Abstract
MRI-based reports of both abnormally increased and decreased amygdala volume in bipolar disorder (BD) have surfaced in the literature. Two major methodological weaknesses characterizing extant studies are treatment with medication and inaccurate segmentation of the amygdala due to limitations in spatial and tissue contrast resolution. Here, we acquired high-resolution images (voxel size=0.55×0.55×0.60mm) using a GE 3T MRI scanner, and a pulse sequence optimized for tissue contrast resolution. The amygdala was manually segmented by one rater blind to diagnosis, using coronal images. Eighteen unmedicated (mean medication-free period 11±10 months) BD subjects were age and gender matched with 18 healthy controls, and 17 medicated (lithium or divalproex) subjects were matched to 17 different controls. The unmedicated BD patients displayed smaller left and right amygdala volumes than their matched control group (p<0.01). Conversely, the BD subjects undergoing medication treatment showed a trend towards greater amygdala volumes than their matched HC sample (p=0.051). Right and left amygdala volumes were larger (p<0.05) or trended larger, respectively, in the medicated BD sample compared with the unmedicated BD sample. The two control groups did not differ from each other in either left or right amygdala volume. BD patients treated with lithium have displayed increased gray matter volume of the cortex and hippocampus relative to untreated BD subjects in previous studies. Here we extend these results to the amygdala. We raise the possibility that neuroplastic changes in the amygdala associated with BD are moderated by some mood stabilizing medications.
Introduction
Volumetric reductions of the amygdala have been reported in MRI studies of both adults (Blumberg et al., 2003, Pearlson et al., 1997a, Rosso et al., 2007) and adolescents (Blumberg et al., 2003, Chang et al., 2005, Dickstein et al., 2005) with bipolar disorder (BD), and these findings are supported by post-mortem data indicative of reduced amygdala volume, neuronal size or neuronal density of some amygdala nuclei (Berretta et al., 2007, Bezchlibnyk et al., 2007). Nevertheless, the volumetric MRI data are inconsistent. While some studies return negative results in adults (Lyoo et al., 2004, Swayze et al., 1992), children (Frazier et al., 2005), and high-risk offspring (Ladouceur et al., 2008, Singh et al., 2008), others report enlargement of the amygdala in adult samples with BD relative to healthy control samples (Altshuler et al., 2000, Altshuler et al., 1998, Brambilla et al., 2003, Strakowski et al., 1999b, Velakoulis et al., 2006).
In this study we addressed two methodological limitations that might explain the diversity of results obtained with structural MRI in BD. Firstly, we applied a high-resolution 3T MRI sequence that enabled more accurate delimitation of the amygdala from adjacent structures than was possible in previous studies. Most previous studies acquired images using MRI scanners of ≤1.5 Tesla (T) field strength at volumetric resolutions ≥1 mm3 (Pearlson et al., 1997b) (Strakowski et al., 1999a) (Altshuler et al., 2000) (Brambilla et al., 2003) (Blumberg et al., 2003) (Lyoo et al., 2004) (Frangou, 2005) (Kogelnik et al., 2006) (Velakoulis et al., 2006). More recently, two studies imaged their subjects using 3T magnets, yet they still acquired images at a resolution of 1.2–1.5 mm3 (Chang et al., 2005, Foland et al., 2008). In images acquired within this range of spatial resolution, the amygdala grey matter (GM) proves difficult or impossible to delimit from some adjacent structures, such as the temporal claustrum, perirhinal cortex, hippocampus, and basal forebrain (Mai et al., 2004), reducing the specificity and reliability of amygdala volumetric measures (Savitz and Drevets, 2009). To increase intra-rater reliability, some studies applied arbitrary boundaries that could be reproducibly applied, while others limited measures to slices where anatomical borders were clear. However, these approaches excluded variable amounts of amygdala tissue, and/or included tissue from adjacent structures, compromising the validity of the amygdala measures.
The identical problem applied to an independent data set of ours that was derived from 1.5T images (voxel size =1 mm3). Despite being segmented by anatomists expert in amygdala morphology (D.L. Price, D. Öngür), we obtained unsatisfactory intra- and inter-rater reliabilities for the amygdala segmentation such that the data were reported only in abstract form (Ongur et al., 1998). Here we resolve this problem with high-resolution 3T MRI images (volumetric resolution=0.4 mm3) that allowed us to segment the amygdala in a reliable and accurate manner.
A second issue that has confounded the psychiatric volumetric MRI literature is treatment with psychotropic medication (Phillips et al., 2008). Lithium, for instance, has been shown to increase whole brain GM volumes by 3% (24 ml) from baseline in BD patients treated for only 4 weeks (Moore et al., 2000); an effect that may extend to other mood stabilizers such as valproate (Mark et al., 1995) (Hao et al., 2004), antidepressants such as tianeptine (McEwen and Olie, 2005), and perhaps even fluoxetine (Stewart and Reid, 2000). The hypertrophic effects of lithium have been reported to extend to the prefrontal cortex (Moore et al., 2009) as well as the amygdala in adult (Foland et al., 2008) and pediatric (Chang et al., 2005) BD samples.
Previous MRI studies of the amygdala either failed to control for medication effects (Altshuler et al., 2000, Frangou, 2005, Lyoo et al., 2004, Strakowski et al., 1999b, Velakoulis et al., 2006), or attempted to address this confound by comparing subjects receiving medication at the time of scanning against subjects not currently taking such agents at scanning (Blumberg et al., 2003, Brambilla et al., 2003, Foland et al., 2008, Usher et al., 2009). The neuroprotection afforded by such agents would, however, be expected to persist for a few weeks following treatment discontinuation (while the drug clears from the brain and the drug effects on gene expression subside), and it is unclear when the pathological processes accounting for a primary abnormality in BD would reassert themselves—manifested by the reappearance of GM abnormalities—following treatment cessation. The current study is the first to address this question by comparing patients who were currently taking medications to patients who had not been not been treated with medication for more than two (mean 11±10) months prior to scanning.
Method
Subjects
Participants met DSM-IV criteria for BD based on the Structured Clinical Interview for DSM-IV (First et al., 1995) and an unstructured interview with a psychiatrist. Eight BD subjects met criteria for Type I (BDI) and 27 for Type II BD (BDII). The following exclusion criteria obtained: left-handedness, significant medical or neurological disorders, past head injury with loss of consciousness, risk of suicide, meeting DSM-IV criteria for substance abuse within the previous 90 days or substance dependence within the previous 5 years, pregnancy, general MRI exclusion criteria or electrolyte disturbance, anemia, or positive drug or HIV screen on laboratory testing. The time period for which the exclusion criterion for substance abuse applied was limited to 90 days since the lifetime prevalence of comorbid substance abuse is so high in BD – approximately 60% (Regier et al., 1990) - that excluding cases for a lifetime history of substance abuse might have introduced a selection bias that would have rendered our sample unrepresentative of the general BD population.
With the exception of the allowances described above for substance abuse disorders, healthy control (HC) subjects (n=35) had no lifetime history of a psychiatric disorder, and no first degree relative with a mood or anxiety disorder, as established using the Family Interview for Genetic Studies (FIGS). One healthy control had a past history of alcohol abuse.
Ages ranged from 23–56 years for the BD group and 23–58 years for the HC sample. Each patient was matched to a control subject of the same sex and similar age (mean age difference per pair = 1.25 ± 1.95 years). The medicated BD group (3 BDI and 14 BDII) and its matched HC group each consisted of 17 matched individuals while the unmedicated BD group (5 BDI and 13 BDII) and its matched HC group each were made up of 18 matched individuals. Age, gender, and other demographic data are shown in Table 1. The participants gave written informed consent to participate, as approved by the NIMH Institutional Review Board.
Table 1.
Demographics and Amygdala Volumes Across Subject Groups [Mean ± SD unless otherwise stated] and Statistical Differences Between Subject Groups.
| Unmedicated BD (n=18) | HC for Unmedicated BD (n=18) | Comparison | Medicated BD (n=17) | HC for Medicated BD (n=17) | Comparison | Comparison of Medicated and Unmedicated BD Groups | Comparison of HC Groups | |
|---|---|---|---|---|---|---|---|---|
| Age at Scan | 35.2±8.4 | 35.2±8.4 | NS | 42.3±9.5 | 42.1±9.6 | NS | Unmedicated BD younger than medicated BD: t=2.4; df=33; p=0.025 | Unmedicated HC younger than medicated HC: t=2.2; df=33; p=0.032 |
| Sex (M/F) | 7/11 | 7/11 | NS | 5/12 | 5/12 | NS | NS | NS |
| BDI/BDII | 5/13 | - | - | 3/14 | - | - | NS | - |
| Family History | 14+4 unknown | - | - | 17+ | - | - | NS | - |
| Age at Illness Onset (years) | 19.5±6.9 | - | - | 17.0±9.0 | - | - | NS | - |
| Duration of illness (years) | 14.8±11.0 | - | - | 25.9±10.7 | - | - | Longer duration of illness in medicated group: t=2.8; df=29; p=0.008 | - |
| MADRS | 23.3±10.3 | 0.43±0.9 | p<0.001 | 30.1±10.0 | 0±0 | p<0.001 | NS | NS |
| YMRS | 4.87±4.10 | - | - | 4.85±3.93 | - | - | NS | - |
| Absolute Left Amygdala Volume (ml3) | 1.369±0.150 | 1.480±0.188 | - | 1.475±0.206 | 1.409±0.204 | - | NS | NS |
| Normalized Left Amygdala Volume | 0.001179±0.000140 | 0.001274±0.000137 | - | 0.001343±0.000163 | 0.001160±0.000152 | - | After controlling for age and duration of illness, left amygdala volumes trended smaller in unmedicated BD (F=3.7; p=0.064) | NS |
| Absolute Right Amygdala Volume (ml3) | 1.333±0.197 | 1.452±0.151 | - | 1.410±0.230 | 1.322±0.204 | - | NS | NS |
| Normalized Right Amygdala Volume | 0.001143±0.000150 | 0.001253±0.000148 | - | 0.001279±0.000157 | 0.001160±0.000152 | - | After correcting for age and duration of illness, unmedicated BD had significant smaller amygdala than medicated BD: F=4.5; p=0.044. | NS |
| Absolute Total Amygdala Volume | 1.351±0.161 | 1.466±0.160 | Amygdala volume smaller in BD: F=5.0; p=0.040 | 1.442±0.211 | 1.365±0.178 | NS | NS | NS |
| Relative Total Amygdala Volume | 0.001161±0.000131 | 0.001263±0.000132 | Amygdala volume smaller in BD: F=10.1; p=0.006 | 0.001311±0.000152 | 0.001198±0.000122 | Larger amygdala volume in medicated BD: F=4.4; p=0.051 | After correcting for age and duration of illness, unmedicated BD had significant smaller amygdala than medicated BD: F=4.7; p=0.039. | NS |
| Left/Right Amygdala Ratio | 1.04±0.13 | 1.02±0.08 | NS | 1.05±0.10 | 1.08±0.20 | NS | NS | NS |
| WBV (ml3) | 1168.5±118.9 | 1166.5±126.9 | NS | 1102.1±121.5 | 1140.5±107.5 | NS | NS | NS |
Abbreviations: MADRS=Montgomery-Asberg Depression Rating Scale; WBV=Whole Brain Volume; NS = Not Significant; + = positive. Total Amygdala Volume = [(Left Amygdala Volume+Right Amygdala Volume)/2]
BD subjects were scanned during either the depressed or partial remission phases. Depression severity was rated using the Montgomery-Asberg Depression Rating Scale (MADRS). Mean MADRS scores of the medicated and unmedicated BD groups were not significantly different from each other (30±10 and 23+10, respectively; p=0.06). Severity of depression was estimated with the MADRS using the criteria of (Hawley et al., 2002). In the unmedicated group, 2 individuals were in the non-depressed range, 1 was mildly depressed, 11 were moderately depressed, and 4 were severely depressed at the time of scanning. The medicated BD group was composed of 1 subject in the non-depressed range, 8 moderately depressed individuals, 7 severely ill patients, and 1 very severely depressed patient. Mania and hypomania was measured with the Young Mania Rating Scale (YMRS). The mean YMRS scores were 5±4 and 5±4 in the unmedicated and medicated BD groups, respectively. Two individuals in the unmedicated BD group and two patients in the medicated BD group showed some evidence of mixed symptomatology at the time of scanning (YMRS scores = 11–12).
The unmedicated BD group consisted of 18 subjects who had not been exposed to psychotropic medications at least two months prior to scanning. Of these, 4 were naïve to psychotropic drugs and the remaining 14 were unmedicated for an average of 11±10 (range 2.5 to 37) months. The medicated group (n=17) consisted of 9 cases taking lithium, 7 cases taking divalproex, and 1 case taking chlorpromazine (therapeutic blood levels documented within several days of scanning). Lifetime drug exposure of both the medicated and (currently) unmedicated BD groups is shown in Table 2.
Table 2.
Life-Time Exposure to Medications in BD Samples.
| Medicated BD Sample (Subject #)* | Medication | Unmedicated Sample (Subject #) | Past medications |
|---|---|---|---|
| 1 | clorpromazine | 1 | lithium, fluoxetine, sertraline, gabapentin, venlafaxine, risperdone |
| 2 | lithium | 2 | sertraline, bupropion, fluoxetine, citalopram |
| 3 | lithium | 3 | fluoxetine, sertraline, paroxetine, bupropion, venlafaxine |
| 4 | lithium, fluoxetine | 4 | olanzepine, methylphenidate, carbamazepine |
| 5 | divalproex, citalopram paroxetine, bupropion | 5 | lamotrigine |
| 6 | lithium | 6 | divalproex, lithium, nortriptyline, olanzepine |
| 7 | lithium, pramipexole | 7 | naïve |
| 8 | divalproex, clonazepam | 8 | bupropion, paroxetine, nefazodone, venlafaxine, phenelzine |
| 9 | Topiramate, alprazolam, clonazepam, carbamazepine, lamotrigine | 9 | lithium, sertraline, paroxetine, citalopram |
| 10 | lithium | 10 | sertraline, bupropion, venlafaxine, lithium, gabapentin, thyroxine |
| 11 | lithium | 11 | lithium |
| 12 | lithium, pramipexole | 12 | sertraline, paroxetine |
| 13 | lithium, sertraline, citalopram | 13 | naïve |
| 14 | divalproex, olanzepine, nefazodone, lithium, sertraline | 14 | naïve |
| 15 | lithium, sertraline | 15 | naïve |
| 16 | bupropion; fluoxetine | 16 | fluoxetine |
| 17 | divalproex | 17 | amitriptyline, olanzepine, bupropion, gabapentin |
| 18 | fluvoxamine |
Note: Medicated BD subjects were not taking more than one type of drug at the time of the scan
Unmedicated patients with BD had the following comorbid conditions: post-traumatic stress disorder (PTSD) and generalized anxiety disorder (GAD) (n=1), social phobia and panic disorder (n=1), and social phobia (n=1). In the medicated BD group the following comorbid conditions were recorded: obsessive compulsive disorder (OCD) (n=1); panic disorder, social phobia, and PTSD (n=2); bulimia (n=2); panic disorder (n=1); and social phobia and PTSD (n=1).
Six medicated subjects with BD had a history of alcohol abuse, 1 patient had abused both alcohol and cannabis, 1 patient had abused cannabis and phencyclidine (pcp), and 1 patient had abused both alcohol and stimulants. Two patients had a prior history of alcohol dependence. Substances abused in the unmedicated BD group were cannabis (n=1); alcohol (n=4); and LSD, cocaine and cannabis (n=1).
Imaging
High-resolution images were acquired using a GE 3T MRI scanner; a standard head radiofrequency coil; and a magnetization-prepared, rapid gradient echo (MP-RAGE) pulse sequence: (echo time [TE]=2.1 msec, repetition time [TR]=7.8msec, prep time = 725 msec, delay time=1400 msec, flip angle=6°). One hundred twenty-four axial slices (slice thickness=0.6 mm) centered on the mesiotemporal lobe were acquired with a 14 cm field-of-view and in-plane resolution of 224×224 voxels, resampled to 256×256×124 voxels for reconstruction, resulting in a displayed resolution of 0.55×0.55×0.6 mm. Three to four 13 minute scans were consecutively acquired, coregistered, and summed to increase signal-to-noise ratio. A second MP-RAGE image of the entire brain also was acquired to measure whole brain volume (voxel size=0.85 × 0.85 × 1.2 mm; TE=4.94msec; TR=11.6 msec, prep time=725 msec; delay time=1400msec).
Image Analysis
Images were corrected for intensity nonuniformity using the minc tool N3, and were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) images using the FSL tool, FAST, in order to measure whole brain volume (WBV).
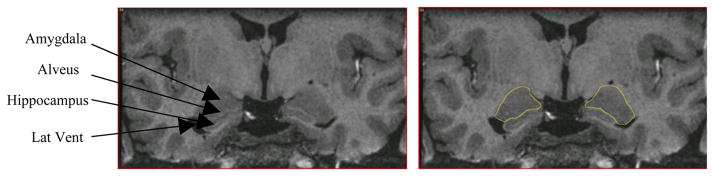
The amygdala was manually segmented by one rater (WB), blind to diagnosis, using MEDx 3.4.1 and published criteria and illustrations (Amaral and Insausti, 1990, Duvernoy, 1998, Hanaway et al., 1998, Mai et al., 2004) (Figure 1). Segmentation was performed on coronal slices, resampled to a slice thickness of 1.1 mm to increase signal-to-noise (horizontal and vertical dimensions of each coronal plane voxel remained at 0.55×0.60 mm) after resampling.
Figure 1.
Coronal section through the amygdala showing the segmentation of the amygdaloid complex (outlined). For anatomical detail see Mai et al. (2004). Lat Vent = Temporal Horn of the Lateral Ventricle.
The anterior boundary of the amygdala was delimited in coronal planes to identify the most anterior MRI slice in which the amygdala GM was apparent. The lateral boundary was defined by the WM between the claustrum and the amygdala. In anterior slices, the dorsomedial boundary was defined by the surface of the semilunar gyrus, the ventromedial boundary was the lateral aspect of the WM adjacent to the entorhinal cortex, and the ventrolateral boundary was delimited by the WM separating amygdala from perirhinal cortex.
In central and posterior slices the ventral boundary was formed by either the alveus or the temporal horn of the lateral ventricle, which delimited amygdala from hippocampus, and the dorsal border was drawn along the GM-WM interface at the dorsal edge of the amygdala (figure 1). In the posterior-most slices, the lateral aspect of the amygdala was delimited from the caudate tail and ventral putamen, and the medial aspect from the parahippocampal cortex.
Data Analysis
Intra-rater reliability of the amygdala segmentation was assessed by repeating amygdala volume measures on 2 separate days in 10 subjects. We calculated an absolute intra-rater reliability score (the degree to which values changed for the same rater segmenting the amygdala on each of two different days) with the Cronbach’s α statistic.
To test our a priori hypothesis that amygdala volumes differ between BD and HC subjects, we carried out both between subject and within subject analyses.
For the comparisons between the medicated BD group and its matched control group, and the unmedicated BD group and its matched control group, separate within subjects analyses in the form of repeated measures ANOVA were performed. For each analysis, significance was evaluated at p<.05, two-tailed. To reduce nonspecific global effects, the absolute amygdala volumes were normalized by dividing by WBV. Unless otherwise stated, all analysis and results pertain to these normalized volumes.
Independent sample t-tests were used for the remaining analyses unless demographic or clinical variables differed between subject groups (p<0.05). In this case the relevant variables were treated as covariates in ANOVA analyses.
The amygdala volumes of the unmedicated and medicated BD groups were compared to their corresponding matched control samples using repeated measures ANOVAs with hemisphere (left versus right) and group (BD versus controls) specified as the within subjects variables. No covariates were entered into the models since the medicated and unmedicated BD groups did not differ from their matched controls on age, sex, or other demographic variables.
We compared the unmedicated and medicated BD groups to each other using an ANCOVA. Age and duration of illness were treated as covariates since these variables differed between the BD groups.
The HC group for the unmedicated BD sample was younger than the HC group for the medicated BD sample. We therefore compared the two HC groups with each other using an ANCOVA with age as a covariate.
We compared the amygdala volumes of the combined BD and HC samples to each other using a two-tailed, independent sample t-test (α =0.05) since the groups did not differ in age, sex, or other demographic variables.
A number of other exploratory post-hoc tests were also conducted:
(5). In order to examine whether patients with BD who were treated with lithium differed from patients with BD who were treated with divalproex, we carried out an independent sample, two-tailed t-test (α=0.05) since the lithium and divalproex groups did not differ on any demographic or clinical variables.
(6). Pearson correlations of amygdala volume with duration of illness, age of onset, and depression at the time of scanning (as measured by the MADRS) were examined in the unmedicated, medicated, and full BD samples.
(7). The correlation between severity of depression at the time of scanning (as measured by the MADRS) and amygdala volume was measured using the Pearson’s statistic (two-tailed, α=0.05) in the medicated, unmedicated, and full BD samples.
(8). The correlation between time to last medication exposure and amygdala volume in the medicated BD group was measured using the Pearson’s statistic (two-tailed, α=0.05).
(9). We tested whether unmedicated patients with BDI differed in amygdala volume from unmedicated patients with BDII. The BDI group differed from the BDII sample in duration of illness so this variable was entered as a covariate in the ANOVA.
Similarly, we tested whether medicated patients with BDI differed from medicated patients with BDII. There were no demographic or clinical differences between these groups and therefore independent sample t-tests (two-tailed, α=0.05) were used for this analysis.
(10). Independent sample t-tests (two-tailed, α=0.05) were used to evaluate whether there were sex differences in amygdala volume in the unmedicated and medicated BD groups, respectively. There were no significant demographic or clinical differences between the male and female samples.
Results
Cronbach’s α scores for the absolute intra-rater reliability of the amygdala tracings were 0.990 (p<0.001) for the left amygdala and 0.993 for the right amygdala.
The general pattern of results was that of reduced amygdala volume in the unmedicated BD patients and enlarged amygdala volume in the medicated BD sample compared to controls. No significant volumetric differences were observed between the two control groups.
Specifically:
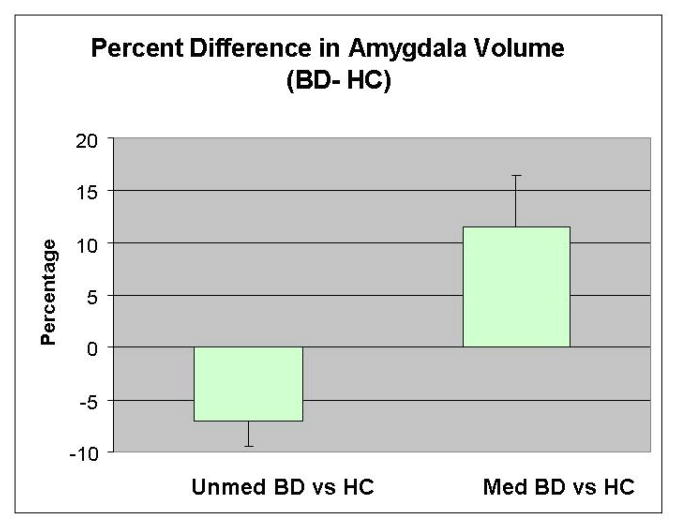
The unmedicated BD group had smaller amygdala volumes (8.2%) than their HC group (F=10.1; p=0.006). The effect of hemisphere was non-significant (F=1.5, p=0.228), and there was no diagnosis by hemisphere interaction (F=0.3; p=0.610). Post-hoc testing showed that the unmedicated BD group had smaller (7.5%) left and right (8.8%) amygdala volumes than their HC group (Table 1). The medicated BD group showed a trend towards a larger amygdala volume (9.4%) than its matched control group (F=4.4; p=0.051) (Table 1 and Figure 2). The hemisphere main effect (F=8.6; p=0.010) but not the hemisphere by diagnosis interaction (F=0.05; p=0.820) was significant, indicating that both the medicated BD group and its healthy control group had larger left than right amygdala volumes.
After controlling for age and duration of illness, total amygdala volume was significantly larger in the medicated BD subjects than their unmedicated counterparts (F=4.7; p=0.039). Mean right amygdala volume was larger in the medicated BD sample compared to the unmedicated BD sample (F=4.5; p=0.044). The left amygdala volume showed a nonsignificant trend towards also being larger in the medicated BD sample than the unmedicated BD sample (F=3.7; p=0.064).
After controlling for age, the two healthy control groups did not differ significantly from each other in either left (F=0.4; p=0.537), right (F=1.4; p=0.249), or total (F=1.1; p=0.312) amygdala volumes.
No significant volumetric difference was found between the combined BD (unmedicated and medicated) and the combined HC samples: left amygdala t=0.1, p=0.931; right amygdala t=0.1, p=0.981; total amygdala t=0.1, p=0.952.
Figure 2.
Mean percentage differences in normalized amygdala volume between the unmedicated and medicated BD samples and their respective control groups. A percentage difference was calculated for each BD-control pair. The average of the percentage difference scores between the pairs of unmedicated BD subjects and their matched controls, and the medicated BD subjects and their matched controls, is shown on the graph. The x-axis through y=0 reflects the amygdala volumes of the matched healthy control groups and the y-axis shows the average percentage deviation in amygdala volume of the BD samples from their respective controls. The bars represent the standard error of the mean (SEM).
Post-hoc analyses
(5). No statistically significant difference in normalized amygdala volumes between the BD subjects treated with divalproex (N=7) and the BD subjects treated with lithium (N=9) was found. Left amygdala volume: 1.498ml (0.246) (divalproex) versus 1.457 ml (0.197) (lithium); p=0.102. Right amygdala: 1.433 ml (0.290) (divalproex) versus 1.389ml (0.204) (lithium); p=0.099. Total amygdala: 1.466ml (0.262) (divalproex) versus 1.423ml (0.193) (lithium); p=0.082. Further, there was no significant difference between the divalproex and lithium groups in WBV: 1056.07ml (100.85) (divalproex) versus 1144.54ml (132.64) (lithium); p=0.166.
-
(6). There was no statistically significant relationship between duration of illness and normalized amygdala volumes in either BD group. Unmedicated BD group: left amygdala r=0.139, p=0.621; right amygdala r=0.077, p=0.785; total amygdala r=0.115, p=0.684. Medicated BD group: left amygdala r=0.077, p=0.778; right amygdala r=−0.187, p=0.489; total amygdala r=0.056, p=0.837. Full BD sample: left amygdala r=0.289, p=0.115; right amygdala r=0.139, p=0.457; total amygdala r=0.225, p=0.224.
No statistically significant correlation between age at illness-onset and normalized amygdala volume was observed in the BD groups: unmedicated BD sample: left amygdala r=0.022, p=0.938; right amygdala r=−0.186, p=0.508; total amygdala r=−0.102, p=0.718; medicated BD sample: left amygdala r=0.010, p=0.970; right amygdala r=0.175, p=0.501; total amygdala r=0.096, p=0.715; full BD sample: left amygdala r=−0.052, p=776; right amygdala r=−0.032, p=0.860; total amygdala r=0.045, p=0.808.
(7). No statistically significant correlation between MADRS score at the time of scanning and normalized amygdala volume was observed in the BD groups (unmedicated BD sample: left amygdala r=0.069, p=0.786; right amygdala r=−0.055, p=0.829; total amygdala r=−0.186, p=0.508; medicated BD sample: left amygdala r=0.091, p=0.737; right amygdala r=0.035, p=0.896; total amygdala r=0.066, p=0.808; full BD sample: left amygdala r=0.218, p=0.216; right amygdala r=0.122, p=0.491; total amygdala r=0.180, p=0.309).
(8). There was no statistically significant correlation between the length of time that an unmedicated subject with BD was off medication and the volume of the left [r=0.312; p=0.278], right [r=−0.249; p=0.390]; or total amygdala [r=0.031; p=0.916)].
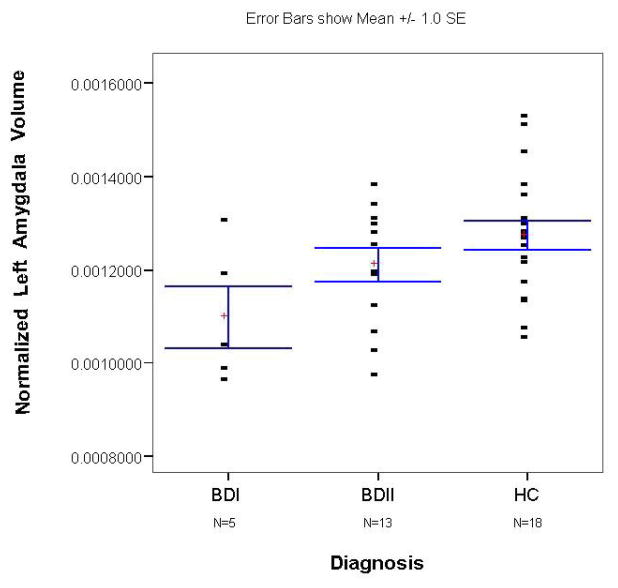
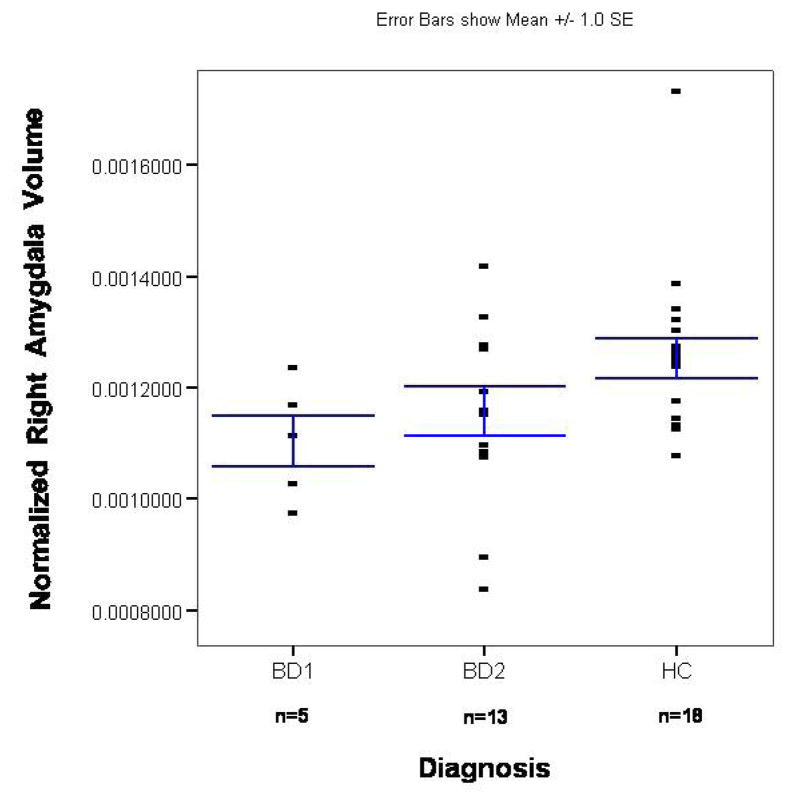
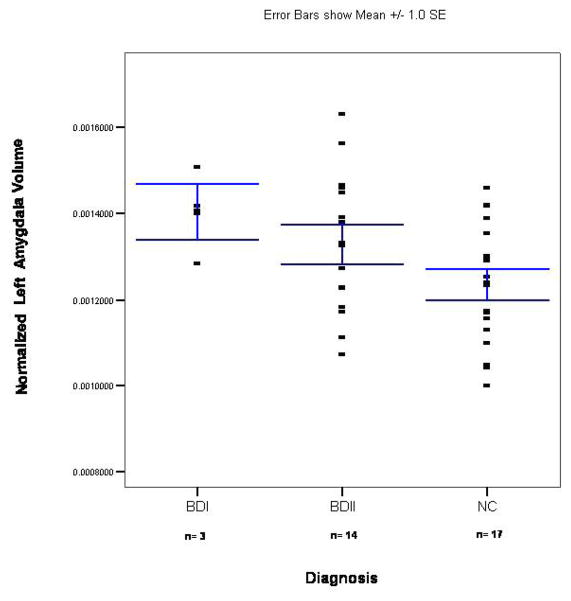
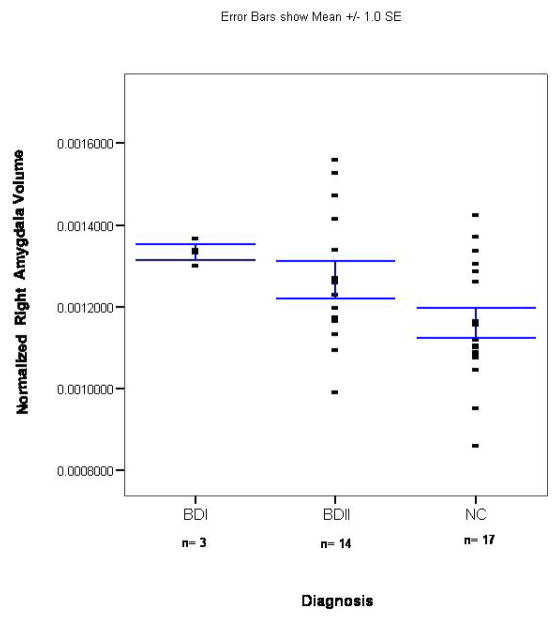
(9). After controlling for duration of illness, we found that the unmedicated BDI sample had a smaller left (F=6.2; p=0.029) but not right (F=1.3; p=0.275) or total (F=3.5; p=0.085) amygdala volume than unmedicated patients with BDII (Figures 3 and 4). There was no significant difference between the medicated BDI and the medicated BDII patients in left (t= 0.7; p=0.493), right (t=1.4; p=0.19), or total (t=0.7; p=0.493) amygdala volume (Figures 5 and 6).
(10). There was no significant difference in the normalized left (t=0.2; p=0.848), right (t=0.4; p=0.705), or total (t=0.3; p=0.751) amygdala volumes between unmedicated BD males and unmedicated BD females. Similarly, no significant sex differences in the normalized left (t=−0.2; p=0.826), right (t=−0.2; p=0.831), and total (t=−0.2; p=0.830) amygdala volumes were observed in the medicated BD group.
Figure 3.
Scatterplot of normalized left amygdala volumes in the unmedicated BD sample and its matched control group. The x-axis shows separate scatterplots for the BDI and BDII groups. The bars represent the standard error of the mean (SEM).
Figure 4.
Scatterplot of normalized right amygdala volumes in the unmedicated BD sample and its matched control group. The x-axis shows separate scatterplots for the BDI and BDII groups. The bars represent the standard error of the mean (SEM).
Figure 5.
Scatterplot of normalized left amygdala volumes in the medicated BD sample and its matched control group. The x-axis shows separate scatterplots for the BDI and BDII groups. The bars represent the standard error of the mean (SEM).
Figure 6.
Scatterplot of normalized right amygdala volumes in the medicated BD sample and its matched control group. The x-axis shows separate scatterplots for the BDI and BDII groups. The bars represent the standard error of the mean (SEM).
Discussion
A Priori Hypothesis Testing
We found that BD subjects who were medication-naïve or had been unmedicated for at least two months displayed smaller amygdala volumes than their matched control subjects. Conversely, the BD subjects who were undergoing treatment with lithium, divalproex or chlorpromazine (n=1) showed a trend towards larger amygdala volumes than their matched HC subjects. After controlling for age and duration of illness, right and total, but not left, amygdala volumes were larger in the medicated BD sample compared with the unmedicated BD sample. In contrast, after controlling for age differences, the two healthy control groups did not differ from each other in either left, right, or total amygdala volume. As expected, given the hypothesized effects of medication on amygdala volume, no statistically significant difference in amygdala volume was found when the medicated and unmedicated BD samples were treated as one group and compared to the full HC sample. The fact that absolute amygdala volumes did not differ significantly across groups may reflect the increased statistical noise of these measurements due to inter-individual differences in WBV.
Preclinical studies have demonstrated that lithium and divalproex administration increases the mRNA expression of several genes involved in synaptic and cytoskeletal plasticity and cellular resilience to physiological stress (Chen et al., 1999a, Chen et al., 1999b, Hao et al., 2004, Harwood, 2005, Manji et al., 2000, McQuillin et al., 2007, Youngs et al., 2006). These findings may explain why imaging studies have found evidence for increased whole brain (Moore et al., 2000) (Sassi et al., 2002), anterior cingulate (Bearden et al., 2007), and hippocampal (Bearden et al., 2008, Beyer et al., 2004, Yucel et al., 2008a) volumes in longitudinal studies of lithium-treated BD patients (Moore et al., 2000, Yucel et al., 2008a) as well as cross-sectional comparisons of lithium-treated subjects with non-lithium treated subjects (Bearden et al., 2007, Sassi et al., 2002), or healthy controls (Bearden et al., 2008, Beyer et al., 2004). Furthermore, treatment with lithium has been associated with an increase in neuronal differentiation and glial cell proliferation in rodents (Chen et al., 2000, Kim et al., 2004, Senatorov et al., 2004); although see (Johnson et al., 2009), who report that lithium treatment prevented stress-induced dendritic hypertrophy of amygdalar pyramidal neurons in rodents.
Partly congruent with these findings, (Foland et al., 2008) carried out a tensor-based morphometric analysis, and observed increased left but not right amygdala volume in a lithium-treated compared with a non-lithium BD control group. These data were difficult to interpret, however, since the non-lithium-treated group was receiving treatment with a variety of other medications (including other mood stabilizers, benzodiazepines, anti-depressants, and/or anti-psychotic agents), no HC group was available for comparison with the two BD samples, and the BD samples were not matched for gender. (Usher et al., 2009) recently found that BD I patients treated with lithium, but not non-lithium treated BD I patients had larger right amygdala volumes than a healthy comparison group. However, the non lithium-treated BD group also displayed larger (but not significantly so) right amygdala volumes than the controls. Again, these data were difficult to interpret because the non lithium-treated group was receiving antipsychotic medication at the time of scanning.
In a similar vein, BD samples with a high percentage of lithium-treated subjects, have yielded evidence of enlarged amygdala volumes (Altshuler et al., 2000, Brambilla et al., 2003). (Strakowski et al., 1999b) reported a correlation between the volume of the amygdala (as well as the hippocampus, prefrontal cortex, thalamus, striatum, globus pallidus) and the use of antipsychotics and mood stabilizers. Conversely, (Chang et al., 2005) reported reduced right amygdala volumes in a medicated pediatric sample with BD. A secondary analysis suggested that past exposure to lithium or valproate protected against GM loss in the left but not right amygdala.
Our data are also partially consistent with two post-mortem studies. (Bezchlibnyk et al., 2007) found evidence of decreased neuronal somal size (suggestive of reduced axodendritic arborization) in the lateral nucleus (LAN) and accessory basal parvocellular nucleus of the amygdala in 11 subjects with BD, relative to schizophrenia, major depressive disorder (MDD), and HC comparison samples. Four out of 11 of the BD subjects in the study had been treated with lithium. (Berretta et al., 2007) reported a 29% decrease in volume of the LAN, a 41% decrease in neuronal number, and a 15% decrease in neuronal density in the LAN in BD samples compared with HC and schizophrenic comparison samples. Eight out of the 10 BD subjects had a lifetime history of lithium and/or valproate treatment, although only 3 of these subjects had been treated with lithium in the 6 months prior to death.
Preclinical studies indicate that significant dendritic remodeling of the amygdala occurs in rodents exposed to repeated stress. The pattern of dendritic remodeling is complex, however, being dependent on the type of stress and the precise anatomical area studied. Chronic, unpredictable stress in rodents has been reported to produce dendritic atrophy in the basolateral amygdala (BLA) (Vyas et al., 2002). Another study reported that chronic restraint stress reduced neuronal spine density in the medial amygdala, but increased spine density in the BLA (Bennur et al., 2007). This pattern of dendritic hypertrophy in response to immobilization stress in the BLA has been confirmed in other studies (Radley and Morrison, 2005) (Vyas et al., 2006), and reportedly persists for at least 21 days (Vyas et al., 2004). Finally, rodents exposed to maternal deprivation showed glial cell reductions in the BLA (Leventopoulos et al., 2007).
Although equivalent human studies are not possible, it is noteworthy that adults receiving chronic corticosteroid therapy (Brown et al., 2007) and children with Cushing’s disease (Merke et al., 2005) reportedly show smaller amygdala volumes than controls. Adrenal steroids appear to facilitate dendritic atrophy in rodents by increasing N-methyl-D-aspartate (NMDA) receptor signaling through the modulation of NMDA and GABA-A receptor gene expression, the activation of voltage-gated calcium channels, and a reduction in the expression of the glutamate transporter (McEwen et al., 2002).
The reason for the general pattern of decreased amygdala volumes in pediatric BD cases apparent in the literature (Pfeifer et al., 2008, Savitz and Drevets, 2009) is unclear since many of these participants are imaged on medication. One possibility is that pediatric BD constitutes a more severe form of illness than that observed in adults. Children with BD are also clinically heterogeneous and often display comorbid conditions such as attention-deficit hyperactivity disorder (ADHD) which may have its own neuropathophysiological sequelae. In addition to raising the issue of medication effects, (Pfeifer et al., 2008) place the reduction in amygdala volume in childhood BD within a developmental framework. In other words, the type of anatomical changes to the amygdala observed in BD may vary from one stage of development to another (Pfeifer et al., 2008). These stage-specific patterns may in part reflect age at illness onset. Nevertheless, in this particular sample, no significant correlation between age at onset and amygdala volume was present.
Post-Hoc Hypothesis Testing
No statistically significant differences in amygdala volume or WBV were found between the BD subjects treated with divalproex and the BD subjects treated with lithium. Although most studies that have investigated the hypertrophic effects of medication have focused on lithium (Moore et al., 2000) (Sassi et al., 2002) (Bearden et al., 2008, Beyer et al., 2004, Yucel et al., 2008a); the neurotrophic effects of divalproex have also been demonstrated in preclinical studies (Yuan et al., 2001) (Hao et al., 2004) (Sugai et al., 2004) (Chen et al., 2006) (Feng et al., 2008) (Wu et al., 2008) (Yasuda et al., 2009).
Divalproex increases the expression of the ERK pathway-regulated anti-apoptotic gene, bcl-2, promotes neuronal growth, and facilitates neurogenesis (Yuan et al., 2001) (Hao et al., 2004). In addition, divalproex administration increases the expression of glial cell line-derived neurotrophic factor (GDNF) and/or brain-derived neurotrophic factor (BDNF) in astrocytes (Chen et al., 2006) (Wu et al., 2008), hippocampal (Frey et al., 2006), and cortical neurons (Yasuda et al., 2009). Compatible with these data, administration of divalproex delays disease onset and prolongs survival in a mouse-model of amyotrophic lateral sclerosis (ALS) (Sugai et al., 2004) (Feng et al., 2008).
(Nakamura et al., 2007) followed patients with first-episode schizophrenia and affective psychosis patients over 1.5 years and found a 6% increase in overall neocortical GM volume in affective psychosis patients treated with lithium or divalproex. No significant difference in cortical GM volume was observed between individuals treated with lithium or divalproex. Consistent with this finding, a group of lithium treated BD patients had greater hippocampal volumes than unmedicated BD patients but the lithium treated BD patients did not differ in hippocampal volume from BD patients treated with lamotrigine or divalproex (Yucel et al., 2008a). Assuming that increases in GM volume play a causal role in remission of BD, the comparative neurotrophic effects of lithium, divalproex, and other medication on brain volume should be tested more rigorously in future studies.
No statistically significant relationship between duration of illness and normalized amygdala volumes was found in our BD samples. There was also no significant correlation between age of illness onset and amygdala volumes. Our data appear compatible with the results of several previous studies that also found no significant association between amygdala volumes and duration of illness, age of onset, number of episodes, or number of previous hospital admissions (Strakowski et al., 1999b) (Blumberg et al., 2003) (Brambilla et al., 2003) (Velakoulis et al., 2006) (Foland et al., 2008) (Usher et al., 2009). These data are congruent with a report of reduced amygdala volume in first-episode BD cases (Rosso et al., 2007), suggesting that the reduction in amygdala volume might be developmental in origin. In contrast, (Altshuler et al., 2000) found that the number of manic episodes was positively correlated with amygdala volume in their BD group without comorbid alcohol abuse but not in their BD patients with a history of alcohol abuse. Nevertheless, no correlation between number of depressive episodes and amygdala volume was observed (Altshuler et al., 2000).
No significant correlation between severity of depression at the time of scanning and amygdala volume was observed. Studies that have reported lithium-associated increases in GM volume in the hippocampus and cortex have made use of samples that were 82% euthymic and 18% depressed (Sassi et al. 2002); 50% depressed or manic and 50% euthymic (Beyer et al. 2004); 70% euthymic and 30% depressed (Bearden et al. 2007); and 81% euthymic and 19% depressed (Bearden et al. 2008). In the two previously published papers that have reported lithium-associated increases in amygdala volume the samples were euthymic (Usher et al. 2009) and ≈ 50% euthymic with the balance made up of depressed and manic patients (Foland et al., 2008). In our sample, all patients were clinically depressed at the time of scanning. Based on these data, it seems reasonable to conclude that the mood stabilizer-associated increase in amygdala volume does not appear to be tightly linked to mood state. The implication of this conclusion is either that dendritic remodeling occurs prior to clinical recovery or that dendritic remodeling occurs regardless of treatment efficacy. The former hypothesis appears more likely to be correct. (Moore et al., 2009) showed that lithium-associated increases in GM volume of the prefrontal cortex were limited to those patients who responded positively to lithium treatment. Nevertheless, this is an issue that should be explored in future studies.
The impact of clinical state on amygdala volume in unmedicated samples is also unclear. We are not aware of other studies that have analyzed amygdala volumes in unmedicated patients with BD. In studies that reported greater amygdala volumes in medicated BD subjects compared to healthy controls, patients were in a manic or mixed mood state (Strakowski et al., 1999b), psychotic (Velakoulis et al., 2006), or euthymic (Altshuler et al., 2000) at the time of scanning. A reduction in amygdala volumes was reported in mostly manic (Rosso et al., 2007); depressed (Chang et al., 2005); and a combination of euthymic, depressed, and hypomanic patients (Blumberg et al., 2003). Thus there does not appear to be a clear relationship between mood state and change in amygdala volume in medicated BD samples. Further studies are needed to determine whether the reduction in amygdala volume observed in our depressed, unmedicated BD sample would be present in a euthymic, unmedicated BD sample.
We also found no significant correlation between the length of time that an unmedicated subject with BD was off medication and amygdala volume. A significant correlation would imply a linear relationship between length of time spent unmedicated and GM volume. However, this relationship is likely to be non-linear since rodent studies suggest that it takes up to 2 months for lesions to reappear after stress induction or chronic corticosterone administration. Another possible reason for the negative result is the large degree of variation in the duration of time that our BD subsample subjects were unmedicated. As noted below, it is also possible that our unmedicated BD subjects were unusual in the sense that they were able to live without medication. It is possible that a greater reduction in amygdala volume over time would have been observed in people with BD for whom medication was indicated, but who did not have access to treatment.
After controlling for duration of illness, we found that the unmedicated BDI patients had a smaller left but not right normalized amygdala volume than unmedicated patients with BDII. However, no significant difference in amygdala volume between BDI and BDII patients was observed in the medicated BD group.
If changes in amygdala volume are a veridical component of the pathophysiology of BD, our data raise the possibility that the degree of reduction in amygdala volume may be a phenotypic correlate of BD nosology/severity in untreated patients. Nevertheless, this finding should be treated with caution because of the small sample size of our BDI samples (n=5 and n=3), which may have led to both type I and type II errors. In particular, our study was underpowered to detect any differences in amygdala volume between BDI and BDII patients. To our knowledge the effect of BD subtype (BDI or BDII) on amygdala volume has not been previously examined. Most studies have not specified the ratio of BDI to BDII subjects in their samples (Pearlson et al., 1997a) (Altshuler et al., 1998) (Altshuler et al., 2000) (Foland et al., 2008) or have only included (or scanned a preponderance of) BDI subjects (Blumberg et al., 2003) (Brambilla et al., 2003) (Lyoo et al., 2004) (Frangou, 2005) (Velakoulis et al., 2006) (Rosso et al., 2007).
Whether bipolar disorders type I and type II fall into distinct nosological categories is unclear. While neuropsychological impairments are widely reported in BD [reviewed in (Savitz et al., 2005)], recent studies suggest that these deficits are either attenuated (Torrent et al., 2006) or not present in patients with BDII (Glahn et al., 2005) (Taylor Tavares et al., 2007) (Savitz et al., 2008). A history of psychosis, which less common in BDII, may be an important variable. A number of researchers have reported that BDI patients with a history of psychosis displayed greater impairment in some cognitive domains than their BDI counterparts without a history of psychosis (Glahn et al., 2007) Antilla et al. (2009) (Savitz et al., 2009). In line with these data, some genetic studies are indicative of overlap in the risk variants for schizophrenia and BD (Barnett and Smoller, 2009) (Lichtenstein et al., 2009) (Moskvina et al., 2009), potentially suggesting a broader diathesis for psychotic breakdown.
The amygdala, with its extensive connections to the medial prefrontal cortex and deeper brain stem nuclei, plays a critical role in evaluating the salience of sensory stimuli and in organizing the emotional and/or behavioral response to such stimuli. In particular, the amygdala mediates the processing of negative emotions such as fear, anger, and sadness (Gloor et al., 1982), and thus dysfunction of the amygdala may adversely affect mood and contribute to the development of affective illness. Specifically, dysregulation of projections from the amygdala to the nucleus basalis, locus ceruleus, and ventral tegmental area (VTA) (Davis and Whalen, 2001) (Sah et al., 2003) may alter cholinergic (ACh), noradrenergic (NE), and dopaminergic (DA) transmission, impacting mood and attention. Dysfunction of the amygdala projections to the bed nucleus of the stria-terminalus (BNST), hypothalamus and periaqueductal gray matter (PAG) (Behbehani, 1995) (Sah et al., 2003) may increase cortisol releasing hormone (CRH) release and anxiety symptoms. Finally, dysregulation of amygdala projections to the ventral striatum (Cardinal et al., 2002) may attenuate or activate reward-seeking and goal-directed behavior, potentially contributing to the anhedonia and amotivation characteristic of depression or the increased involvement in goal-directed or pleasurable activities characteristic of mania or hypomania.
Several limitations of our study design merit comment. Even with high-resolution images, it remains difficult to differentiate the anterior pole of the amygdala from the periamygdalar and temporal claustrum on its lateral border, and the entorhinal cortex on its medial border (Mai et al., 2004). We dealt with this limitation by excluding tissue that could not be delimited clearly from these adjacent structures on the anterior-most coronal plane through the amygdala. The volume of amygdala tissue we measured for this plane thus may have underestimated the true amount of amygdala tissue present, leading to a slight underestimation of the total amygdala volume. However, there is no reason to expect that the extent of this error would differ systematically between BD patients and controls. It is also not possible to delineate subnuclei of the amygdala. In addition, the medication status of the BD subgroups was self-selected, and the groups showed a nonsignificant trend toward differing on depression severity (table 1).
Thirdly, our results may not be generalizable to other studies in the field because the majority of our subjects were diagnosed with BD II while previous studies have either focused on BD I or have not provided a breakdown of the relative percentages of BDI and BDII patients who were scanned. The small number of subjects with BDI in this study may have increased the risk of type II error in any statistical comparisons of BDI and BDII subjects. Further, the BD patients in our study were depressed at the time of scanning and results may differ across studies depending on the mood state of participants.
Finally, we cannot rule out the possibility that a greater number of comorbid conditions in the medicated BD group or a greater level of prior substance abuse or dependence in the medicated BD group contributed to our findings. Nevertheless, studies have reported reduced amygdala volumes in alcohol-dependent (Wrase et al., 2008), cannabis-dependent (Yucel et al., 2008b), and cocaine-dependent (Makris et al., 2004) subjects. Further, conditions such as OCD and PTSD, which were found in greater numbers in our medicated BD sample, have been associated with a reduction in amygdala volume (Szeszko et al., 1999) (Karl et al., 2006) Since our medicated BD sample had larger amygdala volumes than non-medicated BD patients or healthy controls, this suggests that if anything, the increased substance abuse and comorbidity found in our medicated BD sample would have attenuated rather than accounted for our findings.
Ultimately, establishing the impact of medication on cerebral volumes will require longitudinal studies rather than cross-sectional analyses.
Conclusion
We raise the possibility that amygdala volumes are decreased in unmedicated patients with BD but increased in BD patients treated with mood stabilizers such as lithium and divalproex. These medications may modulate the pattern of dendritic remodeling associated with BD. Nevertheless, in rodents the pattern of dendritic remodeling appears complex, with distinct directions of remodeling (atrophy versus hypertrophy) obtaining in different amygdala nuclei under different types of stress. Moreover, the effects of such stress-induced neuroplastic changes on amygdala function remain poorly understood. Our results should therefore be treated with caution until independently replicated.
Footnotes
Disclosure of Interest.
Earl Bain, MD is currently an employee of Abbott Laboratories. Husseini Manji, MD is currently an employee of Johnson and Johnson. In 2006 and 2007, Dennis Charney, MD consulted for Astra Zeneca, Bristol Myers Squibb Company, Cyberonics, Neurogen, Neuroscience Education Institute, Novartis Pharmaceuticals Corporation, and Orexin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–62. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55:663–4. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Hippocampal Formation. In: Paxinos G, editor. The Human Nervous System. Harcourt, Brace, Jovanovich; San Diego, CA: 1990. pp. 711–755. [Google Scholar]
- Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Soares JC. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–38. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–93. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Kuchibhatla M, Payne ME, Moo-Young M, Cassidy F, Macfall J, Krishnan KR. Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry. 2004;12:613–20. doi: 10.1176/appi.ajgp.12.6.613. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Sun X, Wang JF, Macqueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci. 2007;32:203–210. [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–8. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–95. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Brown ES, Woolston DJ, Frol AB. Amygdala Volume in Patients Receiving Chronic Corticosteroid Therapy. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999a;72:1327–30. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–34. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999b;72:879–82. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–25. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–41. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus. Springer; Berlin: 1998. [Google Scholar]
- Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155:567–72. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Dept., New York State Psychiatric Institute; 1995. [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221–4. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S. The Maudsley Bipolar Disorder Project. Epilepsia 46 Suppl. 2005;4:19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–65. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvassori SS, Reus GZ, Quevedo J, Kapczinski F. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79:281–6. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The Neurocognitive Signature of Psychotic Bipolar Disorder. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, Pliszka SR, Olvera RL, Soares JC. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7:546–54. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12:129–44. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- Hanaway J, Woolsey TA, Gado MH, Roberts MP. The Brain Atlas: A Visual Guide to the Human Central Nervous System. Fitzgerald Science Press; Bethesda, M.D: 1998. [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–9. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–26. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- Hawley CJ, Gale TM, Sivakumaran T. Defining remission by cut off score on the MADRS: selecting the optimal value. J Affect Disord. 2002;72:177–84. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163:34–9. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89:324–36. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- Kogelnik AM, Loomis K, Hoegh-Petersen M, Rosso F, Hischier C, Montoya JG. Use of valganciclovir in patients with elevated antibody titers against Human Herpesvirus-6 (HHV-6) and Epstein-Barr Virus (EBV) who were experiencing central nervous system dysfunction including long-standing fatigue. J Clin Virol. 2006;37(Suppl 1):S33–8. doi: 10.1016/S1386-6532(06)70009-9. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, Monk K, Kupfer DJ, Phillips ML. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47:532–9. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–26. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–51. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Elsevier; Amsterdam: 2004. [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–40. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48:740–54. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Ashford JW, Goodman Y, Mattson MP. Anticonvulsants attenuate amyloid beta-peptide neurotoxicity, Ca2+ deregulation, and cytoskeletal pathology. Neurobiol Aging. 1995;16:187–98. doi: 10.1016/0197-4580(94)00150-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM, Reagan LP. Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry. 2002;17(Suppl 3):318–30. doi: 10.1016/s0924-9338(02)00650-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10:525–37. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–17. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- Merke DP, Giedd JN, Keil MF, Mehlinger SL, Wiggs EA, Holzer S, Rawson E, Vaituzis AC, Stratakis CA, Chrousos GP. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J Clin Endocrinol Metab. 2005;90:2531–6. doi: 10.1210/jc.2004-2488. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–2. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, Drevets WC, Manji HK. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, Owen MJ, O’Donovan MC. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009;14:252–60. doi: 10.1038/mp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–83. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Neuroimage. 1998:A12. [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997a;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997b;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–98. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–20. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–87. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Jama. 1990;264:2511–8. [PubMed] [Google Scholar]
- Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61:743–9. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–5. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disord. 2005;7:216–35. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Neuropsychological status of bipolar I disorder: impact of psychosis. Br J Psychiatry. 2009;194:243–51. doi: 10.1192/bjp.bp.108.052001. [DOI] [PubMed] [Google Scholar]
- Savitz JB, van der Merwe L, Stein DJ, Solms M, Ramesar RS. Neuropsychological task performance in bipolar spectrum illness: genetics, alcohol abuse, medication and childhood trauma. Bipolar Disord. 2008;10:479–94. doi: 10.1111/j.1399-5618.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s disease. Mol Psychiatry. 2004;9:371–85. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- Singh MK, Delbello MP, Adler CM, Stanford KE, Strakowski SM. Neuroanatomical characterization of child offspring of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2008;47:526–31. doi: 10.1097/CHI.0b013e318167655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148:217–23. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999a;56:254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999b;56:254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Sugai F, Yamamoto Y, Miyaguchi K, Zhou Z, Sumi H, Hamasaki T, Goto M, Sakoda S. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci. 2004;20:3179–83. doi: 10.1111/j.1460-9568.2004.03765.x. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–40. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–9. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct Profiles of Neurocognitive Function in Unmedicated Unipolar Depression and Bipolar II Depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Torrent C, Martinez-Aran A, Daban C, Sanchez-Moreno J, Comes M, Goikolea JM, Salamero M, Vieta E. Cognitive impairment in bipolar II disorder. Br J Psychiatry. 2006;189:254–9. doi: 10.1192/bjp.bp.105.017269. [DOI] [PubMed] [Google Scholar]
- Usher J, Menzel P, Schneider-Axmann T, Kemmer C, Reith W, Falkai P, Gruber O, Scherk H. Increased right amygdala volume in lithium-treated patients with bipolar I disorder. Acta Psychiatr Scand. 2009 doi: 10.1111/j.1600-0447.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–93. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–84. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang W, Lu RB, Hong JS. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11:1123–34. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–9. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Youngs RM, Chu MS, Meloni EG, Naydenov A, Carlezon WA, Jr, Konradi C. Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J Neurosci. 2006;26:6031–9. doi: 10.1523/JNEUROSCI.0580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–83. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, McKinnon MC, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008a;33:361–7. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008b;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]