-derived perivascular cells. These studies indicate that DLL4-Notch signaling is involved in pericyte/vSMC differentiation and maturation, and that DLL4 may be a new therapeutic target for the treatment of Ewing’s sarcoma.
Acknowledgments
We thank Donna Reynolds, Dr. Krishna Reddy, and Dr. Shu-fang Jia for their teaching and advice.
The project was supported by NIH grant R01 CA103986, core grant CA16672 and Award Number TL1RR024147 from the National Center For Research Resources. The content is the responsibility of the authors and does not represent the official views of the National Center For Research Resources or the National Institutes of Health.
Abbreviations
- BM
bone marrow
- GFP
green fluorescent protein
- αSMA
alpha smooth muscle actin
- VSMC
vascular smooth muscle cell
Footnotes
Statement of Translational Relevance
We have shown that Ewing’s tumor vessels are comprised of bone marrow (BM)-derived and locally derived pericytes. Inhibiting BM cell participation in tumor vessel formation inhibits tumor growth. The molecular mechanisms involved in the formation of BM-derived pericytes are poorly understood. We demonstrate that DLL4 is expressed in Ewing’s sarcoma patient samples and by the BM-derived pericytes/vascular smooth muscle cells in vessels of Ewing’s sarcoma xenograft models. Injection of DLL4 shRNA inhibited DLL4 expression and resulted in decreased BM-derived pericytes in tumor vessels. Reduced numbers of pericytes resulted in less functional vessels which inhibited tumor growth. These results suggest that DLL4 may be a therapeutic target for the treatment of Ewing’s sarcoma.
References
- 1.Lee THBM, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. International Journal of Cancer. 2006;119:839–46. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 2.Bolontrade MFZR, Kleinerman ES. Vasculogenesis Plays a Role in the Growth of Ewing’s Sarcoma in Vivo. Clinical Cancer Research. 2002;8:3622–7. [PubMed] [Google Scholar]
- 3.Krishna Reddy ZZ, Schadler Keri, Jia Shu-Fang, Kleinerman Eugenie S. Bone Marrow Subsets Differentiate Into Endothelial Cells and Pericytes Contributing to Ewing’s Tumor Vessels. Molecular Cancer Research. 2008;6:929–36. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z, Reddy K, Guan H, Kleinerman ES. VEGF165, but not VEGF189, Stimulates Vasculogenesis and Bone Marrow Cell Migration into Ewing’s Sarcoma Tumors In vivo. 2007:1125–32. doi: 10.1158/1541-7786.MCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 5.Hall AP. Review of the Pericyte during Angiogenesis and its Role in Cancer and Diabetic Retinopathy. 2006:763–75. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- 6.Steven Song AJE, Stalcup William, Werb Zena, Bergers Gabriele. PDGFRb+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature Cell Biology. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annika Armulik AA, Betsholz Christer. Endothelial/Pericyte Interactions. Circulation Research. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 8.Gabriele Bergers SS. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iiro Rajantie MI, Alminaite Agne, Ozerdem Ugur, Alitalo Kari, Salven Petri. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du R, Lu KV, Petritsch C, et al. HIF1[alpha] Induces the Recruitment of Bone Marrow-Derived Vascular Modulatory Cells to Regulate Tumor Angiogenesis and Invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mani Valarmathi JD, Yost Michael, Goodwin Richard, Potts Jay. A three-dimensional model of vasculogenesis. Biomaterials. 2009:1–15. doi: 10.1016/j.biomaterials.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Guan H, Zhou Z, Wang H, Jia S-F, Liu W, Kleinerman ES. A Small Interfering RNA Targeting Vascular Endothelial Growth Factor Inhibits Ewing’s Sarcoma Growth in a Xenograft Mouse Model. 2005:2662–9. doi: 10.1158/1078-0432.CCR-04-1206. [DOI] [PubMed] [Google Scholar]
- 13.Ulla-Maj Fiuza AMA. Cell and Molecular Biology of Notch. Journal of Endocrinology. 2007;194:459–74. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 14.Gale NWDM, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffrey S, Scehnet WJ, Ram Kumar S, Krasnoperov Valery, Trindade Alexandre, Benedito Rui, Djokovic Dusan, Borges Cristina, Ley Eric J, Duarte Antonio, Gill Parkash S. Inhibition of DLL4 mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007 doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irene Noguera-Troise CD, Papadopoulos Nicholas J, Coetzee Sanda, Boland Pat, Gale Nicholas W, Lin Hsin Chieh, Yancopoulos George D, Thurston Gavin. Blockade of DLL4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 17.Li JL, SR, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Research. 2007;67:11244–53. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 18.Mats Hellstrom L-KP, Hofmann Jennifer J, Wallgard Elisabet, Coultas Leigh, Lindblom Per, Alva Jackelyn, Nilsson Ann-Katrin, Karlsson Linda, Gaiano Nicholas, Yoon Keejung, Rossant Janet, Luisa Iruela-Arispe M, Kalen Mattias, Gerhardt Holger, Betsholz Christer. DLL4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007:1–5. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 19.Carolina Mailhos JL, Ish-Horowicz David, Modlich Ute, Harris Adrian, Bicknell Roy. Delta4, an endothelial specific Notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135–44. doi: 10.1046/j.1432-0436.2001.690207.x. [DOI] [PubMed] [Google Scholar]
- 20.Rao PK, Dorsch M, Chickering T, et al. Isolation and Characterization of the Notch Ligand Delta4. Experimental Cell Research. 2000;260:379–86. doi: 10.1006/excr.2000.5034. [DOI] [PubMed] [Google Scholar]
- 21.John Ridgway GZ, Wu Yan, Stawicki Scott, Liang Wei-Ching, Chanthery Yvan, Kowalski Joe, Watts Ryan J, Callahan Christopher, Kasman Ian, Singh Mallika, Chien May, Tan Christine, Hongo Jo-Anne S, de Sauvage Fred, Plowman Greg, Yan Minhong. Inihibition of DLL4 signalling inhibits tumor growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 22.Segarra M, Williams CK, Sierra MdlL, et al. Dll4 activation of Notch signaling reduces tumor vascularity and inhibits tumor growth. 2008:1904–11. doi: 10.1182/blood-2007-11-126045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indraccolo S, Minuzzo S, Masiero M, et al. Cross-talk between Tumor and Endothelial Cells Involving the Notch3-Dll4 Interaction Marks Escape from Tumor Dormancy. 2009:1314–23. doi: 10.1158/0008-5472.CAN-08-2791. [DOI] [PubMed] [Google Scholar]
- 24.Caiado F, Real C, Carvalho Tn, Dias Notch Pathway Modulation on Bone Marrow-Derived Vascular Precursor Cells Regulates Their Angiogenic and Wound Healing Potential. PLoS ONE. 2008;3:e3752. doi: 10.1371/journal.pone.0003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazella J, Liang S, Tseng L. Expression of Delta-Like Protein 4 in the Human Endometrium. 2008:15–9. doi: 10.1210/en.2007-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu-Fang Jia LLW, Densmore Charles L, Xu Bo, Zhou Zhichao, Kleinerman Eugenie S. Eradication of osteosarcoma lung metastases following intranasal interleukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene Therapy. 2000;2(9):260–6. doi: 10.1038/sj.cgt.7700432. [DOI] [PubMed] [Google Scholar]
- 27.Guan H, Zhou Z, Gallick GE, et al. Targeting Lyn inhibits tumor growth and metastasis in Ewing’s sarcoma. 2008:1807–16. doi: 10.1158/1535-7163.MCT-08-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Kennard S, Lilly B. NOTCH3 Expression Is Induced in Mural Cells Through an Autoregulatory Loop That Requires Endothelial-Expressed JAGGED1. 2009:466–75. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi H, Iso T, Shiba Y, et al. Notch signaling regulates the differentiation of bone marrow-derived cells into smooth muscle-like cells during arterial lesion formation. Biochemical and Biophysical Research Communications. 2009;381:654–9. doi: 10.1016/j.bbrc.2009.02.116. [DOI] [PubMed] [Google Scholar]