Abstract
Adolescence is a time of increased divergence between males and females in physical characteristics, behavior, and risk for psychopathology. Here we will review data regarding sex differences in brain structure and function during this period of the lifespan. The most consistent sex difference in brain morphometry is the 9-12% larger brain size that has been reported in males. Individual brain regions that have most consistently been reported as different in males and females include the basal ganglia, hippocampus, and amygdala. Diffusion tensor imaging and magnetization transfer imaging studies have also shown sex differences in white matter development during adolescence. Functional imaging studies have shown different patterns of activation without differences in performance, suggesting male and female brains may use slightly different strategies for achieving similar cognitive abilities. Longitudinal studies have shown sex differences in the trajectory of brain development, with females reaching peak values of brain volumes earlier than males. Although compelling, these sex differences are present as group averages and should not be taken as indicative of relative capacities of males or females.
Across species that reproduce by combining genetic material, it is frequently adaptive for one of the pair to contribute DNA from a larger, stationary gamete, and the other member of the pair to contribute DNA from a gamete that is smaller and more mobile. The individual contributing the larger gamete is conventionally designated as the female of the species. Evolutionary forces often favor different characteristics in some domains for males and females, including differences in anatomy. Although the extent of these differences varies widely between species, it is the case that within a given species, the sex of an organism is usually the single greatest determinate of size and shape.
In humans, differing evolutionary forces have led to group average differences in brain and behavior between men and women. Recognition of the importance of the adolescent period in the pathogenesis of common psychiatric disorders such as schizophrenia and depression has gained attention (Kessler et al., 2005) and has stimulated increased interest in how brain development differs between males and females and how this may be contributing to their relative risks for specific disorders.
In this review, we will discuss sex differences in brain structure and function during adolescence, a time of increased divergence between males and females in physical characteristics, behavior, and risk for psychopathology (Häfner, 2003; Kessler et al., 2005). We refer to male/female differences in physiology or behavior as “sex differences” as opposed to “gender differences”, considering gender to refer to the social role adapted by the person.
Overview of the process of sexual differentiation
Sexual differentiation is a cascade of events beginning with the process of sex determination and continuing through different stages of development to establish male or female phenotypes. Following a schema proposed by Phoenix and colleagues in 1959, sex-specific events are often categorized as early occurring organizational effects or later occurring activational effects (McCarthy, Schwarz, Wright, & Dean, 2008; Phoenix, Goy, Gerall, & Young, 1959). Although there are many variations on this basic categorization, including the method of sex determination, timing and number of organizational events, magnitude of differences between the sexes, and which steroid hormones are operative, it continues to serve as a valuable template (McCarthy & Ball, 2008). An important modification to the original hypothesis is that sex steroid exposures during puberty are also associated with organizational effects, and that brain structural modifications in response to changing hormonal levels continue throughout adult life (Sisk & Zehr, 2005).
Sex differences in embryos can be detected as early as the 2nd day after conception, with male embryos in both humans (Ray, Conaghan, Winston, & Handyside, 1995) and mice(Burgoyne et al., 1995) being observed to have more cells and a higher metabolic rate (Wilson & Davies, 2007). Around the 6th week post-conception, the SRY gene on the Y chromosome interacts with products of genes on the X chromosome and autosomes to stimulate the primordial fetal gonad to develop into testes (Koopman, 1999). Testicular hormones, including testosterone and Mullerian inhibiting hormone, are produced beginning between 12-16 weeks gestation, which then triggers the separate processes that will masculinize and defeminize the developing organism. In the absence of these factors, the fetus develops into a female.
The occurrence of sex differences prior to the introduction of hormones implies that genes on the X or Y sex chromosomes have direct effects on sexual differentiation. Sex chromosome genes may directly produce products that contribute to sexual dimorphism or regulate transcription on autosomal genes (Malone & Oliver, 2008; Ober, Loisel, & Gilad, 2008). One method of exploring this hypothesis has been the development of a transgenic mouse model in which the sex chromosome complement is dissociated from the gonadal phenotype. The four potential combinations include XX and XY mice without the SRY genes, who develop ovaries, and XX and XY mice with the SRY gene, who develop testes (Arnold, 2009). Studies of this mouse model have revealed dosages of sex chromosome genes affecting a variety of brain structural and behavioral features, including gene expression in multiple tissues (Arnold, 2009; Reisert & Pilgrim, 1991), regional densities of tyrosine hydroxylase and vasopressin neurons, and behaviors such as habit formation (Quinn, Hitchcott, Umeda, Arnold, & Taylor, 2007) and responses to intruders (Arnold, 2009).
There are several potential mechanisms by which sex chromosome genes can affect phenotype directly (Davies & Wilkinson, 2006). One is through male-specific genes present only on the Y chromosome. A second is through dosage differences of sex chromosome genes between XX and XY organisms. As the X chromosome contains many more genes than the Y chromosome, one avenue is through dosage differences between XX and XY organisms of X and Y chromosome genes. Parity is achieved for a majority of these genes by a process of inactivation of most genes on one of the X chromosomes combined with upregulation of the remaining X chromosome in both sexes (Payer & Lee, 2008). Although most of the genes that escape inactivation of the second X chromosome have functional homologues on the Y chromosome, several do not, raising the possibility of dosage effects. A third means by which X and Y chromosome genes may contribute directly to sex differences is through imprinting. Females receive X chromosomes imprinted by both mother and father, while males only receive a single maternally imprinted X chromosome.
The effects of hormones such as estrogen, testosterone, and their multiple active metabolites proceed by several distinct mechanisms. Testosterone is metabolized to the much more potent dihydrotestosterone (DHT) and then to estradiol. Binding of DHT and estradiol to specific receptors in the cell nucleus affects transcription of a broad variety of genes. Estrogen also promotes neurogenesis and synaptic growth directly or via stimulation of GABAergic neurons (McCarthy, Schwarz, Wright, & Dean, 2008). Early in development, GABA acts as an activating neurotransmitter and induces synaptogenesis. In addition to slower transcriptome-mediated effects, estrogen can act quickly through a membrane bound receptor and through second messenger systems (Balthazart & Ball, 2006; Milner et al., 2001). In addition to effects that are related to GABA transmission, the rate of conversion of testosterone to estradiol by aromatase changes in response to fluctuating glutamate levels. Modulation of local estrogen dosages by the rapid conversion of testosterone to estradiol allows effectiveness of estrogen as a neurotransmitter. Although both males and females have endogenous testosterone, which could serve as a substrate for aromatase, it is not known how the much lower endogenous levels of testosterone in females may affect this pathway.
The organizational processes occuring in utero and during early development set the stage for adrenarche, a rise in adrenal steroids beginning between the ages of 6-8 years that is associated with the development of axillary and pubic hair (Patton & Viner, 2007). Gonadarche is initiated in a separate process by the increasing activation of specialized hypothalamic neurons that secrete gonadotropin releasing hormone (GnRH), typically occurring between ages 8-13 in females and between 9-14 in males (Euling et al., 2008; Senzaki et al., 1993). The rise in GnRH stimulates increased secretion of lutenizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary, triggering the rise of output of the gonadal steroids estradiol and testosterone, which in turn brings about the somatic events associated with sexual maturation. Studies of twins indicate that 60-80% of the 4-5 year variation in onset of gonadarche may be due to genetic factors (Silventoinen et al., 2007; van den Berg & Boomsma, 2007). Physiologic and environmental signals appear to have more of a permissive role that prevents pubertal onset in the presence of adverse conditions such as inadequate nutrition (Roseweir & Millar, 2009), and may accelerate pubertal onset in the presence of some kinds of social stressors (Tither & Ellis, 2008). Kisspeptin is a protein triggering the pubertal upsurge in GnRH production, in part through acting as a mediator by which environmental signals are transferred. However, what signals the up regulation of Kisspeptin is not yet known (Gottsch, Clifton, & Steiner, 2009; Navarro, Castellano, García-Galiano, & Tena-Sempere, 2007).
Sex differences in the brains of adolescents and adults
Total brain volume
Postmortem data (Dekaban, 1977; H. Pakkenberg & Voight, 1964; Witelson, Beresh, & Kigar, 2006), in vivo imaging studies of adults (Allen, Damasio, Grabowski, Bruss, & Zhang, 2003; Andreasen et al., 1993; Goldstein et al., 2001; Good et al., 2001; Nopoulos, Flaum, O'Leary, & Andreasen, 2000), and in vivo imaging studies of children (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997; Reiss, Abrams, Singer, Ross, & Denckla, 1996) (De Bellis et al., 2001) all consistently find a 9-12% greater brain size in males. This difference is not accounted for by body size either in adults, (Ankney, 1992; Ho, Roessmann, Straumfjord, & Monroe, 1980; O'brien et al., 2006; Witelson et al., 2006) or in children, where larger brain volumes in males are observed despite the minor sex differences in height and weight characteristic of prepubertal development.
Allometry, the relationship between size and shape, is highly relevant to studies of sexual dimorphism. At least some changes in proportions of different brain regions occur solely as a factor of increasing brain volume (Finlay & Darlington, 1995). For example, it has been reported that the ratio of gray matter (GM) to white matter (WM) is larger in females (Allen et al., 2003; Gur, Gunning-Dixon, Bilker, & Gur, 2002). However, studies within and between species have found that WM volume increases more quickly than GM following a 4/3 power law (Zhang & Sejnowski, 2000). Studies directly comparing male-female differences to effects of overall brain size have found that sex differences in the GM/WM ratio are minimal once overall brain size differences have been accounted for, meaning that males and females with equal brain volumes will also have equal gray/white matter ratios (Leonard et al., 2008).
The complex relationship of cortical morphometry and brain size is highlighted by a recent report in healthy adults relating brain volume to cortical features including thickness, surface area and gyrification (Im et al., 2007a). Consistent with previous reports from postmortem data (Pakkenberg & Gundersen, 1997), they found that increases in gray matter volume were driven primarily by increased surface area rather than cortical thickening. They also found that increased brain size was associated with a marked increase in folding of the cortical surface. There were no sex differences after accounting for differences in total brain size. This is inconsistent with some previous studies which found relatively thicker cortex and greater cortical complexity in females if differences in total brain volume were taken into account (Luders et al., 2004; Luders et al., 2006; Sowell et al., 2006), although the authors speculated that this may have been due to the use of a linear scaling method in the previous studies, which did not completely account for brain size differences. If different brain regions do not scale linearly, it is possible that contrasts in some areas may be ascribed to sexual dimorphism when they are related instead to overall differences in brain volume (Brun et al., 2009).
With respect for the complexities of interpretation introduced by allometric issues, we will next discuss reports of sexual dimorphism in measures adjusted in some way for differences in total brain volume (TBV).
Sexual dimorphism of brain regions
Besides overall differences in brain volume, there are specific areas of the brain which show differences in male and female adolescents and adults. It would seem logical that these may be areas that contain significant populations of sex steroid receptors. Estrogen, androgen, and progesterone receptors are all found in the hypothalamus, consistent with its central role in the control of sexual and reproductive function (Cameron, 2001). Many of the areas with strong connections to the hypothalamus also contain dense numbers of one or more of the sex steroid receptors. Prominent among these regions are the amygdala, bed nucleus of the stria terminalis, and parts of the nucleus of the solitary tract and parabrachial nucleus. Other regions observed to contain sex steroid receptors include the basal ganglia, hippocampus, and cerebellum (Goldstein et al., 2001; Simerly, Chang, Muramatsu, & Swanson, 1990).
Sex steroids impact on cortical function through both direct and indirect pathways. Dopaminergic neurons in the midbrain and elsewhere have been shown to be sensitive to sex steroid activity(Creutz & Kritzer, 2004; Kritzer & Creutz, 2008; Stewart & Rajabi, 1994). Serotonergic neurons in areas such as the dorsal raphe nucleus have also been identified as containing sex steroid receptors.(Bethea, Lu, Gundlah, & Streicher, 2002; Vanderhorst, Gustafsson, & Ulfhake, 2005). Both dopaminergic and serotonergic nuclei project diffusely to wide areas of the cortex. In addition to effects mediated through these systems, sex steroid receptors have within many cortical regions, (Montague et al., 2008), adding the potential for direct effects of sex steroids on cortical development. Among the cortical areas with high densities of steroid receptors are areas of the frontal cortex, motor and somatosensory cortex, posterior parietal cortex, agranular insular cortex and parahippocampal regions (Goldstein et al., 2001).
Our picture of the role of sex steroids in different parts of the brain is still incomplete. More recent studies using assays such as immunohistochemistry methods and in situ hybridization techniques have extended the range of brain regions, cell types, and cellular locations in which sex steroids have been found (Guerriero, 2009; Sarkey, Azcoitia, Garcia-Segura, Garcia-Ovejero, & DonCarlos, 2008). Besides questions relating to the “non-classic” functions of receptors outside of the nucleus, we are just beginning to explore how splice variations in steroid receptor transcription may impact both function and the ability of existing assays to register their presence (Weickert et al., 2008; Weiser, Foradori, & Handa, 2008). There is also limited data regarding developmental changes in sex steroid receptor expression, particularly during puberty (Sugiyama et al., 2009).
Nevertheless, there does appear to be some consistency between regions of the brain with structural sexual dimorphism and those found to have high numbers of sex steroid receptors. After covarying for TBV, orbitofrontal and caudate volumes have been reported to be larger in adult females (Filipek, Richelme, Kennedy, & Caviness Jr., 1994). Goldstein et al (2001) found several frontal and medial paralimbic brain regions to be relatively larger in women, whereas the frontomedial cortex, hypothalamus, amygdala, and angular gyrus were proportionately larger in men. The differences with the greatest effect sizes were also areas that are richly endowed with sex steroid receptors during development as determined by animal models (Goldstein et al., 2001).
In three independent pediatric cohorts, the caudate nucleus has been found to be relatively larger in females (Giedd et al., 1997; Sowell, Trauner, Gamst, & Jernigan, 2002; Wilke, Krägeloh-Mann, & Holland, 2007). Findings of sexual dimorphism in other regions have been less consistent. These include reports of relatively larger regions in the temporal lobes, thalamus, and basomesial diencephalons in females (Sowell et al., 2002), inferior frontal gyrus gray matter (Wilke et al., 2007) and relatively larger measures of total white matter (Wilke et al., 2007) and globus pallidus white matter (Giedd et al., 1997) in males.
Neufang and colleagues used voxel based morphometry to explore sex differences, including the influences of sex steroid levels and pubertal stage to GM and WM densities in a sample of 46 males and 46 females aged 8-15 years (Neufang et al., 2009). In girls, the hippocampus was larger bilaterally, as was the right striatum. In boys, a region of the amygdala was larger in males. With the exception of higher levels of serum testosterone in older males, there were no differences in sex steroid levels in their sample. They first examined the relationship of steroid levels and pubertal stages to brain structure in regions that had already been demonstrated to be sexually dimorphic; they found that GM intensity in the amygdala was predicted by testosterone levels in both males and females. Testosterone levels also predicted hippocampal size in females, but with younger females having larger hippocampi. In a whole brain regression analysis, testosterone was positively associated with increased GM density in right sided diencephalic structures in males, and negatively correlated with parietal GM volume in males. Estradiol levels were positively correlated with greater GM density in the uncus and parahippocampal gyri in girls only. Although this study did not look at effects of sex steroids and pubertal stages separately from effects of chronologic age, the sex-specific effects of testosterone and estrogen are consistent with findings that sex steroids continue to have organizational effects on brain structure during puberty. For more discussion of the effects of pubertal timing on behavior in adolescents, please see the paper by Dahl and Forbes, this issue.
Reports regarding sex dimorphism and pubertal effects on brain development are beginning to appear from a large cohort of typically developing twins currently being followed longitudinally by researchers in the Netherlands (Peper et al., 2009; Peper et al., 2008). Lutenizing hormone (LH) levels were measured as an indication of onset of puberty in 57 male twins (age 9.20 +- 0.10) and 47 female twins (age 9.21 +-0.12), and voxel based morphometry was used to relate LH levels to regional gray and WM densities (Peper et al., 2008). LH levels were found to predict WM volumes when both sexes were looked at together, although not when they were looked at separately, potentially due to the loss in power from the smaller sample sizes. A second analysis in an overlapping cohort of 10-15 year olds, also using voxel based morphometry, compared brain gray and WM density and their relationships to estradiol and testosterone levels (Peper et al., 2009). Males (n=37; age 11.6 +- 1.0 yrs) had overall larger brain volumes than females (n=41, age=12.2 +-1.2yrs) after correction for age differences, although no difference was seen in the ratio of gray to WM between the sexes. The largest regional sex differences were in the putamen, insula, and amygdala, all larger in males. Total GM volumes correlated negatively with estradiol levels in females and positively with testosterone levels in males. A limitation of this study was that the female twin pairs were significantly older than the males. While a factor to account for age was included in all analyses, the authors note that it is possible that males were on the upward portion of their developmental trajectory in brain volume while females were on the downward trajectory, making exact comparisons difficult.
The relationship between WM development and testosterone was examined in a cross sectional sample of 408 healthy adolescents (204 males; age range 12-18)(Perrin et al., 2008). They measured serum testosterone levels and genotyped the androgen receptor, which contains a CAG repeat that affects testosterone activity. They further sought to separate out developmental changes in myelination by measuring changes in the magnetization transfer ratio (MTR), a measure sensitive to macromolecular structure and composition of tissue. In the absence of overt pathology such as edema, the predominant factor affecting MTR in WM is the amount of myelin, and thus developmental changes in MTR are thought to indicate changes in myelination. Similarly to previous studies, they found a much more rapid increase in WM volume in males than females. Although levels of bioavailable testosterone did not add significantly to chronologic age in explaining variation in WM volume in the overall group, it appeared to have a stronger effect in the subset of males with the AR genotype having fewer CAG repeats, a variation associated with higher transcriptional activity of the AR gene and suggesting an interaction of testosterone level with genotype (see Figure 1 in the paper by Paus, this issue). Despite the increasing WM volume, the MTR ratio decreased with age, with age explaining a greater proportion of the variance in the MTR ratio in males (8%) than in females (1%). This pattern suggests that the rapid increase in WM volume in males may be related to other structural elements such as axonal volume rather than myelination.
Figure 1.
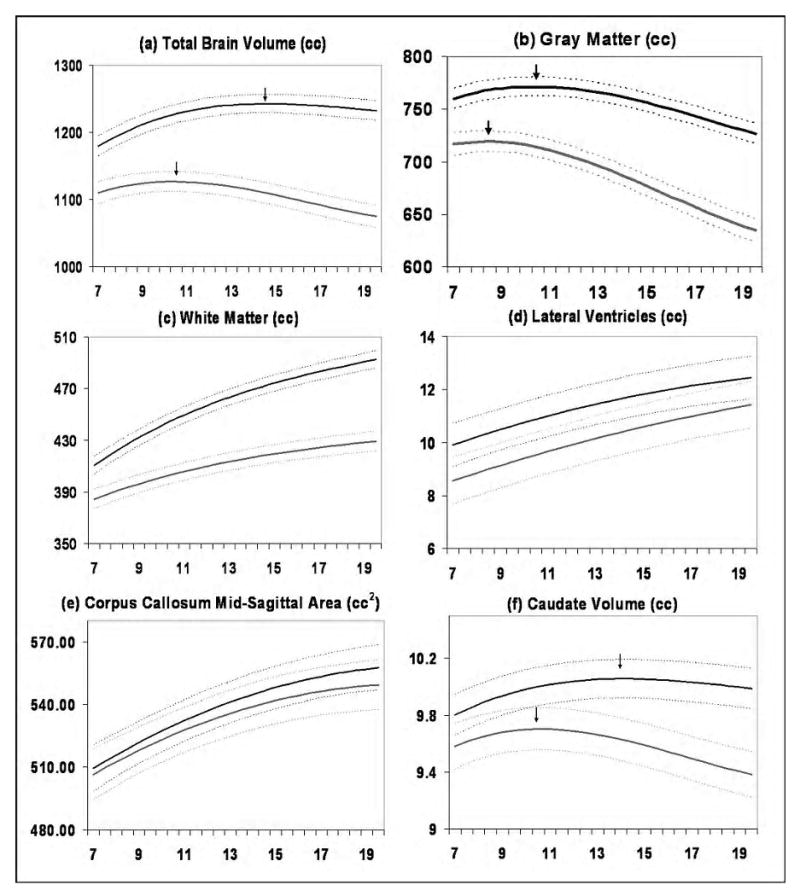
Mean volume by age in years for males (N = 475 scans) and females (N = 354 scans). Middle lines in each set of three lines represent mean values, and upper and lower lines represent upper and lower 95% confidence intervals. All curves differed significantly in height and shape. Figure adapted from Lenroot et al., 2007.
A follow-up study in the same group of adolescents found that males had significantly greater apparent GM density (aGMD) in the putative cortico-spinal tract, a region containing fibers emanating from Brodman's area 4 and thought likely to be involved in motor control (Herve et al., 2009). Males showed a significant increase in aGMD with age, while females did not, such that the sex differences in volume were only present in older adolescents. Rising levels of bioavailable testosterone in males contributed to the variance in the aGMD even after accounting for age effects; females did not show a similar rise in testosterone levels or a similar relationship to aGMD.
Diffusion tensor imaging
In recent years, more studies have begun to appear using imaging methods that aim to quantify brain tissue characteristics directly rather than by measuring volumes of gray and white matter in anatomic structures. One of these is magnetization transfer imaging, discussed above. Another is diffusion tensor imaging (DTI), which provides information about the properties of diffusion of water through different regions of the brain(Mori & Zhang, 2006). If unconstrained, water molecules will randomly diffuse in all directions, whereas water molecules interacting with tissue components such as cell membranes or large molecules will be more likely to diffuse in particular directions. In DTI, a tensor can be calculated for each voxel which represents the overall distribution of the diffusion of the water molecules in that area (Le Bihan et al., 2001). Although many potential measures of water diffusion can be obtained from diffusion imaging data, the most frequently reported metrics are general diffusivity, a measure of how quickly water diffuses in any direction, and fractional anisotropy (FA), which refers to the proportion of water molecules within a certain brain region that are diffusing in the same direction. Factors that increase FA include organization of tissue into tightly packed unidirectional structures and increased myelination; thus structures such as the corpus callosum and interior capsule tend to have high fractional anisotropy values. Mean diffusivity tends to be decreased in the presence of these same factors. Tissues such as the cortex will have lower FA levels despite their highly organized states because tissue elements are going in different directions, a phenomenon termed fiber crossing (see paper by Schmithorst, this issue).
In general, neurodevelopmental studies of diffusion tensor imaging parameters have found FA to be increasing and mean diffusivity to be decreasing in key WM tracts throughout adolescence (Barnea-Goraly et al., 2005; Mukherjee et al., 2002; Schmithorst & Holland, 2007; Schmithorst, Holland, & Dardzinski, 2008; Snook, Paulson, Roy, Phillips, & Beaulieu, 2005). This pattern has been most often interpreted in relation to the increases in myelination associated with maturation previously observed in postmortem studies, although the magnetization transfer findings described above suggest that it may also be related to changes in other structural components.
In the largest study in adolescents thus far, Schmithorst et al measured anisotropy and diffusivity in a group of typically developing subjects (104, 52 male, mean age 12.3 +- 3.5 years)(Schmithorst et al., 2008). They found higher FA and lower mean diffusivity in females in the splenium of the corpus callosum, while in males, FA was higher and mean diffusivity was lower in bilateral frontal WM regions, the right arcuate fasciculus, and left parietal and parieto-occipital WM. Correlations of FA with age also differed between brain regions in males and females; for example, left frontal lobe FA was positively correlated with age in boys, but negatively correlated with age in girls.
Adjusting for presumed earlier maturity in females by comparing females with boys who were approximately two years older resulted in even more pronounced effects with the exception of the splenium, where the effects size of the difference decreased. As increases in FA and decreases in mean diffusivity with development had been reported in a similar population by this group as well as others, the finding of greater FA values in males, despite their later maturation, suggested that sexually dimorphic processes are at work beyond differences in maturational rates. The authors speculated that this may be related to more extensive fiber crossing in females. A study in 21 adolescents (9 male, mean age 12.3 +- 2.9yrs) also found that males overall had higher FA values in left frontal WM regions than females (Silveri et al., 2006). These results are different than those found in a study in adults, which reported greater anisotropy in frontal regions in females (Szeszko et al., 2003). It is possible that relative anisotropy values in males and females may change as maturation completes, a question awaiting the availability of longitudinal data. Further information on changes in diffusion tensor imaging parameters during adolescence can be found elsewhere in this issue.
Cortical morphometry
Postmortem studies in adults have consistently found sex differences in the cortical cytoarchitecture, including higher neuronal densities in granular cortical layers in females (Witelson, Glezer, & Kigar, 1995), higher overall neuronal densities and numbers in males (Rabinowicz, Dean, Mcdonald-Comber Petetot, & De Courten-Myers, 1999), and more neuropil in females(Rabinowicz et al., 2002) without overall differences in cortical thickness (Mayhew, Mwamengele, & Dantzer, 1996). A greater number of neurons in the brain and a thicker cortex has also been reported in males regardless of overall body size (Pakkenberg & Gundersen, 1997) as well as higher synaptic density in males throughout the cortex(Alonso-Nanclares, Gonzalez-Soriano, Rodriguez, & DeFelipe, 2008). Regional differences include a larger visual cortex in males (Amunts et al., 2007). Language-related areas such as the superior temporal cortex and Broca's region have been reported larger in females (Harasty, Double, Halliday, Kril, & McRitchie, 1997), and one study found the cortex to be thicker in females (Henery & Mayhew, 1989). Cortical complexity has been reported as similar in males and females (Zilles et al., 1998). There are currently no equivalent postmortem studies in pediatric subjects.
Results from neuroimaging studies have been mixed. Some neuroimaging studies have not found sex differences in cortical thickness (Nopoulos et al., 2000; O'Donnell, Noseworthy, Levine, & Dennis, 2005; Salat, 2004) after covarying for total brain volume, or they have found trends towards greater thickness in males (Salat, 2004). Others have found thicker cortex in females after taking differences in overall brain volume into account. Luders and colleagues measured cortical thickness in sixty healthy right handed adults (females aged 24.32 +- 4.35 years; males 25.45 +- 4.72 years), using a method in which spatially homologous regions of the cortex were aligned between individuals by matching patterns of cortical landmarks (Luders et al., 2006). They found that when total brain volume was covaried, the cortex was thicker in females across nearly the entire lateral surface of the brain. If unadjusted values were used, a similar but less widespread pattern of greater thickness in females was found, which was most pronounced in the left inferior and superior frontal gyri, and then to a lesser extent in the superior pre- and post-central regions and occipital lobe. In contrast, males had an area of increased thickness in the left posterior temporal lobe. Surface area was significantly larger in females when scaled data were used and was larger in males using the original unscaled data. Gyrification, measured through determining the degree of curvature at thousands of points across the brain, was higher in females in several regions in the frontal, parietal, and temporal lobes. The areas of greatest differences were in the anterior regions of the frontal lobe. No regions had greater gyrification in men(Luders et al., 2004; Luders et al., 2006).
Using the same cortical-pattern matching method of comparing cortical thickness measurement as Luder et al, a cross-sectional study in 176 subjects who ranged from 7 to 87 years of age reported females to have thicker cortices in the right inferior parietal and posterior temporal regions (Sowell et al., 2007). In this study, the authors accounted for scaling issues related to differences in total brain size by creating a subsample of 36 adult subjects (18 males and 18 females) who had been individually matched on total brain volume and age. They found that even when overall brain volume and GM volumes were identical, females had thicker cortex in the right lateral frontal, temporal, and parietal cortices. These were in similar regions as found when using the entire sample, except even more statistically robust, despite the smaller sample size. While there were also significant changes in cortical thickness with age, these were not in the same regions as where sex differences were identified, leading the authors to suggest that these sex differences may exist prior to the age of the youngest participants in their study, which was age 7.
Sex Differences in Developmental Trajectories
An emerging theme from longitudinal studies is that in neuroimaging, as in life, the journey is often as important as the destination. This is exemplified in MRI studies where developmental trajectories of morphometry (i.e. size by age curves) show discriminating features not found with static measures for predicting cognitive parameters (Shaw et al., 2006), separating clinical groups (Shaw et al., 2007), and predicting good and bad outcomes (Mackie et al., 2007). Understanding the sexual dimorphism of developmental brain trajectories may also clarify some of the allometric issues previously discussed.
In 1989, the Child Psychiatry branch at the NIMH initiated a large scale longitudinal study of typical brain development, which to date has acquired data regarding brain development and function from over 1000 typically developing children (including twins and siblings) scanned from 1-7 times at approximately two year intervals. A study of a subset of these data, which included 829 scans from 387 unrelated individuals (age range 3-27, 209 males), demonstrated that neurodevelopmental trajectories were significantly different between males and females (Lenroot et al., 2007). Total brain size followed an inverted U trajectory in both sexes, with peak total brain size occurring at approximately 10.5 years in females and 14.5 years in males. Regional GM volumes also followed an inverted U shaped maturational curve and peaked earlier in females {SEE FIGURE 1}.
WM volumes continued to increase in both males and females throughout the age range of the study. Consistent with a prior report from an independent cohort of 188 children and adolescents (De Bellis et al., 2001), WM in males grew more rapidly, resulting in increasingly larger volumes relative to females with age. After covarying for total brain volume, many of the regional size differences disappeared. Differences were still present in the frontal lobe, in which GM volume was proportionately larger in females, and which had sex differences in rates of growth for GM and WM. The lateral ventricles were larger in males, while the corpus callosum was relatively larger in females (Lenroot et al., 2007).
In an earlier study from the NIMH sample, males had a more rapid increase in amygdala size, while the hippocampus grew more quickly in females (Giedd et al., 1997). Suzuki and colleagues also found sexual dimorphism in the growth of the hippocampus, but in this case, they reported more pronounced growth in males. They measured age and sex effects on volumes of the hippocampus and parahippocampus in a group of 23 adolescents (13-14 yrs age, 10 male) and 30 young adults (18-21 years, 15 males)(Suzuki et al., 2005). Hippocampal volumes were significantly larger in the adult males than in the adolescents, but there were no differences in females. The authors speculated that the discrepancy could be related to the older age of participants in this study compared to previous reports, suggesting that hippocampal growth may occur earlier in girls than boys, consistent with the pattern seen in other brain regions (Lenroot et al., 2007).
Sex differences in brain physiology
Studies have reported sex differences in brain activation levels in the presence of equal cognitive ability (Bell, Willson, Wilman, Dave, & Silverstone, 2006). Some have suggested that, at least in adulthood, females tend to have more bilateral activity during tasks, and males more regional activation (Shaywitz et al., 1995). One study found that females had less relative activation for a given level of task performance than males, possibly indicating greater efficiency (Christova, Lewis, Tagaris, Uğurbil, & Georgopoulos, 2008). Although there has been very little investigation yet into sex differences in functional brain development during adolescence, one study looking at an interaction of sex and age during adolescence with regards to responses to viewing different types of faces found that females and males had similar responses to angry faces during childhood. However, females showed greater responses to angry faces after puberty, while males did not change. This pattern is possibly related to sex-related changes in HPA axis functioning, which is discussed in more detail below (Mcclure, 2004).
A hint towards the potential relevance of sex steroid differences to brain activity can be suggested by studies looking at brain activation across the different phases of the menstrual cycle. Brain activation levels themselves change as a function of menstrual phase (Goldstein, 2006). The relation to cognitive function also may change. For example, several studies have found that performance and brain activation fluctuate across the menstrual cycle on tasks including spatial ability (Hausmann & Gunturkun, 2000; Schöning et al., 2007) and semantic performance (Konrad et al., 2008). Tests of learning and memory also show fluctuations across the menstrual cycle, suggesting that temporary changes in sex steroid exposure can affect neuronal plasticity (Farage, Osborn, & Maclean, 2008; Sherwin, 2003). A recent study examining interhemispheric inhibition found that the influence of left hemispheric regions is much stronger during the menses, while lateralization decreases during the follicular phase as estradiol levels rise (Weis et al., 2008). Although brain imaging studies in general rarely explicitly account for influences such as menstrual phase, the opportunity to study the interaction of changing levels of steroid hormones with neuronal function may provide an invaluable window onto the processes underlying neuronal plasticity and how it may change in response to the hormonal changes that characterize adolescence.
Sex differences in brain development during adolescence: impact on function
Sex differences in cognitive ability are modest (McCarthy & Konkle, 2005). Patterns of social interaction typically show much stronger contrast, extending in varying form and degrees of magnitude across species. In general, species having the greatest differences in roles in procreation tend also to have the most marked behavioral differences (McCarthy, 2008). In humans and most other mammalian species, females have generally been characterized as being more sensitive to social cues and stresses, such as perception of rejection. Evolutionarily this has been tied to adaptation of social roles to facilitate bearing offspring and having primary responsibility for care of the very young, including the capacity for attunement needed to foster cognitive and social development of the neonate (Cyranowski, Frank, Young, & Shear, 2000). The relationship of the sex differences in brain development described by the neuroimaging studies above to these functional differences in social behavior is as yet largely unexplored. It is intriguing to speculate that a better understanding of the neurodevelopmental processes underlying sex differences in social cognition may also provide a key to another functional aspect of sex differences during adolescence: the disparity in rates of onset, course, and symptomatology of the common psychiatric disorders whose incidence begins to rise during this time.
The clearest example of this increased incidence is major depression. Prior to the onset of puberty, males and females have approximately equal rates of depression at 5%. With the onset of puberty, rates in females double, while males stay approximately the same (Angold, Costello, & Worthman, 1998). Many factors may play a role in this pattern, including different stresses associated with gender expectations, the higher incidence of exposure to trauma in young females, and differences in social cognitive function such as rejection sensitivity (Cyranowski et al., 2000; Zahn-Waxler & Shirtcliff, 2006). One potential mechanism is sex differences in the development of the HPA axis. Studies indicate that the increase in incidence of depression is linked to pubertal maturation rather than increases in chronological age (Angold et al., 1998). In females, there is an increased response of the HPA axis to stress with advancing puberty, while in males, the response is decreased, possibly associated with increased testosterone levels (Mccormick & Mathews, 2007).
Schizophrenia is another disorder whose incidence rises markedly during adolescence and whose presentation shows significant sex differences (Castle, Sham, & Murray, 1998; Grossman, Harrow, Rosen, Faull, & Strauss, 2008; McGlashan & Bardenstein, 1990). Schizophrenia is slightly more common in males (McGrath, Saha, Chant, & Welham, 2008). Males also have a distinct peak age of onset during late adolescence and young adulthood, while the peak in females is later and more gradual, and there is a second rise in incidence around the time of menopause (Angermeyer & Kuhn, 1988). If schizophrenia is linked to abnormalities in adolescent brain development, this would seem counter-intuitive based on the behavioral and brain imaging data that females mature earlier than males. It has been suggested that the pubertal surge in estrogen levels seen in females but not males has a neuroprotective effect, possibly serving to delay onset of the disorder and ameliorate some of its effects (Kulkarni et al., 2008). Women with schizophrenia also tend to continue to have better outcomes, linked at least in part to better social functioning than males (Castle & Murray, 1991; Grossman et al., 2008; Thorup et al., 2007). It has also been observed that males are more likely to have a history of lower functioning prior to their first psychotic break, suggesting that males may be more at risk for earlier abnormal neurodevelopmental processes that could interact with other processes during adolescence to facilitate the onset of schizophrenia (Castle & Murray, 1991; Thorup et al., 2007).
Conclusion
In summary, male adolescents, as a group, have larger brain volumes than females. The longitudinal data show that adolescent males reach their peak volumes later than females, such that volumes become increasingly divergent as males and females reach adulthood, particularly for WM. Assessing regional differences is complicated by the observation that scaling with increased volume is not necessarily linear, leading to regional differences that could be attributable to variations in brain size alone (Im et al., 2007b; Leonard et al., 2008). The complexities of comparing brain measurements in cross-sectional data across development are highlighted by findings in the hippocampus and the corpus callosum, in which studies done at different ages have found different patterns of sex differences (Giedd et al., 1997; Lenroot et al., 2007; Suzuki et al., 2005). If brain regions are growing at different rates, the size or even direction of the difference between them could depend on the age at which measurements are made.
The regions most frequently reported by imaging studies as showing morphological sex differences include the basal ganglia and limbic structures. The caudate has been reported as proportionately larger in females by several studies across different ages and using different methodologies (Filipek, Richelme, Kennedy, & Caviness, 1994; Giedd et al., 1997; Sowell et al., 2002), which is intriguing given the involvement of the basal ganglia in disorders with pronounced sex differences in incidence such as Attention Deficit/Hyperactivity Disorder and Tourette's syndrome. The other areas most frequently reported as being different even after accounting for overall differences in brain size are the hippocampus and amygdala (Giedd et al., 1997; Goldstein et al., 2001; Suzuki et al., 2005; Wilke et al., 2007), with larger size or more rapid growth of the hippocampus typically reported in females, and of the amygdala in males. These findings appear consistent with observations of greater densities of androgen receptors in the amygdala versus higher levels of estrogen receptors in the hippocampus, as well as with the preliminary data combining steroid levels and brain volumes, which seem to indicate more sensitivity of the amygdala to testosterone levels and of the hippocampus to estrogen. These areas have also been associated with disorders such as depression and anxiety, disorders which show distinct differences between the sexes (Becker et al., 2007; McEwen, 2001; Romeo, Waters, & Mcewen, 2004).
DTI and magnetization transfer imaging have also shown sex dimorphism in measurements sensitive to the brain's microstructural features such as myelination and tissue organization. Studies of brain activity have shown different patterns of activation in the presence of equal cognitive performance, suggesting that male and female brains may follow slightly different paths to achieve similar levels of function.
Do sex differences in brain structure or function during adolescence explain sex differences in functional capacity and behavior? Answering this question is one of the next pressing tasks for better understanding how the processes of sexual differentiation affect behavior or risk for psychopathology. Separating out effects of development from those of sex is challenging, particularly in cross-sectional data, given the enormous variability within the normal range of both brain structural features and ability. For example, if brain volumes in the frontal lobe appear to peak two years earlier in females, suggesting more rapid development in females, is matching populations on chronological age the most appropriate method, or should developmentally equivalent groups be chosen? And if the latter, what measure of development should be used? Pubertal stage is one possibility, as seen in some of the studies here, but the timing of pubertal maturation relative to other aspects of adolescence varies between individuals. Moreover, different systems may not mature at the same rate even within the same person, and not all cognitive maturation is
Understanding how sex differences interact with other factors that lead to vulnerability or resilience for neuropsychiatric disorders could throw unexpected light on relevant pathways. A consequence of sex differences in brain development is the possibility that treatments may not have the same effects in males and females, which may be important to explore further for the goal of optimizing individual treatment strategies (Hodes, Yang, Van Kooy, Santollo, & Shors, 2009). Although a better understanding of how sex differences develop during childhood and adolescence may eventually help to guide interventions such as treatment and education, it should be remembered that all the findings discussed in this paper represent group averages with substantial overlap between groups. Causality has not yet been established between any normal variation of brain development and functional ability. Neuroimaging findings should be taken as clues pointing us towards different processes affecting male and female brain development rather than definitive statements about the capabilities of male or female individuals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18(4):880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Alonso-Nanclares L, Gonzalez-Soriano J, Rodriguez JR, DeFelipe J. Gender differences in human cortical synaptic density. Proc Natl Acad Sci U S A. 2008;105(38):14615–14619. doi: 10.1073/pnas.0803652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Armstrong E, Malikovic A, Homke L, Mohlberg H, Schleicher A, et al. Gender-specific left-right asymmetries in human visual cortex. J Neurosci. 2007;27(6):1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, 2d, O'Leary DS, Alliger R, Cohen G, et al. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. see comments. [DOI] [PubMed] [Google Scholar]
- Angermeyer MC, Kuhn L. Gender differences in age at onset of schizophrenia. An overview. Eur Arch Psychiatry Neurol Sci. 1988;237(6):351–364. doi: 10.1007/BF00380979. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1998 doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Ankney CD. Differences in brain size. Nature. 1992;358:532. doi: 10.1038/358532c0. letter; comment. [DOI] [PubMed] [Google Scholar]
- Arnold A. Mouse Models for Evaluating Sex Chromosome Effects that Cause Sex Differences in Non-Gonadal Tissues. J Neuroendocrinol. 2009;21(4):377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball G. Is brain estradiol a hormone or a neurotransmitter? Trends in Neurosciences. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Becker J, Monteggia L, Perrot-Sinal T, Romeo R, Taylor J, Yehuda R, et al. Stress and Disease: Is Being Female a Predisposing Factor? Journal of Neuroscience. 2007;27(44):11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30(2):529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Brun CC, Lepore N, Luders E, Chou YY, Madsen SK, Toga AW, et al. Sex differences in brain structure in auditory and cingulate regions. Neuroreport. 2009;20(10):930–935. doi: 10.1097/wnr.0b013e32832c5e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350(1333):253–260. doi: 10.1098/rstb.1995.0159. discussion 260-251. [DOI] [PubMed] [Google Scholar]
- Cameron JI. Effects of sex hormones on brain development. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, Mass.: MIT Press; 2001. p. xvi.p. 685. [Google Scholar]
- Castle D, Sham P, Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res. 1998;33(3):179–183. doi: 10.1016/s0920-9964(98)00070-x. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychological medicine. 1991;21(3):565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- Christova P, Lewis S, Tagaris G, Uğurbil K, Georgopoulos A. A voxel-by-voxel parametric fMRI study of motor mental rotation: hemispheric specialization and gender differences in neural processing efficiency. Exp Brain Res. 2008;189(1):79–90. doi: 10.1007/s00221-008-1405-x. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476(4):348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Davies W, Wilkinson L. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126(1):36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Tables of cranial and orbital measurements, cranial volume, and derived indexes in males and females from 7 days to 20 years of age. Ann Neurol. 1977;2:485–491. doi: 10.1002/ana.410020607. [DOI] [PubMed] [Google Scholar]
- Euling S, Herman-Giddens M, Lee P, Selevan S, Juul A, Sorensen T, et al. Examination of US Puberty-Timing Data from 1940 to 1994 for Secular Trends: Panel Findings. Pediatrics. 2008;121(Supplement):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Farage M, Osborn T, Maclean A. Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch Gynecol Obstet. 2008;278(4):299–307. doi: 10.1007/s00404-008-0708-2. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: An MRI-based morphometric analysis. Cerebral Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Hormones and Behavior. 2006;50(4):612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of aging in 465 normal adult human brains. NeuroImage. 2001;14(1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gottsch M, Clifton D, Steiner R. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30(1):4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman LS, Harrow M, Rosen C, Faull R, Strauss GP. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49(6):523–529. doi: 10.1016/j.comppsych.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G. Vertebrate sex steroid receptors: evolution, ligands, and neurodistribution. Ann N Y Acad Sci. 2009;1163:154–168. doi: 10.1111/j.1749-6632.2009.04460.x. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12(9):998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28 2:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54(2):171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Gunturkun O. Steroid fluctuations modify functional cerebral asymmetries: the hypothesis of progesterone-mediated interhemispheric decoupling. Neuropsychologia. 2000;38(10):1362–1374. doi: 10.1016/s0028-3932(00)00045-2. [DOI] [PubMed] [Google Scholar]
- Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. J Anat. 1989;167:167–180. [PMC free article] [PubMed] [Google Scholar]
- Herve PY, Leonard G, Perron M, Pike B, Pitiot A, Richer L, et al. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KC, Roessmann U, Straumfjord JV, Monroe G. Analysis of brain weight. I. Adult brain weight in relation to sex, age, and race. Arch Pathol Lab Med. 1980;104:635–639. [PubMed] [Google Scholar]
- Hodes GE, Yang L, Van Kooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009;163(2):609–617. doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Size and Cortical Structure in the Adult Human Brain. Cong Rec. 2007a;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Im K, Lee J, Lyttelton O, Kim S, Evans A, Kim S. Brain Size and Cortical Structure in the Adult Human Brain. Cerebral Cortex. 2007b;18(9):2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Konrad C, Engelien A, Schöning S, Zwitserlood P, Jansen A, Pletziger E, et al. The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm. 2008;115(9):1327–1337. doi: 10.1007/s00702-008-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P. Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci. 1999;55(67):839–856. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28(38):9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955–960. doi: 10.1001/archpsyc.65.8.955. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C, Towler S, Welcome S, Halderman L, Otto R, Eckert M, et al. Size Matters: Cerebral Volume Influences Sex Differences in Neuroanatomy. Cerebral Cortex. 2008;18(12):2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr K, Thompson P, Rex D, Jancke L, Steinmetz H, et al. Gender differences in cortical complexity. Nat Neurosci. 2004;7(8):799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr K, Thompson P, Rex D, Woods R, Deluca H, et al. Gender effects on cortical thickness and the influence of scaling. Human Brain Mapping. 2006;27(4):314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164(4):647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Malone JH, Oliver B. The sex chromosome that refused to die. Bioessays. 2008;30(5):409–411. doi: 10.1002/bies.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Mwamengele GL, Dantzer V. Stereological and allometric studies on mammalian cerebral cortex with implications for medical brain imaging. J Anat. 1996;189(Pt 1):177–184. [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Ball GF. The neuroendocrine control of sex specific behavior in vertebrates: lessons from mammals and birds. Curr Top Dev Biol. 2008;83:213–248. doi: 10.1016/S0070-2153(08)00407-9. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Frontiers in neuroendocrinology. 2005;26(2):85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Schwarz JM, Wright CL, Dean SL. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol. 2008;20(6):777–783. doi: 10.1111/j.1365-2826.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclure E. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55(11):1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Mccormick C, Mathews I. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology Biochemistry and Behavior. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Genome and hormones: gender differences in physiology. J Applied Physiol. 2001;18 doi: 10.1152/jappl.2001.91.4.1485. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Bardenstein KK. Gender differences in affective, schizoaffective, and schizophrenic disorders. Schizophr Bull. 1990;16(2):319–329. doi: 10.1093/schbul/16.2.319. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429(3):355–371. [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20(7):893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Castellano J, García-Galiano D, Tena-Sempere M. Neuroendocrine factors in the initiation of puberty: The emergent role of kisspeptin. Rev Endocr Metab Disord. 2007;8(1):11–20. doi: 10.1007/s11154-007-9028-2. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O'Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- O'brien L, Ziegler D, Deutsch C, Kennedy D, Goldstein J, Seidman L, et al. Adjustment for Whole Brain and Cranial Size in Volumetric Brain Studies: A Review of Common Adjustment Factors and Statistical Methods. Harvard Review of Psychiatry. 2006;14(3):141–151. doi: 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- O'Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24(4):948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Ober C, Loisel D, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384(2):312–320. [PubMed] [Google Scholar]
- Pakkenberg H, Voight J. Brain weight of the Danes. Acta Anat. 1964;56:604–606. [Google Scholar]
- Patton G, Viner R. Pubertal transitions in health. The Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- Peper J, Brouwer R, Schnack H, Van Baal G, Van Leeuwen M, Van Den Berg S, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Peper J, Brouwer R, Schnack H, Vanbaal G, Vanleeuwen M, Vandenberg S, et al. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33(7):909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Sex chromosome complement regulates habit formation. Cong Rec. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean D, Mcdonald-Comber Petetot J, De Courten-Myers G. Gender Differences in the Human Cerebral Cortex: More Neurons in Males; More Processes in Females. J Child Neurol. 1999;14(2):98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Petetot JM, Gartside PS, Sheyn D, Sheyn T, de CM. Structure of the cerebral cortex in men and women. J Neuropathol Exp Neurol. 2002;61(1):46–57. doi: 10.1093/jnen/61.1.46. [DOI] [PubMed] [Google Scholar]
- Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil. 1995;104(1):165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons--genetic or epigenetic? Trends Neurosci. 1991;14(10):468–473. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Waters E, Mcewen B. Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. NGB. 2004;1(03):11. doi: 10.1017/S1740925X05000086. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2009;15(2):203–212. doi: 10.1093/humupd/dmn058. [DOI] [PubMed] [Google Scholar]
- Salat D. Thinning of the Cerebral Cortex in Aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm Behav. 2008;53(5):753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35(1):406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöning S, Engelien A, Kugel H, Schäfer S, Schiffbauer H, Zwitserlood P, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45(14):3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Senzaki A, Okubo Y, Abe T, Matsuura M, Moriiwa M, Asai K, et al. Quantitative magnetic resonance imaging in patients with temporal lobe epilepsy. Journal Of Epilepsy. 1993;6(4):243–249. [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. see comments. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24(2):133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Bartels M, Posthuma D, Estourgie-van Burk GF, Willemsen G, van Beijsterveldt TC, et al. Genetic regulation of growth in height and weight from 3 to 12 years of age: a longitudinal study of Dutch twin children. Twin Res Hum Genet. 2007;10(2):354–363. doi: 10.1375/twin.10.2.354. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn Reson Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology. 2005;26(34):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson L, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sowell E, Peterson B, Kan E, Woods R, Yoshii J, Bansal R, et al. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age. Cerebral Cortex. 2006;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Res. 1994;646(1):157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Andersson S, Lathe R, Fan X, Alonso-Magdalena P, Schwend T, et al. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14(2):223–232. 117. doi: 10.1038/mp.2008.118. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, et al. Male-specific volume expansion of the human hippocampus during adolescence. Cereb Cortex. 2005;15(2):187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, et al. Sex differences in frontal lobe white matter microstructure: a DTI study. Brain Imaging. 2003;14(18):2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Ohlenschlaeger J, Christensen T, Krarup G, et al. Gender differences in young adults with first-episode schizophrenia spectrum disorders at baseline in the Danish OPUS study. J Nerv Ment Dis. 2007;195(5):396–405. doi: 10.1097/01.nmd.0000253784.59708.dd. [DOI] [PubMed] [Google Scholar]
- Tither JM, Ellis BJ. Impact of fathers on daughters' age at menarche: a genetically and environmentally controlled sibling study. Dev Psychol. 2008;44(5):1409–1420. doi: 10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- van den Berg SM, Boomsma DI. The familial clustering of age at menarche in extended twin families. Behav Genet. 2007;37(5):661–667. doi: 10.1007/s10519-007-9161-4. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488(2):152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V, et al. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet. 2008;17(15):2293–2309. doi: 10.1093/hmg/ddn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Hausmann M, Stoffers B, Vohn R, Kellermann T, Sturm W. Estradiol modulates functional brain organization during the menstrual cycle: an analysis of interhemispheric inhibition. J Neurosci. 2008;28(50):13401–13410. doi: 10.1523/JNEUROSCI.4392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57(2):309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krägeloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 2007;178(3):296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133(2):331–359. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129(Pt 2):386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. Journal of Neuroscience. 1995;15(5):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff E. Childhood Disorders: Gender and Psychopathology. Annu Rev Clin Psychol. 2006 doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(10):5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Langemann C, Amunts K, Morosan P, Palomero-Gallagher N, et al. Quantitative analysis of sulci in the human cerebral cortex: Development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Human Brain Mapping. 1998;5(4):218–221. doi: 10.1002/(SICI)1097-0193(1997)5:4<218::AID-HBM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]


